
Office of Water
EPA
822R23003
May 2023
Report on the 2
nd
Five-Year Review of
EPA’s Recreational Water Quality
Criteria
i
Disclaimer
While this document cites statutes and regulations that contain legally binding requirements, it
does not itself impose legally binding requirements on EPA, states, tribes, other regulatory
authorities, or the regulated community. EPA, state, tribal, and other decision makers retain the
discretion to adopt approaches on a case-by-case basis that differ from those discussed in this
document as appropriate and consistent with statutory and regulatory requirements. This
document does not confer legal rights or impose legal obligations upon any member of the
public. This document does not constitute a regulation, nor does it change or substitute for any
Clean Water Act (CWA) provision or EPA regulations. EPA can update this document as new
information becomes available. EPA and its employees do not endorse any products, services, or
enterprises. Mention of trade names or commercial products in this document does not constitute
an endorsement or recommendation for use.
Acknowledgments
This report was developed by the U.S. Environmental Protection Agency’s (EPA’s) Office of
Science and Technology (OST), Office of Water (OW). OST/OW authors are Katie Bentley
(formerly EPA Region 3, currently at OWM), John Ravenscroft, Lars Wilcut, Tracy Bone,
Shamima Akhter, Adrienne Keel, Lesley D’Anglada (formerly at OST, currently at OITA),
Betsy Behl, Sara Hisel-McCoy, Susan Euling, Shari Barash (formerly at OST, currently at
OPPT), and Menchu Martinez. EPA authors from the Office of Research and Development
(ORD) are Orin Shanks, Tim Wade, and Rick Greene. EPA contributors, including to systematic
literature review screening, and section reviewers include Kevin Oshima (ORD), Donna Hill
(ORD), Elizabeth Hilborn (ORD), Jingrang Lu (ORD), Aabir Banerji (ORD), Armah de la Cruz
(ORD), Blake Schaeffer (ORD), Toby Sanan (ORD), Dan Tettenhorst (ORD), Asja Korajkic
(ORD), Alison M. Franklin (ORD), Rich Haugland (ORD), Marika Schulhof (former AAAS
Fellow in OST), Czarina Cooper (OST), Sharon Nappier (OST), and Tom Glazer (OGC).
Literature searches and screening and technical editing was conducted by ICF International LLC
(EPA contract number: 68HERC19D0003, Task Order number: 68HERC21F0443). ICF Task
Order Managers: Audrey Ichida and Lucas Rocha Melogno. ICF Technical Contributors: Kaedra
Jones, Laura Tuhela-Reuning, Caelen Caspers, Nidhi Patel, Madison Lee, Ryan Gan, Hannah
Eglington, Afroditi Katsigiannakis, Connie Wanchen Xiong, Jaycee Mayer, Chenyang Wang,
Lauren Browning, and Sara Schwarzkopf. ICF subcontractors: Jeff Soller and Mary Schoen.
ii
Contents
I. Introduction ............................................................................................................................. 1
II. Background .............................................................................................................................. 3
A. Brief Description of the 2012 RWQC and Key Aspects of Implementation .................... 3
1. Criteria Magnitude, Duration, and Frequency: Geometric Mean and Statistical
Threshold Value ........................................................................................................ 3
B. How the 2
nd
Five-Year Review Was Conducted ............................................................... 5
1. Scope and Methods of the Review ............................................................................ 5
2. Inventory of Scientific Information Published Since 2016 ....................................... 5
3. Summary of Recreational Criteria Implementation Tools Developed and EPA
Outreach and Training Since 2016 ............................................................................ 5
4. Sources of Information and How Information Was Accessed .................................. 6
5. A Systematic Review of Available Peer-Reviewed Literature ................................. 6
III. Findings of the 2
nd
Five-Year Review ..................................................................................... 9
A. New Information on Health Effects after Exposure to Recreational Waters .................... 9
1. Recreational Water Epidemiological Studies ........................................................... 9
2. Use of QMRA to Understand Risks in Recreational Water Settings ...................... 16
3. Outbreak Studies of Illnesses in Ambient Recreational Waters Associated with
Enteric Pathogens .................................................................................................... 22
4. New Information on Children’s Exposure in Recreational Waters ........................ 25
5. Summary of Major Findings from New Information on Health Effects and
Children’s Exposure ................................................................................................ 27
B. New Information on Coliphages ..................................................................................... 28
1. Research on Coliphages .......................................................................................... 29
2. New Epidemiology Studies Including Coliphages ................................................. 32
3. QMRA ..................................................................................................................... 33
4. Studies of Wastewater Treatment Efficacy ............................................................. 33
5. Summary of New Information on Coliphages ........................................................ 34
C. New Information on Cyanotoxins ................................................................................... 34
1. RWQC Guidance Values and Health Advisories for Cyanotoxins ......................... 35
2. Health Studies ......................................................................................................... 36
3. Summary of New Information on Cyanotoxins ...................................................... 43
D. New Information on Antimicrobial Resistance ............................................................... 44
1. EPA AMR Research ............................................................................................... 44
2. Summary of New Information on AMR ................................................................. 45
E. New Information on Human and Non-Human FSI ......................................................... 45
1. Human FSI Research .............................................................................................. 46
2. Non-Human FSI Advances ..................................................................................... 48
3. Community-based FSI ............................................................................................ 49
4. Summary of New Information on FSI .................................................................... 50
F. New Information on Analytical Methods for Recreational Waters ................................. 51
1. FIB .......................................................................................................................... 51
iii
2. Coliphage Methods ................................................................................................. 53
3. Cyanotoxin Methods ............................................................................................... 55
4. AMR Bacteria ......................................................................................................... 56
5. Human FSI .............................................................................................................. 57
6. Summary of New Findings on Analytical Methods ................................................ 58
G. New Implementation Tools ............................................................................................. 58
1. Implementation of the 2012 RWQC ....................................................................... 58
2. Cyanotoxins ............................................................................................................ 60
3. Predictive Modeling Research ................................................................................ 61
4. Process Modeling Research .................................................................................... 61
5. Outreach and Grants ................................................................................................ 62
6. BEACH Act Grant Funding .................................................................................... 62
IV. Summary of Major Findings and Priorities for Further Work ............................................... 64
A. Summary of Major Findings ........................................................................................... 64
1. Summary of Findings Identified in New Epidemiological Studies ........................ 64
2. Summary of New Information on Coliphages ........................................................ 65
3. Summary of New Information on Cyanotoxins ...................................................... 65
4. Summary of New Information on AMR ................................................................. 65
5. Summary of New Information on FSI .................................................................... 66
6. Summary of New Findings on Analytical Methods ................................................ 66
B. Assessment of the Need to Revise the 2012 RWQC ....................................................... 66
C. Priorities for Further Work .............................................................................................. 67
V. References ............................................................................................................................. 71
Appendix A. Literature Search and Review Strategies .............................................................. A-1
Appendix B. Summaries of Studies Reviewed by EPA .............................................................. B-1
iv
Tables
Table 1. 2012 RWQC Recommended GM and STV Values for 36 and 32 Illnesses/1,000
Recreators (NEEAR-GI Illness [NGI]) for Marine and Fresh Waters ............................. 3
Table 2. Recreational Criteria or Swimming Advisory Recommendations for Microcystins and
Cylindrospermopsin ....................................................................................................... 36
Figures
Figure 1. Process for Identifying Literature for Some of the Five-year Review Topics: 2016–
2021 ................................................................................................................................ 7
v
Acronyms
AFO animal feeding operation
ALT alanine aminotransferase
AMR antimicrobial resistance
aOR adjusted odds ratio
ARG antimicrobial resistant genes
aRR adjusted risk ratio
ATX anatoxin-a
AWQC ambient water quality criteria
B. theta Bacteroides thetaiotaomicron
BASINS Better Assessment Science Integrating Point and Nonpoint Sources
BAV Beach Action Value
BEACH Beaches Environmental Assessment and Coastal Health
BMAA β-N-methylamino-L-alanine
BNR biological nutrient removal
BTB blood-testes barrier
BUN blood urea nitrogen
bw body weight
CAFO combined animal feeding operation
CAT Catellicoccus
CCE calibrator cell equivalent
CDC Centers for Disease Control and Prevention
CE cell equivalents
CFR Code of Federal Regulations
CFU colony forming unit
CI confidence interval
CSO combined sewer overflow
CWA Clean Water Act
DAL double agar layer
dcNEOSTX decarbamoyl neosaxitoxin
dhATX dihydroanatoxin-a
D-HFUF dead-end hollow-fiber ultrafiltration
DMF direct membrane filtration
DNA deoxyribonucleic acid
dPCR digital polymerase chain reaction
E. coli Escherichia coli
eDNA environmental deoxyribonucleic acid
EFSA European Food Safety Authority
EPA Environmental Protection Agency
ESBL extended spectrum beta-lactamase
EU European Union
FDA Food and Drug Administration
vi
FIB fecal indicator bacteria
FR Federal Register
FSI fecal source identification
g grams
GC gene copies
GI gastrointestinal
GI.1 norovirus genogroup I, genotype 1
GII.4 norovirus genogroup II, genotype 4
GJs gap junctions
GM geometric mean
GTX gonyautoxin
HAB harmful algal bloom
HDA helicase-dependent amplification
HESD Health Effects Support Document
HSPF Hydrological Simulation Program-Fortran
HPyVs human polyomaviruses
HUC Hydrologic Unit Codes
IC inhibitory concentration
IEM integrated environmental monitoring
IgG Immunoglobulin G
Inv-IMS/ATP inversely coupled immunomagnetic separation/adenosine triphosphate
i.p. intraperitoneal
kg-d kilograms per day
L liter
LAMP loop-mediated isothermal amplification
LD
50
lethal dose 50 percent (median lethal dose)
LDH lactate dehydrogenase
LRV log reduction value
LOAEL lowest-observed-adverse-effect level
LWTX lyngbyatoxins
M. smithii Methanobrevibacter smithii
MC microcystin
mL milliliter
MPN most probable number
MRA-IT microbial risk assessment-interface tool
MSC male-specific coliphage
MST microbial source tracking
MTT 3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyltetrazolium bromide
µg microgram
NEEAR National Epidemiological and Environmental Assessment of Recreational
Water
NGI NEEAR gastrointestinal
nmol/kg nanomoles per kilogram
vii
NIST National Institute of Standards and Technology
NPDES National Pollutant Discharge Elimination System
NOAEL no-observed-adverse-effect level
NORS National Outbreak Reporting System
ODH Ohio Department of Health
OHEPA Ohio Environmental Protection Agency
OR odds ratio
ORD Office of Research and Development (U.S. EPA)
PDV phocine distemper virus
PFU plaque forming unit
PI3K/AKT phosphatidylinositol 3 kinase/protein kinase B
PRISMA Preferred Reporting Items for Systematic Reviews and Meta-Analyses
PSP Paralytic Shellfish Poisonings
QMRA quantitative microbial risk assessment
qPCR quantitative polymerase chain reaction
R-squared Pearson's correlation coefficient squared
RBT risk-based threshold
RfD reference dose
RNA ribonucleic acid
rRNA ribosomal ribonucleic acid
ROS reactive oxygen species
RPMI Roswell Park Memorial Institute
RT-PCR reverse transcriptase-polymerase chain reaction
RT-qPCR reverse transcriptase-quantitative polymerase chain reaction
RVA Group A Rotavirus
RWQC Recreational Water Quality Criteria
SAL single-agar layer
SCCWRP Southern California Coastal Water Research Project
SDWA Safe Drinking Water Act
spp. species (plural)
SRM Standard Reference Method
STEC Shiga toxin-producing E. coli
STV statistical threshold value
STX saxitoxin
STX-Cou Fluorescent coumarin-coupled STX
STXeq saxitoxin equivalents
SWAT soil and water assessment tool
SWCNT single-walled carbon nanotubes
SWCNT-COOH carboxylated single-walled carbon nanotubes
Ti/Abs title and abstracts
TJs tight junctions
TMDL Total Maximum Daily Load
TSM Technical Support Materials
viii
TMRNEL Tetramethylrhodamine nick end labeling
UK United Kingdom
U.S. United States
UV ultraviolet
WHO World Health Organization
WHOI Woods Hole Oceanographic Institution
WOS Web of Science
WQS water quality standards
WRRF water resource recovery facility
WWTP wastewater treatment plant
ix
Executive Summary
The Beaches Environmental Assessment and Coastal Health (BEACH) Act amendments to the
Clean Water Act (CWA) Section 304(a)(9)(B) require the United States (U.S.) Environmental
Protection Agency (EPA) to conduct a review of the current 304(a) National Recommended
pathogen and pathogen indicator Recreational Water Quality Criteria (RWQC) every 5 years,
and, as necessary, revise the RWQC. In conducting this review, EPA considered several factors,
including the availability and evaluation of the latest scientific knowledge and additional
implementation support needs. EPA conducted a systematic review and evaluation of scientific
information and collection of updated information on recreational criteria implementation tools
since the previous 2017 five-year review. Specifically, EPA evaluated health and
epidemiological studies related to indicators of fecal pollution, studies related to children’s
health, new data on coliphages as indicators of enteric viruses, cyanotoxins health studies,
antimicrobial resistance, fecal source identification, studies addressing advances in analytical
methods, and implementation materials including advances in the use of models to predict water
quality and assess risk. This report contains extensive information in these topic areas along with
priorities for further work. EPA has completed a detailed review of the latest scientific
knowledge and has determined that current science supports a revision of the 2012 RWQC and
that there are several additional implementation tools that EPA can make available to manage
recreational waters.
The available science demonstrates an increased health risk for children compared to adults
when recreationally exposed to fecal contamination. Further, studies indicate that the use of
culturable fecal indicator bacteria alone to evaluate recreational waters impacted by human
sources can result in under-protection of human health. In these waters quantitative polymerase
chain reaction (qPCR)-based criteria (i.e., based on a molecular testing method using qPCR)
offer greater protection. Since 2017, EPA has published nationally validated protocols for qPCR
methods and codeveloped (along with the National Institute of Standards and Technology
[NIST]) a standard reference material for national use that supports the use of qPCR
technologies. To address these issues, first EPA plans to develop additional criteria
recommendations for qPCR-enumerated enterococci protective of children, which would also be
protective of all recreators. Second, EPA plans to continue to develop recommendations for
coliphages to help address potential risks from human enteric viruses in ambient waters. Third,
EPA plans to explore how best to use human fecal source identifiers, such as HF183, for water
quality management. Being able to demonstrate that a waterbody has been impacted by human
fecal contamination will enable risk managers to use the appropriate tools to evaluate and
manage risks for waters impacted by human sources.

1
I. Introduction
United States (U.S.) Environmental Protection Agency (EPA) has conducted a five-year review
of its 2012 Recreational Water Quality Criteria (RWQC), as required by the Beaches
Environmental Assessment and Coastal Health (BEACH) Act amendments to the Clean Water
Act (CWA) Section 304(a)(9)(B). The last review was conducted in 2017. In conducting this
review, EPA considered several factors, including the availability and evaluation of the latest
scientific knowledge and additional implementation support needs. The Agency used the
information in this “state-of-the science” report to assess whether new or revised RWQC are
necessary at this time.
The development of the 2012 RWQC and five-year reviews are requirements of the BEACH Act
of 2000. Through that Act, EPA has provided grants to states, territories, and tribes to implement
water quality monitoring and notification programs for coastal recreation waters
1
(including the
Great Lakes) since 2002. The 2012 RWQC included development of a beach advisory threshold
for use in posting swimming advisories and the recommended ambient water quality criteria
(AWQC). Swimming advisory decisions based on water quality monitoring are intended to
reduce the risk to recreators and other users of these waters from illness associated with exposure
to human fecal contamination and provide the public with information to make decisions about
their actions. AWQC developed under CWA Section 304(a) are recommendations based on the
latest science which states and authorized tribes can adopt as part of their water quality standards
(WQS). In the case of the 2012 RWQC, EPA’s recommendations were designed to protect
primary contact recreation, in all coastal and non-coastal waters designated for recreational use.
The criteria, once adopted by states and authorized tribes and approved by EPA under CWA
section 303(c), become part of the regulatory structure of the state or authorized tribe for
protection of primary contact uses for the applicable waters. The recreational criteria values that
are part of a state or authorized tribe’s approved WQS have a direct bearing on the issuance of
National Pollutant Discharge Elimination System (NPDES) discharge permits, waterbody
assessments, the decisions regarding attainment of WQS under CWA Sections 303(d) and
305(b), and the development of targets for total maximum daily loads (TMDLs) for restoring
impaired waters.
The criteria values specified in the RWQC are for densities of culturable fecal indicator bacteria
(FIB) in water. The FIB, enterococci, and Escherichia coli (E. coli) are not pathogenic under
usual circumstances, but their presence in water above specified levels can indicate the presence
of fecal contamination potentially containing viral, bacterial, or protozoan pathogens associated
1
The BEACH Act of 2000 defines coastal recreation waters as follows:
The term “coastal recreation waters” means:
(i) The Great Lakes; and
(ii) Marine coastal waters (including coastal estuaries) that are designated under section 303(c) by a Stat
e
fo
r use for swimming, bathing, surfing, or similar water-contact activities.
The term “coastal recreation waters” does not include:
(i) Inland waters; or
(ii) Waters upstream of the mouth of a river or stream having an unimpaired natural connection with the
open sea.
2
with an elevated risk of illness. Therefore, ensuring that the RWQC are consistent with the
current state of the science and are protective of human health is key to protecting the health of
users of all waters designated for primary contact recreation.
EPA identified the following objectives for this review of the 2012 RWQC:
• Identify the latest science and information available since the publication of the 2017
five-year review that may impact the 2012 criteria.
• Discuss the status of adoption of the RWQC.
• Include the latest science and information on health effects relevant to RWQC,
coliphages, cyanotoxins, antimicrobial resistance (AMR), fecal source identification, and
analytical methods that have the potential to impact recreational uses.
• Identify additional indicators and microbial methods including those that have become
more refined or feasible and include this information.
• Provide information on the implementation materials for the 2012 criteria including site-
specific tools, implementation materials for cyanotoxins, predictive and process modeling
for both fresh and marine waters, outreach, and training.
An important goal of this review is to evaluate whether revisions to the 2012 RWQC are
necessary based on the overall review of new information provided in the previous 5 years,
described later in this report.

3
II. Background
A. Brief Description of the 2012 RWQC and Key Aspects of Implementation
The 2012 RWQC, which use enterococci and E. coli as predictors of gastrointestinal (GI)
illnesses in recreational waters, are described below along with several other important aspects of
how the criteria can be implemented.
1. Criteria Magnitude, Duration, and Frequency: Geometric Mean and Statistical
Threshold Value
The 2012 RWQC consist of three primary components: magnitude, duration, and frequency.
Magnitude: The magnitudes of the bacterial indicators are the measured densities of the FIB
from the water quality density distribution used for the criteria, expressed both as a geometric
mean ([GM] 50th percentile value) and as a statistical threshold value ([STV] 90th percentile value).
Duration: The duration is the period over which excursions of the magnitude values are recorded
and calculated. EPA recommended a duration of 30 days in the criteria for both the GM and the STV.
Frequency: The frequency is how often the GM or the STV are exceeded. EPA recommended no
exceedances for the GM over the period of the duration.
Because the STV reflects the 90th percentile of the distribution of values used to determine the
RWQC, the RWQC allowed for a 10 percent exceedance of the STV. EPA selected the estimated
90th percentile of the water quality distribution to account for the expected variability in water
quality measurements, while limiting the amount of time allowed to exceed the STV as a
threshold of water quality impairment.
EPA was clear that “both the GM and the STV would be part of the WQS, and therefore both
targets would be used to determine whether a waterbody attains the WQS for primary contact
recreation” (U.S. EPA, 2012a).
Table 1. 2012 RWQC Recommended GM and STV Values for 36 and 32 NGI
Illnesses/1,000 Recreators for Marine and Fresh Waters
CRITERIA
ELEMENTS
Recommendation 1
Estimated Illness Rate (NGI): 36/1,000
Recommendation 2
Estimated Illness Rate (NGI): 32/1,000
Indicator
GM
(CFU/100 mL)
a
STV
(CFU/100 mL)
a
GM
(CFU/100 mL)
a
STV
(CFU/100 mL)
a
Enterococci
(marine and fresh water)
35 130 30 110
E. coli (fresh water)
126 410 100 320
Duration and frequency: The waterbody GM should not be greater than the selected GM magnitude in any 30-day
interval. There should not be greater than a 10 percent excursion frequency of the selected STV magnitude in the
same 30-day interval. NEEAR = National Epidemiological and Environmental Assessment of Recreational Water;
NGI = NEEAR gastrointestinal.
a
EPA recommends using EPA Methods described at https://www.epa.gov/cwa-methods to measure culturable
enterococci and E. coli. Units are colony forming units (CFU) per milliliter (mL).
4
Criteria values were provided for culture-enumerated FIB at two illness rates, 32 and 36 NGI
illnesses per 1,000 swimmers. Based on EPA’s analysis of the available information, either set of
thresholds protects the designated use of primary contact recreation and, therefore, protects the
public from the risk of exposure to harmful levels of pathogens from fecal contamination. The
two sets of numeric concentration thresholds included in the 2012 RWQC provide states and
authorized tribes flexibility to make their own risk-management decisions. The recommendations
for criteria illness rate apply to both marine and fresh waters, regardless of the intensity of use of
the beach.
In addition to recommending criteria values, EPA also provided states and authorized tribes with
Beach Action Values (BAVs) for use in notification programs. The BAV was defined as the 75th
percentile of the water quality distribution of values of E. coli and Enterococcus species (spp.) in
the epidemiological studies used by EPA to establish a health link between gastrointestinal (GI)
illness and levels of culturable FIB. EPA’s intent was to provide the BAV for states and
authorized tribes as a precautionary tool for beach management decisions. EPA recommended
the BAVs for use by the states for issuing beach notifications/advisories in their public health
programs, but not as part of the 2012 RWQC recommendations under CWA Section 304(a).
States, territories, and authorized tribes continue to make progress adopting RWQC. Thirty-one
jurisdictions have adopted, and EPA has approved, revised RWQC for all primary contact
waters. Three additional jurisdictions have adopted, and EPA has approved, revised RWQC for
their coastal recreation (i.e., BEACH Act) waters only. One jurisdiction only includes fecal
coliform as FIB in its WQS. Seven additional jurisdictions use fecal coliform as FIB for some
but not all of their waters designated for primary contact recreation (e.g., they use enterococci as
the FIB in their marine waters and fecal coliform in their fresh waters).
Although identified in 2017 as a possible barrier to adoption of the 2012 RWQC, criteria that are
based on use intensity does not appear to be hindering continued adoption of the 2012 RWQC, as
states and authorized tribes with coastal recreation waters have continued to adopt the 2012
RWQC without the inclusion of use intensities. More than half the states have adopted the 2012
RWQC or an equivalently protective criteria in all their waters designated for primary contact
and an additional three states have adopted the 2012 RWQC for their coastal recreation waters;
all authorized tribes with coastal recreation waters have adopted the 2012 RWQC.
While the culture-enumerated FIB at two illness rates were the recommended criteria in 2012,
EPA also included quantitative polymerase chain reaction (qPCR)-based values as additional
information. EPA developed and validated a molecular testing method using qPCR as a rapid
analytical technique for the detection and quantitation of enterococci in recreational water (EPA
Method 1611, EPA 2012b). EPA included qPCR-based values for the GM, STV, and BAV for
both illness rates protective of the general population in the 2012 RWQC document. Because of
potential matrix interference issues in water types other than those studied at the NEEAR
effluent-affected beach sites, EPA’s Office of Research and Development (ORD) continued
research to characterize and refine the qPCR methodology (Haugland et al., 2016) resulting in
the publication of Method 1609.1 (EPA Method 1609.1; U.S. EPA, 2015c).

5
Following publication of the 2012 recreational criteria, EPA provided additional information on
tools for evaluating and managing recreational waters, such as predictive modeling and sanitary
surveys, and stressed the need for a tiered approach to developing beach monitoring plans in the
2014 National Beach Guidance and Required Performance Criteria for Grants. The Agency also
provided Technical Support Materials for developing site-specific criteria and for the use of
alternative indicators or methods at recreational beaches (see
https://www.epa.gov/wqc/recreational-water-quality-criteria-and-methods).
B. How the 2
nd
Five-Year Review Was Conducted
1. Scope and Methods of the Review
This section describes the measures EPA has taken to assess advances in the state of the science
supporting the 2012 RWQC since 2016, the date of the last five-year review’s literature cut-off
year (published in 2018). It also addresses advances in implementation. The measures include an
inventory of the relevant scientific information published since 2016, a description of
recreational criteria implementation tools applied at recreational settings, and information on
sources of information and how information was accessed.
2. Inventory of Scientific Information Published Since 2016
A thorough inventory of scientific information published since 2016 for topics central to
recreational waters monitoring and assessment is the core of this review (Figure 1). Several
general categories of relevant information were identified:
i. Health studies, including epidemiological studies, outbreak studies, children’s health
studies, and the application of quantitative microbial risk assessment (QMRA) to water
quality data and complex settings at recreational beaches.
ii. Summary of advancements in coliphage health studies and methods since 2016.
iii. Cyanotoxins health studies, methods, and criteria and implementation since 2016.
Note that cyanotoxin recreational criteria are not subject to the BEACH Act because they
are neither pathogens nor pathogen indicators. EPA included cyanotoxins in the literature
reviews since EPA has recreational criteria for two cyanotoxins.
iv. Summary of scientific advancements in AMR since 2016.
v. Fecal source identification (also called microbial source tracking in the literature),
including human and non-human fecal source markers and tracking.
vi. Performance, implementation, and updates of microbial methods for FIB and
alternative indicators 2016 to present.
3. Summary of Recreational Criteria Implementation Tools Developed and EPA Outreach
and Training Since 2016
A further category of activities and tools related to water quality monitoring and contextual
assessment of beach settings was identified as highly relevant to the implementation of the
BEACH Act and activities related to the 2012 RWQC. This category of implementation tools
includes:

6
i. Site-specific implementation tools
ii. Sanitary surveys and watershed assessments
iii. Cyanotoxins implementation tools
iv. Predictive modeling
v. Process modeling
4. Sources of Information and How Information Was Accessed
The collection and analysis of information in each of these categories included accessing post-
2016 information from two broad sources:
• EPA recreational water research and publications relating to that research.
• External (non-EPA) academic research conducted by researchers at academic
institutions and government organizations that have focused on recreational water
activities and science related to the BEACH Act.
5. A Systematic Review of Available Peer-Reviewed Literature
EPA performed systematic searches of the peer-reviewed literature for articles pertaining to
health studies, including epidemiological studies of recreational water-contact activities,
outbreak studies, advances in recreational exposure descriptions for children, characterization of
children’s illness susceptibility, observed illness rates in children upon exposure, and the
application of QMRA to water quality data and complex settings at recreational beaches;
advances in human and non-human fecal source identification (microbial source tracking); and
advances in molecular methods used in recreational waters to measure indicators of fecal
contamination. Multiple sets of search terms applicable to the topic were applied to references in
Web of Science and PubMed (http://www.ncbi.nlm.nih.gov/pubmed) (see Appendix A for
detailed search terms). Searches for gray literature were also performed. Abstracts were screened
for relevance to the scope of the search. The literature search was limited to English-language,
peer-reviewed citations published between January 2016 and November 2021, with the exception
of searches pertaining to children’s health studies. Searches pertaining to advances in
recreational exposure descriptions for children, characterization of children’s illness
susceptibility, and observed illness rates in children upon exposure were limited to articles
published between January 2018 and November 2021. Following the abstract screening, the full
text of articles passing scope was reviewed for specific information related to each topic (see
Appendix A for details). Diagrams of the process and results are provided in Figure 1, below.
The steps of a systematic review as described in the Preferred Reporting Items for Systematic
Reviews and Meta-Analyses (PRISMA) protocol, which defines the minimum set of items for
reporting in systematic reviews. The search strategy, search terms, screening criteria, and
PRISMA diagrams are provided in Appendix A. Results of the systematic reviews and
summaries of studies reviewed are included in Appendix B.

7
Figure 1. Process for Identifying Literature for Some of the Five-year Review Topics: 2016–2021
a
Three literature searches (Methods Progress; Health Studies; Children’s Risk) were conducted (Appendix A) to
identify articles for four relevant topics: Microbial source tracking (MST); Molecular methods; Health studies; and
Children’s risk. The MST topic includes fecal source identification (FSI) and bacterial source tracking. Note that the
literature on three additional topics, AMR, cyanotoxins, and coliphages, that is described in those sections was
identified from additional literature searches conducted using different methods and is not shown here.
b
Articles were cross-tagged to other document topics/sections when applicable, resulting in some articles being
counted for more than one topic.
c
Pre-screening prioritization of the Title/Abstracts (Ti/Abs) for Health Studies was conducted; 1,293 Ti/Abs were
reduced to 162 based on machine learning supervised clustering using 37 seed title/abstracts (see Appendix A).
Fifty-one cross-tagged articles were added after prioritization, resulting in 213 studies.
Identification
Screening
Eligibility
Records Identified via
Database Searching
b
Method Progress n = 732
Health Studies
c
n = 213
Children’s Risk n = 495
Records Identified via
Gray Literature Searching
b
Method Progress n = 37
Health Studies n = 61
Children’s Risk n = 30
Total number of titles identified for Ti/Abs review
b
Method Progress n = 769
Health Studies n = 274
Children’s Risk n = 525
Full-text articles reviewed
b
Method Progress n = 107 (MST); 120 (Methods)
Health Studies n = 118
Children’s Risk n = 38
Relevant articles reviewed for 5-year review
b
Method Progress n = 85 (MST); 80 (Methods)
Health Studies n = 44
Children’s Risk n = 12
PubMed
n = 1,746
WOS
n = 1,490
Gray
Literature
n = 128
Databases
Total Records Identified
a
8
EPA also performed systematic searches of the peer-reviewed literature for articles pertaining to
characterizing the human health effects of cyanotoxins from all routes of exposure. The
cyanotoxins of interest for this topic included: anatoxins, cylindrospermopsin, microcystins,
nodularins, and saxitoxins. The literature searches for anatoxins, microcystins, and
cylindrospermopsin built upon work previously conducted by the Office of Water and covered
the period from January 2014 to April 2021. The literature searches for all other cyanotoxins
were conducted with no start date to April 2021. Multiple sets of search terms applicable to the
topic were applied to references in Web of Science (WOS), PubMed, and Scopus (see Appendix
A for search terms). Abstracts were screened for relevance to the scope of the search. Following
the abstract screening, the full text of articles passing scope was reviewed for specific
information related to each topic. Search strategy, search terms, screening criteria, and PRISMA
diagrams are provided in Appendix A. Results of the systematic reviews are included in
Appendix B.
9
III. Findings of the 2
nd
Five-Year Review
A. New Information on Health Effects after Exposure to Recreational Waters
The systematic literature review identified 44 health studies and 12 children’s risk studies
(Figure 1) that were evaluated for the Second Five-Year Review report (Appendix B). Additional
relevant literature was identified via an ad hoc process (e.g., provided by subject matter experts).
From this ad hoc process, twelve references relevant to health study information (Teunis et al.,
2008, 2016, 2018, 2020; U.S. EPA. 2010, 2019; Boehm et al., 2015; Wade et al., 2019; Vanden
Esschert et al., 2020; Egorov et al., 2021; Goh et al., 2021; California Water Boards, 2022) and
two references relevant to children’s risk (U.S. EPA, 2011 and Dufour et al., 2017) were
identified.
Health studies of recreational water exposure broadly fall into three categories: epidemiological
studies, QMRA-based studies, and outbreak reports or studies (also referred to as surveillance
summaries).
• Epidemiological studies quantify associations between reported health symptoms (or
biomarkers of infection) in participants and a measure of water quality, such as an
indicator of fecal contamination, on the day of swimming exposure. These studies
generally provide information on the level of illness experienced by recreators, and
many have observed statistically significant associations between illness and measures
of water quality (e.g., FIB). Epidemiological studies have informed the development of
recreational water guidelines in the United States and elsewhere.
• QMRA-based studies characterize the probability of illness by focusing on the
pathogens that can cause illness and, in this context, can provide risk-based thresholds
(RBTs) for measures of water quality (including traditional and alternative surrogates
of fecal contamination) that are based on the fecal sources affecting a waterbody.
• Outbreak studies are retrospective investigations that describe the conditions and
consider possible etiologic agents resulting from clusters of reported illnesses. All three
types of studies inform human health effects that recreators experience due to fecal
contamination of recreational waters and may inform the understanding of the
waterborne pathogens responsible for causing illness. This section presents studies
identified in the published scientific literature in each of these three study categories,
followed by a summary of major findings.
1. Recreational Water Epidemiological Studies
New Epidemiological Studies Conducted in the United States: 2016–2022
Children’s Health Studies
The systematic literature search (see Figure 1 and Appendix A for method details) that identified
new literature since the first five-year review (i.e., 2016–2021) found four recreational water
epidemiology studies conducted in the United States that included enterococci data (Wade et al.,

10
2022; Arnold et al., 2016, 2017; Benjamin-Chung et al., 2017). Several studies evaluated the
children’s health risk from exposure to fecal contamination in the beach environment.
Wade et al. (2022) analyzed a pooled data set of over 80,000 beachgoing children, adolescents,
and adults from 13 beach sites across the United States to compare illness risks and water quality
by collecting measures of both culture- and qPCR-enumerated enterococci, exposure levels,
beach sites, and health endpoints for different age groups. Sites previously studied by EPA
included: West Beach, Huntington Beach, Washington Park Beach, Edgewater Beach, Fairhope
Beach, Goddard State Park Beach, Surfside Beach, and Boquerón Beach. California sites studied
by the University of California, Berkeley and the Southern California Coastal Water Research
Project (SCCWRP) included: Mission Bay, Doheny Beach, Malibu Beach, and Avalon Beach.
As part of the analysis, beach sites studied were categorized by the likelihood of human fecal
contamination and whether the human source was a point source. Seventy percent of all
participants had at least some contact with water and 27 percent stayed in the water 60 minutes
or more. Children 12 years of age and under comprised approximately 25 percent of the total
study population from all sites. The authors used odds ratios (ORs) among swimmers to
characterize associations between levels of Enterococcus spp., measured by culture and qPCR,
for different age groups across different exposures, beach category, and health endpoints (GI and
respiratory illness). The authors found that GI symptoms were the most sensitive health endpoint
associated with fecal contamination across all age groups, exposures, and site categories.
Children were at higher risk of swimming-associated illness compared to older adolescents and
adults with the highest mean ORs observed for GI illness among children under 10 years old at
beaches affected by effluent. Statistically significant associations were consistently observed
with Enterococcus qPCR cell equivalents (CE) across sites impacted by human fecal
contamination. The highest odds ratios (OR = 2.32, 95% confidence interval [CI]: 1.33–4.06)
were between Enterococcus qPCR and children 6 years old and under who spent at least 60
minutes in the water; ORs were similar for children in the “’10 and under” and “’12 and under”
age groups. Respiratory illness was significantly associated with Enterococcus qPCR exposures
among children 4 and under who spent 60 minutes or more in the water at sites affected by
effluent. Cough was also associated with Enterococcus CFU at sites affected by effluent among
children 4 and under, although small sample sizes resulted in imprecise estimates for these
associations. Combining data across all sites, the association between GI illness and culturable
enterococci, although positive, was not statistically significant for the general population (i.e.,
data from all participants combined). ORs for culturable enterococci and GI illness increased at
“likely human-impacted” sites, particularly among those who swallowed water (1.37, 95% CI:
1.04–1.82) and there was evidence for increasing risk with younger aged children at sites
affected by effluent. For example, among children under 6 years old at the sites affected by
treated effluent the OR was 1.82 (95% CI: 1.14–2.91), which was significantly higher compared
to adults 18 years old and over. ORs for severe GI illness
2
among swimmers, which accounted
for approximately 20 percent of all reported GI illness, were statistically significant for
Enterococcus qPCR (1.16, 95% CI: 1.03–1.31) and higher for children under 6 years old (1.50,
2
Severe GI illness was defined as a GI illness episode that lasted three of more days or resulted in a visit to the
hospital, doctor’s office, or emergency room.
11
95% CI: 1.13–1.99), but not for culturable enterococci. The study conclusions were that age,
source of fecal contamination, and the intensity of swimming exposure are important factors
affecting the association between Enterococcus spp. Exposure from swimming-associated
illnesses.
Arnold et al. (2016) conducted a meta-analysis of 13 beach sites and nearly 90,000 subjects. The
study combined individual level-data from studies conducted by EPA and the University of
California, Berkeley, which were coordinated to ensure similar designs and measurement
methods. Data from 13 prospective cohorts were combined and integrated into a single data set.
The beaches represented a range of recreational water conditions across the United States: four
freshwater beaches were in the Great Lakes region, four marine beaches were in Southern
California, and the remaining five marine beaches were spread along the Gulf Coast, Eastern
Seaboard, and Puerto Rico. Nine of the beaches were located near a known source of treated
sewage discharge and the remaining four beaches had more diffuse contamination from urban
runoff. The primary outcome was incident diarrhea defined as three or more loose or watery
stools in 24 hours. Swimmers were defined as those who immersed their body in water and were
further classified on the basis of water quality, that is, whether the FIB, Enterococcus, exceeded
the revised EPA guideline value of 35 CFU per 100 ml.
Compared to non-swimmers, diarrhea incidence increased with swimming exposure.
Enterococcus levels were positively associated with diarrhea risk within age strata, with the
largest absolute increases in risk among children 0 to 4 years old. Swimmers exposed to
Enterococcus above EPA guidelines had higher diarrhea incidence compared to non-swimmers
and compared to swimmers exposed to water below the guideline, but only at beaches with a
known point source of human fecal pollution. Swimmers exposed to Enterococcus levels above
the EPA guidelines had higher diarrhea incidence compared to non-swimmers as well as with
swimmers exposed below the guideline in all age groups. The finding was more pronounced in
children: 0 to 4 years old exposed to water above regulatory guidelines reported 103 episodes per
1,000 compared to 48 episodes per 1,000 among non-swimmers at beaches with a known point
source. For swimmers that recreated in waters below the guideline level, the illness rate was 65
episodes per 1,000 compared, which is significantly higher than non-swimmers (48/1,000) but
lower than the rate for swimmers who recreated in waters above the guideline level (103/1,000).
The authors noted the uncertainties about the causes of diarrhea, which include non-infectious
causes of diarrhea (e.g., swallowing excess saltwater), outcome reporting bias, or pathogens
present in recreational waters that do not covary with Enterococcus. Children 0 to 4 and 5 to 10
years old had the most water exposure, stronger associations between levels of water quality and
illness, and the largest attributable health burden. Overall, recreational water exposure was
associated with increased risk of acute gastroenteritis resulting in missed daily activities with the
highest risk and burden among young children.
Arnold et al. (2016) also performed a comparative analysis for association to health effects for
FIB and qPCR. They found that the exposure-response relationship was more consistently
monotonic for Enterococcus measured using qPCR methods versus culture methods across
different analyses, even when restricted to beaches without a known point source of pollution.
12
Arnold et al. (2017) conducted a longitudinal cohort study of adult surfers (18 years old and
older), a group of recreators with potentially high exposure profiles, in California waters to
evaluate health impacts associated with water quality, measured by culturable FIB, under dry and
wet weather conditions. The study collected illness information on GI illness, sinus infections,
ear infections, fever, skin rashes, and infected wounds. Under dry conditions, recreational
exposures were associated with an increased incidence of all health outcomes compared to the
nonexposed group (adjusted incidence rate ratio = 1.86; 95% CI: 1.27, 2.71). With the exception
of infected wounds, illness symptoms were not associated with increases in culturable
enterococci levels. Culturable enterococci levels were strongly associated with multiple health
outcomes after wet-weather exposure. In the wet weather flows discharging to the beaches,
human fecal contamination from urbanized areas was identified. FIB (culturable enterococci,
fecal coliforms, and total coliforms) were significantly associated with illness up to 3 days after
rainfall. The median wet weather-associated excess risk at 35 CFU enterococci per 100 mL was
16 GI illnesses per 1,000 (95% CI: 5, 27) or less than half the rate of illness associated with the
EPA’s RWQC recommendations. The significance of this study is that it identifies wet weather
as another factor that can affect loading of fecal contamination to recreational waters and can
result in increased human health risk to recreators. The results support posting of beaches after
rainstorms when human fecal sources are likely to affect recreational waters and management
actions that would reduce fecal loading in urban runoff.
Benjamin-Chung et al. (2017) conducted a pooled analysis of six prospective cohort studies at
coastal beaches in California, Alabama, and Rhode Island. Water quality was measured using
culturable enterococci and coliphages. Knowledge of contamination inputs was used to interpret
indicator monitoring. Human fecal pollution was unlikely on all study days at two of the beaches
(Mission Bay and Malibu). At Fairhope and Goddard beaches, human fecal pollution was likely
on all study days. The remaining two beaches (Doheny and Avalon) had evidence of variable
human input such as groundwater influx conveying sewage through the sand or a berm that
blocked direct discharge of contaminated water at the beach. Among all participants across the
study sites and under all conditions combined, there was no association between GI illness and
levels of culturable enterococci or coliphages. However, associations between culturable
enterococci or coliphage levels and GI illness were observed when human fecal pollution was
“likely present” and there was some evidence that the association with illness for male-specific
coliphage (MSC; also known as F+ or F-specific) was stronger than for enterococci.
Studies Assessing Antibody Response
Four health effects studies were identified that characterized seroconversion, an immune
response to infection, in recreators (Wade et al., 2018, Augustine, et al., 2020, Egorov et al.,
2018, and Egorov et al., 2021).
Wade et al. (2018) collected saliva samples as part of the U.S. EPA Boquerón Beach
epidemiological study to test for immunoglobulin G (IgG) responses to genogroup I.1 (GI.1) and
genogroup II.4 (GII.4) noroviruses. Immunoconversions for noroviruses were observed in 34
subjects, or 2.6 percent of the study population (n = 1,298). Swimmers who reported submerging
their heads while swimming had a significantly higher rate of immunoconversion compared to
13
non-swimmers. Immunoconversion was not statistically associated with GI symptoms. The
results of this study demonstrate the potential for transmission of noroviruses among recreators
at a marine beach. Another study by Wade et al. (2019) found similar results.
Augustine et al. (2020) demonstrated the use of a rapid salivary antibody assay to assess the
prevalence of salivary antibodies against hepatitis A virus and immunoconversions in a
population of beachgoers at Boquerón Beach, Puerto Rico. Results showed an
immunoprevalence rate of approximately 16 percent among participants, which is less than half
the overall immunoprevalence rate among U.S.-born persons 2 years old or older. Only 1.43
percent of the participants who provided all three samples were found to have hepatitis A virus
immunoconversions. There was no statistically significant association between hepatitis A
immunoconversions and any of the demographic or exposure risk factors tested. Previous health
studies conducted at this location reported low rates of GI illness (e.g., <2 NGI/1,000 recreators),
low levels of pathogens, and good water quality based on levels of enterococci below the EPA
RWQC (U.S. EPA, 2010; Soller et al., 2016). This study demonstrated the potential of rapid
assays for immunoconversions to a waterborne virus like hepatitis A virus to inform the
protection of recreational water and public health (Augustine et al., 2020).
Egorov et al. (2018) conducted a prospective cohort study recruiting local families with children
in Lawrence, Massachusetts (n = 1986) that overrepresented children (1,170 children <18 years;
58.8%). The results were that 12.5 percent of participants that immunoconverted to
Cryptosporidium, tested through salivary immunoassay, were symptomatic. Immunoconversions
by study participants to Cryptosporidium after recreational exposure to natural waterbodies or
swimming pools was associated with adjusted odds ratios (aORs) of 2.3 (0.4;15.4) and 4.9 (1.6;
15.5), respectively. No association between immunoconversion with noroviruses and swimming
in natural waterbodies (i.e., rivers, lakes, or oceans) was observed. However, only 3.6 percent of
participant responses indicated swimming in pools and 2.5 percent in natural waterbodies,
reducing the sample size included in analysis. For all oral exposure pathways analyzed,
incidence rates of Cryptosporidium immunoconversions declined steadily with age: 8.5 per 100
person-years in children between 1 and 10 years of age, 5.6 per 100 person-years for age 11 to 20
years, 4.2 per 100 person-years for age 21 to 40, and 1.5 per 100 person-years in adults ages 41
to 85 years. Norovirus genogroups I and II also showed lower rates of immunoconversions in the
group age 41 to 85 compared to ages 1 to 10. This community-level study demonstrated the
utility of the salivary antibody immunoassay methodology for the detection of infections with
Cryptosporidium and norovirus. In this study, the immunoconversions were related to potential
exposure pathways, thus providing useful information for improving public health protection.
Egorov et al. (2021) conducted a prospective salivary antibody study at a Lake Michigan beach
to study seroconversion to norovirus and Cryptosporidium. Among 872 study participants, there
were seven cases of seroconversion, including six individuals with seroconversion to noroviruses
and two to Cryptosporidium, with one study participant seroconverting to both pathogens. Those
that seroconverted to norovirus were more likely to experience vomiting within 4 days of a beach
visit (p = 0.003). This study provided further evidence that recreational water exposure can be
associated with symptomatic illness and asymptomatic waterborne infections.
14
New Non-U.S. Epidemiological Studies: 2016–2022
Three recreational water epidemiology studies conducted outside the United States and one
review of epidemiological data were identified (Joosten et al., 2017; Kuhn et al., 2018, and
Verhougstraete et al., 2020; Young, 2016).
Joosten et al. (2017) conducted a prospective cohort study to assess risk factors for health
complaints (GI illness, respiratory illness, and complaints associated with the skin) associated
with recreational exposure to urban waters affected by wet weather-associated sewage overflows
during canal swimming events in two Netherland cities in 2015. Prior to the events, E. coli and
enterococci monitoring data from the canals were below the European Union (EU) thresholds.
By the afternoon of the day of the event, E. coli levels greatly exceeded the EU threshold in one
of the two cities while enterococci remained below the EU threshold. Thirty-one percent of
swimmers (427 of 1,375 swimmers) reported GI illness symptoms following exposure to canal
waters affected by sewage overflows resulting in an adjusted risk ratio (aRR) of 6.3 (95% CI:
4.1–9.5). Five out of seven stool samples provided by participants tested positive for various
norovirus genotypes. One water sample from one of the canals tested positive for genogroup I
norovirus and two water samples tested positive for genogroup II norovirus. The conclusion of
this study is that the outbreak of acute GI illness in swimmers was related to the presence of
norovirus, which was possibly linked to wet weather-associated sewage overflows affecting the
canals.
Kuhn et al. (2018) conducted a prospective case-control study among Danish persons that
included 446 children ages 1 to 5 years old (from a total of 3,119 study participants ages 1 to 30
years old) to identify risk factors for campylobacteriosis. Study participants provided information
by completing an online questionnaire. Consumption of contaminated food, animal contact,
bathing in fresh waters, contact with beach sand, and bathing in a paddling pool were identified
as significant risk factors for increased risk of campylobacteriosis. The authors estimated that
4 percent of sporadic Danish campylobacteriosis cases may be caused by recreational water
contact (Kuhn et al., 2018). The authors indicated that, based on their estimates, recreational
water contact and contact with sand was likely linked to a large proportion of campylobacteriosis
in Denmark’s children and young adults.
Verhougstraete et al. (2020) developed adjusted risk difference models (excess gastrointestinal
illness per swimming event) for children (<10 years of age) and “non-children” (≥10 years of
age) across five Brazilian beaches using epidemiological data collected in the United Kingdom
(UK) and Brazil. Water quality at the Brazilian beaches exceeded the maximum fecal
streptococci levels measured in the UK studies 11.8 percent of the time. Risks associated with
the elevated indicator levels equated to 53 and 96 NGI per 1,000 recreators for non-children and
children, respectively. The study concluded that the World Health Organization (WHO)
recreational water quality guidelines, based largely on the epidemiological study performed at
UK beaches, were not appropriate as a basis for guideline development in tropical settings where
there is minimal wastewater treatment. Pathogen profiles distinct to tropical waters and point
15
source discharges with minimal treatment were two factors identified for supporting the
development of more regionally specific guidelines.
Young (2016) reviewed studies published before 2015 of infectious disease transmission in
marine bathing waters, sources of pathogens in marine waters, and epidemiological evidence for
the association between marine bathing and infectious disease. Numerous studies demonstrated
an increased risk of gastrointestinal illness associated with marine swimming compared to non-
swimming. However, an association between levels of FIB and illness among swimmers was not
consistently found across the studies reviewed. The authors suggest that traditional FIB may not
be predictive of human health impacts when human waste was not the predominant source of
pathogens. In one study, 71 percent of gastrointestinal episodes in Southern California were
estimated to occur when the water quality was considered safe for bathing, potentially implying
that most cases of illness associated with marine bathing could arise from the lowest risk
exposures, combined with high numbers of people exposed. The authors identified the need for
research to identify additional markers for human health risk at non-point source beaches and the
use of rapid methods to improve public health protection.
Summary of Findings Identified in New Epidemiological Studies
These studies vary in their focus, but conclusions highlight that:
• While FIB can be a useful indicator in waters impacted by raw and poorly treated
sewage, the inconsistent performance of culture-enumerated FIB related to health
outcomes was demonstrated in multiple studies, which suggests that other indicators
(e.g., Enterococcus measured by qPCR) are more predictive of risk of illness.
• qPCR-enumerated enterococci performed better than FIB as a predictor of risk in waters
dominated by human fecal sources, and there was some evidence that culture-enumerated
enterococci were associated with increased GI illness when human fecal pollution was
suspected based on knowledge of point sources.
• Some waters receiving human fecal contamination have measured viral pathogens at
levels expected to lead to health outcomes and yet also be below EPA recommended
water quality level for culturable FIB.
• Health risk in recreational waters is greatest in children 0 to 10 years old compared to
both adolescents and adults.
• The health risk from recreating during or following wet weather compared to dry weather
can be due to changes in fecal loading dynamics to a waterbody.
• Use of salivary assays to estimate seroconversion to waterborne pathogens can provide
early information on infection by specific pathogens by recreators exposed to fecal
contamination. Studies found evidence of seroconversion to norovirus and
Cryptosporidium among swimmers and also found that those who seroconverted were
16
more likely to have gastrointestinal symptoms compared to those who did not
seroconvert.
2. Use of QMRA to Understand Risks in Recreational Water Settings
QMRA studies published between 2016 and 2022 included studies that characterized human
health risk from recreating in waters contaminated by human and non-human fecal sources; used
QMRA to develop or evaluate risk-based water quality values for alternative indicators; and
evaluated the importance or sensitivity of specific data as input to QMRA for recreational water
purposes. The new studies described below report several important trends with respect to the
use of QMRA to study recreational water risks. As with prior QMRA studies, the risks evaluated
in these studies characterize risk of GI illness and do not include other health endpoints, such as
respiratory illness.
Characterization of Risks from Human Fecal Sources
Soller et al. (2016) conducted a QMRA that included fecal indicator and pathogen monitoring
concurrently with an epidemiological study to characterize the risk of GI illness at Boquerón
Bay, Puerto Rico. Results of the QMRA were used to improve interpretation of a recreational
water epidemiological study. Results of the water quality study component of the QMRA
demonstrated low levels of pathogens and good water quality. The QMRA findings provide a
plausible explanation for the lack of relationship between fecal indicator organism detection and
swimming-related illness in the epidemiological study. The QMRA estimated a swimming-
associated risk of less than 2 NGI per 1,000 recreation events, which was below the level of
illness that the epidemiological study was designed to detect.
Vergara et al. (2016) conducted a QMRA to estimate the GI illness risk to a population from
primary and secondary contact recreation in an urban catchment in Singapore using field
measurements of norovirus, adenovirus, and Cryptosporidium. Land use in the catchment was
mostly residential with some industrial and office areas and human contamination can be
attributed to non-point sources of pollution (Vergara et al., 2016). Norovirus was detected more
frequently and at higher levels compared to adenovirus. QMRA results showed a higher illness
risk associated from exposure to norovirus compared to adenovirus. Risks for children (<18
years), based on children-specific recreational water ingestion data, were higher compared to
adults and exceeded 36 NGI per 1,000 recreators approximately 5.6 percent of the time. The
higher prevalence and illness risk of norovirus supported the use of norovirus as a reference
pathogen for QMRA in recreational waters in Singapore.
Lapen et al. (2016) conducted a QMRA of freshwater recreation in rivers in Ontario, Canada.
Using Cryptosporidium as the reference pathogen, two river basins in Ontario were monitored
for Cryptosporidium oocysts levels and species/genotype data were collected. The QMRA
included two approaches: one assumed all observed oocysts were infectious to humans, and for
the second, risk was based on the fraction of oocysts that were Cryptosporidium hominis and/or
Cryptosporidium parvum, which are the predominant human infectious forms of the parasite.
Compared to assuming all oocysts are infective to humans, the estimated infection risk was one
17
order of magnitude lower when only human infectious forms were selected, and fluctuations in
risk were also observed depending on seasonality. Results of this study demonstrate the
variability in potential risk when different pollution sources can occur. Also, the availability of
pathogen data at the species/genotype level can help inform more accurate estimates of potential
human infectious risk in a QMRA.
Eregno et al. (2016) coupled discharge-based hydrodynamic modeling with QMRA to estimate
the risk of infection from swimming in marine waters following a rainfall event with combined
sewer overflow events (CSOs). Of the simulated bacteria, protozoa, and virus infection risks, the
virus risk dominated, as represented by norovirus, and exceeded the infection health benchmark
of 19 GI illnesses per 1,000 swimmers in the days after the rainfall event. This modeling result
corroborates the findings of epidemiology studies (described above) that identified wet weather
and sewage overflow as factors leading to increased pathogen levels and infection risks. The
study demonstrates the potential utility of combining discharge-based hydrodynamic modeling
with health modeling in QMRA to estimate risk under wet weather conditions to inform beach
management conditions.
Soller et al. (2017) used QMRA to predict the GI illness risk to adult surfers associated with wet
weather at marine beaches in Southern California impacted by urban stormwater. In the QMRA,
sanitary survey information, monitoring data for fecal indicators, human microbial source
markers, reference pathogens, site-specific dilution estimates, and literature-based data for
incidental ingestion, pathogen dose-response, and morbidity were considered together to
generate risk estimates associated with wet weather stream flows affecting coastal nearshore
waters. As part of a broader “Surfer Health Study,” data collection for the QMRA was conducted
in parallel with a longitudinal recreational water epidemiological study focusing on surfer
exposure (Arnold et al., 2017). Water quality monitoring showed the presence of human fecal
contamination and fecal-associated pathogens in the stormwater discharging to the coastal water
where surfers were exposed. The results of the health modeling demonstrated that enteric
viruses, as indexed by norovirus, were an important etiologic agent of GI illness among surfers.
The QMRA was bolstered by a sensitivity analysis characterizing the uncertainty associated with
using published norovirus dose-response information. QMRA risk estimates from this study
corroborated the reported epidemiological results (Arnold et al., 2017). Predicted average illness
levels were lower at higher levels of FIB when compared to the epidemiological data that
informed EPA’s national recommendations. For both the QMRA (Soller et al., 2017) and
epidemiological (Arnold et al., 2017) components of the “Surfer Health Study,” the predicted
and reported GI illness levels represent a specific short-term high-risk scenario associated with
exposure to human fecal contamination present in coastal stream discharges containing urban
stormwater. The QMRA methodology used in this study and the reported results can be useful in
developing alternative health-based water quality criteria specific to risks associated with wet
weather impacts in an urbanized setting.
Bortagaray et al. (2020) assessed the risk of infection and illness for Group A Rotavirus (RVA)
in the Santa Lucia and Uruguay watersheds in Uruguay that are affected by raw sewage from two
cities. The authors performed qPCR on surface water samples and developed a QMRA
18
framework for people who use surface waters from the rivers. RVA was detected at all sampling
locations in both watersheds with an approximate detection frequency of 40 percent of samples.
Detection frequency increased during the coldest month of the year. The mean level of RVA was
1.3 × 10
5
genomic copies per liter (L). Due to the frequency and occurrence of RVA, QMRA
results demonstrated that populations using both rivers had comparable risks of infection and
illness.
Shoults et al. (2021) conducted a reverse QMRA for a natural swimming pool relying on
biological treatment processes, including a biofilm filter, a zooplankton filter, a hydro-botanic
filter, and a submerse filter in constructed wetlands coupled with ultraviolet (UV) irradiation
prior to the treated water being returned to the pool. The authors found that of the four reference
pathogens included (norovirus, Campylobacter, Cryptosporidium, and Giardia), only norovirus
exceeded the median recreational water risk benchmark. Log reduction values (LRVs) for the
reference pathogens were developed. The authors indicated that more than 1 day of treatment
would be needed to achieve the risk benchmarks after heavy bather use. Finally, the authors
concluded that ultraviolet disinfection had little effect on reducing the treatment time required.
Abia et al. (2016) conducted a QMRA to evaluate the public health risk associated with exposure
to pathogenic bacteria in polluted river water under undisturbed conditions and conditions of
sediment resuspension in the Apies River, Gauteng, South Africa. River water was monitored for
E. coli levels and the presence or absence of three feces-associated enteric pathogens: Salmonella
spp., Shigella spp., and Vibrio cholerae. The authors assumed that 8 percent of generic E. coli
counts were pathogenic. Ingestion rates of 1 mL, 50 mL, and 100 mL were used to represent
different levels of ingestion exposure. The results indicated that risks of infections increased
during the wet season. A 2-log increase in water E. coli count following sediment disturbance led
to approximately 10 times higher probability of infection than when sediments were undisturbed.
Liao et al. (2016) linked a watershed-scale microbial fate and transport model with a stochastic
dose-response model in a QMRA to predict human health risks from different fecal sources in an
urban watershed for comparison with regulatory benchmarks in order to prioritize remediation
efforts. Results indicated that human illness risks were consistently higher than 36 illnesses per
1,000 people for the study watershed, even when the predicted FIB levels were in compliance
with the E. coli GM standard of 126 CFU per 100 mL. Sanitary sewer overflows were associated
with the greatest risk of illness, which is of particular concern, given increasing indications that
sewer leakage is ubiquitous in urban areas. Uncertainty analysis suggested the accuracy of risk
estimates would be improved by additional site-specific pathogen data. The authors recommend
integrating this QMRA with water quality management planning to provide greater clarity to
stakeholders and decision makers (Liao et al., 2016).
Characterization of Risks from Non-Human Fecal Sources and Mixed Sources
Gitter et al. (2020) used QMRA to estimate the probability of GI illness from exposure to a
freshwater creek impacted by a mixture of sources. Pathogen concentration was estimated based
on FIB MST source apportionment of human, wildlife, cattle, and domestic animals, assuming
all detected FIB were from fresh contamination. The results indicate that the risk of illness from
19
norovirus, representing human fecal sources, contributed the greatest risk to human health. The
most frequent sources of E. coli at the sites monitored were from non-human sources (Gitter et
al., 2020).
Lim et al. (2017) conducted a source-apportionment QMRA to assess risk of GI illness at a
Southern California beach (Baby Beach, City of Dana Point) that did not have a known source of
human fecal contamination. Dry weather inputs of enterococci included bather shedding and
birds. Wet weather inputs of enterococci included the potential presence of sewage in stormwater
outflows (up to 20% of enterococci loading), animal feces from wildlife and dogs, and
nonpathogenic sources, such as plant and soil associated sources. Historical enterococci
measurement data were used to evaluate risks in dry and wet weather scenarios. During dry
weather, the median recreational waterborne illness risk at this beach is below the EPA’s RWQC
target illness rate of 36 illness cases per 1,000 bathers regardless of the fecal source contributing
enterococci. During wet weather, the median recreational waterborne illness risk predicted by the
QMRA depends on the assumed level of human waste associated with stormwater; the risk is
below the EPA RWQC illness risk benchmark 100 percent of the time, provided that <2 percent
of the FIB in stormwater are of human origin.
Evaluating Alternative Water Quality Surrogate Measures and Derivation of Risk-Based
Thresholds
Boehm et al. (2018) investigated the risk of gastrointestinal illness associated with swimming in
surface waters with aged sewage contamination. A systematic literature search and meta-analysis
compiled decay rate constants for reference pathogens and human feces-associated source
markers, which were incorporated into the QMRA. The QMRA evaluated HF183, a human-
associated fecal source marker, as an index for sewage present and thereby provided insight into
how risk relates to HF183 concentrations in surface water. Risks were modeled for aged sewage
and sewage of unknown age. The risks from recreational exposure to fresh and aging sewage
were primarily attributed to the reference pathogen norovirus. Based on the range of decay
values (n = 52) from the literature, HF 183 was shown to decay (~0.4 log
10
d
-1
) faster than
norovirus as sewage ages. Therefore, the risk associated with a fixed number of gene copies of
HF183 increases with the age of contamination. A HF183 risk-based water quality threshold for
fresh sewage was 9,700 copies per 100 mL and 900 copies per 100 mL after sewage aged for
2.5 days.
Ahmed et al. (2018a) estimated concentrations (genome copies [GC]) of sewage-associated
markers in 100 mL of beach water sample contaminated with either fresh untreated or secondary
treated sewage that exceeded the median illness rate of 36 per 1,000 people at a single event for
reference pathogens norovirus and human adenovirus 40/41. The concentration of 3.22 × 10
3
GC
of the HF183 in 100 mL of water sample corresponded to a risk above the GI illness benchmark
value when beach water was contaminated with fresh untreated sewage and roughly the same for
waters contaminated by secondary treated effluent.
Wu et al. (2020) identified RBTs (corresponding with 36 illnesses per 1,000 swimmers) within a
QMRA context in a hypothetical waterbody contaminated by a continuous loading of both
20
human and non-human fecal contamination. Their results indicated that a larger difference in
decay rates between pathogen and indicator leads to an increased miscalculation of
gastroenteritis risk compared to calculations that do not account for differential decay. Given the
continuous loading scenario, the difference in RBT between fresh and aged contamination was
less for human marker HF183 in the human contamination scenarios than for animal markers in
the animal contamination scenarios. The median RBT for human contamination was
approximately 3.5 to 3.7 log
10
gene copies per 100 HF183.
Goh et al. (2021) estimated RBT of human polyomaviruses (hPyVs), Bacteroides
thetaiotaomicron (B. theta), and Methanobrevibacter smithii (M. smithii) that correspond to the
acceptable GI illness risks associated with recreational activities in Singapore using a QMRA
approach. Among the three MST markers, hPyVs showed the highest specificity (100%) to
sewage samples, followed by M. smithii (97%) and B. theta (90%). All MST markers showed
100 percent sensitivity toward sewage contamination, with B. theta present in highest abundance
in sewage, followed by hPyVs and M. smithii. Field data showed that the MST markers at
threshold concentrations were able to classify the safe level in more than 83 percent of the
samples. This study presents the range of threshold concentrations in MST markers in freshwater
and seawater matrices by considering two scenarios (i.e., decay and dilution) under different
exposures (i.e., minimum, average, and maximum ingestion rates for swimming activity).
Brown et al. (2017) interpreted measured concentrations of Catellicoccus (CAT) genes (from
gull contamination) in recreational waters. The authors took 37 gull fecal samples to measure the
CAT gene concentrations and integrated the measurements in a QMRA framework using dose-
response functions from reference pathogens. They estimated that when the level of CAT
surpasses 4 × 10
6
copies per 100 mL of water, the median predicted illness exceeds three
illnesses per 100 swimmers.
Boehm and Soller (2020) incorporated updated data for microbial decay rates and aging of
mixtures of human sewage contamination to derive a risk-based water quality threshold for the
human marker HF183 in ambient waters using QMRA. The authors identified a threshold of
1–525 HF183 copies per 100 mL that corresponded with the health benchmark of 32 illnesses
per 1,000 for different contamination scenarios. This threshold was estimated for a scenario in
which human contamination in sewage of different ages co-occurs with contamination from gull
feces.
Schoen et al. (2020) used QMRA to explore the RBT of various markers for different
recreational water quality scenarios based on a series of assumptions and uncertainties. For
example, they found that fresh sewage RBT estimates were not always protective when aged
sewage was present, and aged sewage RBT estimates often fell below the marker lower limit of
quantification. Conservative RBT estimates of 9.3 × 10
2
and 9.1 × 10
3
(copies/100 mL) for
HF183/BacR287 and CPQ_056, respectively, were predicted when fresh sewage was greater (by
volume) than aged at the time of measurement. Conversely, genetic markers may not be effective
indicators when aged sewage contributes the majority of pathogens but minimal marker levels,
relative to fresh contamination. This study describes the importance of taking into account the
21
impact of a potentially slower decay rate and higher abundance when modeling public health
risk. The results highlight the utility of QMRA that incorporates pollutant age and mixture
scenarios, and the potential influence of site-specific factors on estimating RBT values. The
approach discussed in this study has been refined in multiple previous studies (Boehm et al.,
2015, 2018; Boehm and Soller, 2020) and supports establishing health protective RBTs for
alternative indicators.
Van Abel et al. (2017) conducted a QMRA to assess risk in surface waters used for drinking,
domestic, and recreational purposes in South Africa. Water monitoring results for three rivers
demonstrated a frequency of occurrence of norovirus genogroup I (50%) and genogroup II
(74%), with genogroup I occurring at lower levels than genogroup II. Risk estimates were based
on the occurrence of norovirus, exposure route and the dose-response model selected. Risk of
norovirus infection was sensitive to the choice of dose-response model chosen. Recreational risk
varied with the level of exposure (i.e., swimming, playing on the shore adjacent to the river, and
boating) with swimming yielding the highest risk. Depending on the dose-response model, river
waters frequently exceeded an annual recreational water illness benchmark of 0.03 (genogroup I:
46%–96%, genogroup II: 74%–96%). The risk burden was greater for individuals from
vulnerable subpopulations who may utilize untreated surface waters for multiple uses.
Sunger et al. (2019) used QMRA incorporating literature values of pathogen densities in
secondary treated wastewater effluents to simulate the risk of illness from swimming in
freshwater receiving wastewater effluent. Data from both disinfected and non-disinfected
secondary effluent were used. Enteric viruses, as indexed by norovirus, dominated the estimate
risk of GI illness.
Dose-response modeling has advanced for several waterborne pathogens that are commonly used
as reference pathogens in recreational water QMRAs. Adenovirus (Teunis et al., 2016),
norovirus (Teunis et al., 2020), and Campylobacter (Teunis et al., 2018) dose-response modeling
has been further developed by combining data sets and conducting Bayesian analyses to better
characterize infection and illness rates. Teunis et al. (2020) reported a meta-analysis of clinical
challenge studies for norovirus and norovirus outbreaks from consumption of oysters. Data
included were from 14 challenge studies using six different norovirus inocula and nine
outbreaks. The study showed how challenge studies and “natural experiments” (outbreaks) can
be combined in a multilevel, hierarchical dose-response framework. Overall, the results of the
study confirm the high infectivity of norovirus reported by earlier studies (Teunis et al., 2008).
Teunis et al. (2020) indicate that an updated dose-response model may be applied in QMRA,
with the potential benefit of differentiating between norovirus genogroups and host secretor
status.
Evaluating Parameter Selection and Sensitivity Analyses
Ballesté et al. (2018) examined the impact of decay rates of fecal source identification (FSI)
markers and FIB from different sources. The authors concluded that it is important to take into
consideration the rate of inactivation of bacteria and FSI markers in QMRA or other models used
to model pollution sources in freshwaters. Decay rates were found to be affected by changes in
22
temperature (seasons), sunlight, predation rates, presence of organic matter, fecal source, and
alkalinity. Intrinsic characteristics of indicators and markers for specific sources influenced
seasonal changes in decay rates.
Federigi et al. (2019) used PRISMA guidelines to review QMRAs of recreational waters
published between 2003 and 2018. PRISMA guidelines define the minimum set of items for
reporting in systematic reviews. They found research gaps included: 1) a lack of epidemiological
data on the main pathogens of concern in specific geographic areas; 2) a lack of site-specific
water quality data; 3) little consideration of recovery efficiency or infectivity of pathogens; 4)
lack of consideration of host-specific factors (like previous immunity or immunodeficient); and
5) lack of QMRA model validation. The authors conclude that the use of QMRA is “very
promising for management of recreational waters and as a mean[s] to improve the regulations,”
but additional research in the areas identified could improve the reliability of results.
Poma et al. (2019) evaluated type of methodology used for finding the best fit for pathogen data
and reported that this is a critical consideration in QMRA, especially in data sets with numerous
non-detects, because different approaches can result in very different estimations of risk. Five
different alternatives to represent censored data were evaluated. They found that the decision on
how to treat censored data, especially when they are abundant in the data set, is crucial. These
findings led the authors to suggest that, if in doubt about which approach to use, one should
adopt a more protective approach, such as using half the detection limit.
Summary of Findings from New QMRA Studies
• Additional evidence shows that waters affected by human fecal inputs can pose higher
health risks compared to non-human fecal sources.
• Several studies utilized norovirus as a primary reference pathogen to characterize risk
from human enteric viruses in waters affected by human fecal pollution.
• QMRA combined with epidemiological data can be a robust approach for
characterizing elevated health risks in acute exposure recreational scenarios.
• Children-specific exposure data can be used in a QMRA to estimate potential illness
risk to recreating children.
• Across analyses, the age of fecal contamination was identified as an important factor
affecting risk estimates and calculation of RBT values for alternative indicators.
3. Outbreak Studies of Illnesses in Ambient Recreational Waters Associated with Enteric
Pathogens
Outbreaks of illness in recreational waters are defined as the occurrence of similar illnesses in
two or more persons, epidemiologically linked by location and time of exposure to recreational
water or to pathogens, toxins, or chemicals aerosolized or volatilized from recreational water into
the surrounding air (Graciaa et al., 2018). The National Outbreak Reporting System (NORS) is a
23
passive reporting system through which state and local health officials voluntarily report
outbreaks to the Centers for Disease Control and Prevention (CDC). CDC reported NORS
information on leading etiologies causing outbreaks nationally in ambient recreational waters
between 2000–2014 (Graciaa et al., 2018). Vanden Esschert et al. (2020) discussed three
outbreaks in detail including reported FIB monitoring data for two of the outbreaks.
Additionally, four recreational water-related outbreaks of illness occurring outside the United
States were identified in the peer-reviewed scientific literature (Mosnier et al., 2018; Polkowska
et al., 2018; Schets et al., 2018; Sips et al., 2020). During 2000–2014, CDC reported 140
untreated recreational water-associated outbreaks resulting in at least 4,958 cases and two deaths
of which 95 outbreaks were confirmed to have a pathogen etiology, including five outbreaks
with multiple etiologies (Graciaa et al., 2018). The 95 outbreaks represented at least 3,125 cases;
enteric pathogens caused 80 (84%) resulting in 2,704 cases, with norovirus linked to 21
outbreaks (22%), pathogenic E. coli linked to 19 (20%), Shigella linked to 14 (15%), and
Cryptosporidium linked to 12 (13%). Of the eight outbreaks related to toxins or chemicals, seven
(88%) were caused by algal toxins associated with harmful algal blooms (HABs). Most
outbreaks were associated with freshwater venues (84%) and occurred during the June–August
timeframe (81%). Statistical analyses indicated the outbreak number was not significantly
different over the 15-year reporting period (Graciaa et al., 2018).
CDC recorded 119 outbreaks associated with ambient recreational water for the period 2009–
2019. In 69 of the outbreaks (58%), enteric pathogens were identified as the cause: norovirus (19
[22%]; 1,858 cases); Shiga toxin-producing E. coli (STEC) (19 [22%]; 240 cases);
Cryptosporidium (27 [19%]; 237 cases); and Shigella (14 [16%]; 713 cases) (Vanden Esschert et
al., 2020). In July 2018, 97 people reported becoming ill with GI symptoms after swimming at
Woods Pond Beach in Maine. Recreators reporting head submersion or swallowing water were
approximately three times more likely to be ill. Two stool specimens collected from four ill
people tested positive for norovirus genogroup I although the source of contamination was not
identified (Vanden Esschert et al., 2020). In August 2019, 69 cases of STEC infection were
identified in persons who reported swimming at a public lake in Minnesota. No E. coli
monitoring results from this outbreak exceeded state recreational water criteria during April–
October. No evidence of a point source of fecal contamination was identified, but investigators
did report multiple swimmers and lifeguards had contact with the lake while ill (Vanden Esschert
et al., 2020). In July 2019, 24 cases of shigellosis were identified in California following contact
with Santa Ana River water. E. coli concentrations in Santa Ana River water exceeded
acceptable levels and ranged from 350 to 1,600 most probable number (MPN) per 100 mL. Flow
in the Santa Ana River consists of discharges from multiple wastewater treatment plants
(WWTPs) and non-point sources of pathogens affect the quality of the river (California Water
Boards, 2022).
Four international outbreak investigations that demonstrated an increased risk burden among
children recreating in contaminated recreational water and/or provided specific etiological
information on the cause of the outbreak were found in the literature search. Norovirus was
identified as a cause of outbreaks at public beaches (Polkowska et al., 2018; Schets et al., 2018)
24
or other untreated recreational water venues (Sips et al., 2020). One study characterized a
Cryptosporidium-related outbreak among children in an isolated village with poor sanitation
(Mosnier et al., 2018).
Outbreaks of norovirus affected at least 1,093 people swimming at beaches in Finland in 2014
reported via a web-based survey (Polkowska et al., 2018) and 100 people swimming in a lake in
the Netherlands in 2012 (Schets et al., 2018); norovirus was detected in 19 of 23 (Polkowska et
al., 2018) and 4 of 5 (Schets et al., 2018) stool samples, respectively. No point sources of human
fecal contamination were identified at the lakes involved in either outbreak.
Polkowska et al. (2018) conducted a retrospective cohort study on an outbreak of gastroenteritis
in Tampere, Finland, which included children from 0 to 17 years old. Beach water monitoring
demonstrated acceptable levels of FIB (range from 0 to 5 CFU enterococci per 100 mL and 1 to
27 CFU E. coli/100 mL). The authors developed a web-based survey to identify infection sources
and risk factors. Cases in the general population included children who visited beaches at four
different lakes. Identified risk factors included exposure by getting water into the mouth while
swimming, swallowing water while swimming, submersing the head under water, and playing at
the beach. Generally, children 0 to 17 years old were more frequently affected, which the authors
related to higher exposure associated with recreating activities. Risks associated with submerging
the head under water were higher for children 0 to 4 years compared to the general population
(risk ratio 3.55 vs. 3.24). For other risk factors, the risk ratio was either lower for children
compared to the general population or not significant. The potential sources of enteric pathogens
detected (norovirus, Campylobacter jejuni, and rotavirus) were infected beachgoers.
Environmental health officers reported poor hygienic conditions at public beaches, including
children urinating or defecating on the beaches, overflowing trash receptacles, and used diapers
observed on the beach. (Polkowska et al., 2018). In both outbreaks, infected individuals may
have contaminated lake waters and contributed to secondary transmission of norovirus between
recreators. The authors suggested a need for new indicators of water quality for norovirus and
development of evidence-based recommendations regarding timing of safe reopening of
recreational water venues associated with outbreaks.
Schets et al. (2018) reported an outbreak of norovirus infection in Gelderland, the Netherlands,
among swimmers at a freshwater lake. Most cases were children with gastroenteritis symptoms,
and 82 percent of the cases had been in the water for 30–60 minutes. An epidemiological
investigation, combined with detection of norovirus genogroup I in stool samples of patients and
sand samples collected at the lake, suggested that exposure to the recreational lake resulted in the
outbreak. The most likely source of contamination was determined to be an infected human and
the authors concluded that active communication about human shedding of viruses during and
after diarrhea is needed along with guidance to refrain from swimming when contamination is
suspected.
Outbreaks of recreational water-associated GI illness reported since 2017 show that children can
be disproportionately susceptible to health effects from exposure to pathogens in recreational
waters compared to adults (Mosnier et al., 2018; Schets et al., 2018; Sips et al., 2020).
25
Mosnier et al. (2018) describe an increase in cryptosporidiosis reported over a 6-month period
among children living in a remote area along the Maroni River in French Guiana. Questionnaires
were distributed to collect data on demographics, food consumption, river interaction (play,
washing), symptoms, and outcome. Stool samples were collected and analyzed 3 months after
the onset of symptoms. Mosnier et al. (2018) report on a specific outbreak cluster of 14 cases, all
children, aged between 4.5 and 38 months, and all linked to Cryptosporidium hominis. The
outbreak was notable because it represented a peak in occurrence compared to previous sporadic
cases and occurred during a dry period. Human behavior and activities in the river area were
suspected to be the driver for the outbreak among the children. Numerous playgrounds on the
riverside included clothes washing, bathing, cooking/eating, playing, and defecation. Fecal
contamination and high turbidity were noted at water supply locations. Although this outbreak
likely resulted via multiple factors occurring at this location, it does provide evidence that
children, who have immature immune systems, can be at a disproportionate risk of contracting
severe infectious diseases when pathogen prevalence increases in a population.
Sips et al. (2020) described a norovirus outbreak in a natural playground in the Netherlands
where untreated water flows from a nearby river into a recreational lake. The Public Health
Service received 21 case notifications via the national online reporting system and an additional
100 cases (mostly children) were identified following an online query of the local health-related
Facebook platform. Water and fecal samples from humans and birds were tested and human
introduction of norovirus was identified as the most likely cause of the outbreak.
Summary of Findings from New Outbreak Reports
• Outbreak data document increased risk to children in recreational waters
• Outbreak surveillance in the United States and elsewhere continues to indicate the
importance of human enteric viruses as a leading etiologic agent of illness in ambient
recreational waters.
• Some outbreaks documented high levels of FIB in waters linked to the outbreak, but
some outbreaks occurred despite low densities of FIB.
4. New Information on Children’s Exposure in Recreational Waters
In 2019, EPA published Recommended Human Health Recreational Ambient Water Quality
Criteria or Swimming Advisories for Microcystins and Cylindrospermopsin, which used data
from Dufour et al. (2017) to characterize incidental ingestion for children ages 6 to 10 years old
and 7 to 11 years old compared to adults (Cyanotoxin AWQC, U.S. EPA, 2019). Section 4.2.3.1
of the Cyanotoxin AWQC includes graphical visualizations of ingestion volumes for different
age groups, information on the length of time an individual spends in the water (duration of
exposure), and a calculation of the daily incidental ingestion rate. Children ages 6 to 10 years old
have a daily incidental ingestion rate higher than any other age group. Section 7.3.2 of the
Cyanotoxin AWQC discusses exposure factors for children younger than 6 years old. Data for
ingestion by children <6 years old is more qualitative in nature and there are large uncertainties
26
given the lack of measured incidental ingestion data specifically for this age group, thus it is
challenging to compare this group directly to other age groups. However, using assumptions and
estimates for exposure for younger children from EPA’s 2011 Exposures Factor Handbook and
the data reported from Schets et al. (2011), the daily ingestion rate for children <6 years old was
estimated to be lower than the 6- to 10-year-old age group. Section 7.2.1 of the Cyanotoxin
AWQC presents data on duration of recreational exposures from both DeFlorio-Barker et al.
(2017) and EPA’s Exposure Factors Handbook (U.S. EPA, 2011).
DeFlorio-Barker et al. (2018) compiled self-reported swimming durations from epidemiological
study surveys from 12 beaches in which participants were asked to estimate, in minutes, the total
time they spent in the water. The study combined the data for time spent in the water with
incidental ingestion volumes reported in Dufour et al. (2018) to estimate the incidental ingestion
of water swallowed per swimming event by age group. Parents or guardians were responsible for
answering survey questions assessing exposures such as getting water in the mouth or
swallowing water, on behalf of their minor children. The study results represent 7,534 children
ages 8 to 12 years old and 5,998 children ages 4 to 7 years old recreating in fresh and marine
water. Marine recreators spent more time in the water compared to freshwater recreators. The
authors suggest that behaviors may have been influenced by the warmer water at most of the
marine sites (California and Gulf Coast) compared to the freshwater sites in the Great Lakes.
Children ages 4 to 7 and 8 to 12 years old had the highest exposures. Children 6 to 12 years old
were estimated to have the highest ingestion amounts and male children ingested more water
compared to females. This study provides additional evidence that children have higher exposure
in recreational water compared to adults and supports the results of other studies that show
children have higher risks of GI illness from recreational exposures.
The Influence of Children’s Behavior on Exposure to Recreational Waters
Recent studies of children’s behavior support other conclusions that they spend more time in
water and engage in more vigorous activity. Ferguson et al. (2019) conducted an observational
study focused on children ages 1 to 6 years old and their behavior patterns at recreational marine
beaches in Florida and Texas. Analyzing data from more than 400 parental surveys in both
locations, the authors found that over 46 percent of the study participants stay for 3 hours or
longer at the beach when going with their children, increasing their potential time of exposure
compared to adults who stay for less than 3 hours. Survey respondents also indicated that
children ages 1 to 6 years old spent 31–43 percent of the time in the water, 27–35 percent in the
intertidal zone, and 34–45 percent in dry sand. The authors found that age was the most relevant
factor in how and where children play (gender being less influential), with dry sand being the
most common zone for children 1 to 6 years old, although 6-year-old children spent the most
time in water. Hygiene practices like washing hands before eating at the beach significantly
varied by region (p < 0.001), indicating that only 37 percent of children washed their hands
before eating in Florida compared to 63 percent in Texas. Lastly, the authors observed that the
time spent digging in the sand was longer than previously published estimates. Although the data
were self-reported, they provide insights about children’s behaviors in marine coastal beaches.
27
Ferguson et al. (2021), used a virtual timing device to quantify real-time, sequential micro-
activity pattern data collected from videos of 120 children at four different beaches. This study is
the first to use this approach extensively for collecting data on children’s behavior at beaches.
Across all sexes, age groups, and beaches, wading was the most common activity and seawater
was the most common location where children played. For all age groups (0 to 6 years old), the
majority of time was time spent wading (activity) for both male (40.7%) and female (41.5%),
which corresponds to the seawater location where the most time was spent by both female
(45.8%) and male (47.5%) children. The fraction of time spent wading was 38.9 percent for
children 0 to 24 months, 37.6 percent for children 25 to 48 months old, and 45.1 percent for
children greater than 48 months. Although the study did not address ingestion directly, the
authors reported mouth contacts and for the majority of time, the mouth was not in contact with
any surface. When the mouth did contact a surface, the most common item was food (2%), the
next common was drinks (1.2%). The authors concluded that, based on activity patterns,
exposures for young females and males may be similar, and for dermal exposure and ingestion
exposure, the right hand is influential.
Summary of Findings from New Studies on Children’s Exposure
New studies on children’s exposure indicate that risk differences can be influenced by one or
more of the following:
• Children’s recreational exposure is greater than any other age group. In fact, studies
showed that ages 6 to 12 years old had greatest total volume of ingestion of
recreational water, independent of body size.
• Children’s greater exposure is due to behaviors such as increased time spent in water,
length of time spent playing in the sand, and more vigorous activity, which are
associated with greater health risk compared to adolescents and adults.
5. Summary of Major Findings from New Information on Health Effects and Children’s
Exposure
• Recent publications have advanced understanding and further confirm that
epidemiological and outbreak data demonstrate that children 0 to 10 years have a
higher risk of illness compared to adults when exposed to fecal contamination in
recreational waters.
• Children’s behavior, such as increased time spent in water, length of time spent playing
in the sand, and more vigorous activity, can increase their potential exposure to fecal
contamination and is associated with greater health risk compared to other life stages,
especially those 18 and over. Children’s recreational exposure is greater than
adolescents and adults because they ingest more water in proportion to their body
weight than the other age groups.
28
• Studies demonstrate that human fecal contamination can pose higher risks of illness in
recreators relative to some non-human fecal sources.
• While FIB can be a useful indicator where raw and poorly treated sewage dominates
water quality, multiple studies discuss the inconsistent performance of culture-
enumerated FIB related to health and point to other indicators to better represent the
potential risk of illness (e.g., Enterococcus measured by qPCR).
• Waters receiving human fecal contamination can have viral pathogens present at
health-relevant levels and yet also be below recommended water quality levels for
culturable FIB.
• The health risk of recreating during or following wet weather compared to dry weather
can be different due to changes in fecal loading dynamics to a waterbody.
• Recent QMRA studies corroborate past findings that waters affected by human fecal
inputs can pose higher health risks compared to non-human fecal sources.
• Several QMRAs utilized norovirus as a primary reference pathogen to characterize risk
from human enteric viruses in waters affected by human fecal pollution.
• Across QMRA studies, the age of fecal contamination was identified as an important
factor affecting risk estimates and calculation of RBT values for alternative indicators.
• Outbreak surveillance in the United States and elsewhere continues to indicate the
importance of human enteric viruses as a leading etiologic agent of illness in ambient
recreational waters, even where low densities of FIB occur.
• Multiple outbreak investigations demonstrate the increased health burden children
experience when exposed to fecal pollution in ambient waters.
B. New Information on Coliphages
Since the finalization of the first five-year review, new peer-reviewed scientific literature on
coliphages has been published. In particular, EPA has focused on literature assessing coliphages
as indicators of fecal contamination and their ability to act as a surrogate for enteric viruses in
human fecal contamination that impacts recreational waters. The sources of the new literature for
this section on coliphages include studies cited in the literature review performed by Nappier et
al. (2019a) and thorough discussions with experts (e.g., U.S. EPA, 2017a) and colleagues.
In 2019, a systematic literature review and meta-analysis characterizing the occurrence of
coliphages in raw wastewater and ambient waters was published (Nappier et al., 2019a). The
review identified studies published up to January 2017 of coliphages occurrence in raw
wastewater and ambient waters of sufficient quality and study design to support estimating
coliphage distributions accounting for geographic region and season. Study results reviewed
29
consistently found that somatic coliphages occur in significantly higher numbers compared to
MSC in both raw wastewater and ambient waters, which is consistent with other published
results. In ambient waters, somatic coliphages were more prevalent compared to MSC. Analysis
of the available quantitative data and distribution estimates showed higher densities of somatic
coliphages compared to MSC in raw wastewater, but this difference is not significant when
accounting for geographic region or season. Seasonal trends for both somatic and MSC were
noted with a winter peak for both coliphage types, which is similar to the seasonal variation
reported in the literature for noroviruses. The distributions for somatic and MSC reported in
Nappier et al. (2019a) could be used to support various risk assessment applications, such as
water reuse, shellfish, and understanding recreational water risk.
Additional studies found in the published scientific literature since 2017 have emphasized the
need for a viral surrogate to evaluate risk of human fecal contamination affecting ambient
waters. Culturable FIB are more effectively reduced by conventional WWTP processes than are
enteric viruses, such as adenovirus and norovirus, and protozoa (Dias et al., 2018; Korajkic et al.,
2022). Because FIB are disproportionately inactivated by most water treatment and
environmental degradation processes compared to viruses, Farkas et al. (2020) concluded that
FIB are “poor indicators of viral infection risk” and should not be used as the sole indicator of
human fecal contamination in water quality monitoring programs. Furthermore, Farkas et al.
(2020) pointed out that somatic coliphages and male-specific ribonucleic acid (RNA)
bacteriophages are used to assess wastewater contamination but are not always specific to human
feces. While some studies corroborated the finding that FIB are useful as a fecal contamination
indicator, the level of FIB may not be a good indicator of waterborne viral pathogens (Dias et al.,
2018 Korajkic et al., 2018).
1. Research on Coliphages
Since the first five-year review, a series of studies were published measuring coliphage
occurrence trends in recreational waters and wastewater (Wanjugi et al., 2018, Korajkic et al.,
2020a; Jones et al., 2022), environmental decay and fate and transport characteristics of
coliphages (Korajkic et al., 2019a; McMinn et al., 2019; McMinn et al., 2020; Boehm et al.,
2019), wastewater disinfection effectiveness on coliphages (Korajkic et al., 2022; Jones et al.,
2022), co-occurrence of coliphages and common host-associated fecal source markers (Li et al.,
2021), and wastewater treatment process efficacy on the removal of pathogens and indicators
(Ryu et al., 2021).
Wanjugi et al. (2018) characterized the incidence of culture-enumerated MSC and somatic
coliphages, E. coli and enterococci in recreational waters in the Great Lakes basin. FIB
concentrations were consistently higher than the concentration of MSC and somatic coliphages.
Somatic coliphage levels were consistently higher compared to MSC. Ultraviolet light absorption
and water temperature, but not FIB levels, were closely associated with coliphage concentrations
suggesting different persistence trends between FIB and coliphages in Great Lake waters. In
general, physico-chemical properties and recreational area parameters (e.g., meteorological)
30
were better predictors of FIB compared to coliphages at Great Lake recreational sites tested in
this study (Wanjugi et al., 2018).
Korajkic et al. (2020a) reported geospatial trends of culturable bacteriophages (MSC, somatic,
total coliphages, and GB-124 phage), and multiple fecal pollution-related genetic markers in
49 primary influent wastewater samples collected from rural and urban WWTPs across the
United States. Total coliphages were enumerated using the CB390 host. Like the pattern reported
for the Great Lakes region by Wanjugi et al. (2018), coliphages consistently occurred at levels
lower than FIB at a national scale. On average, there was a trend of higher somatic coliphage
levels compared to MSC levels, however, log
10
plaque forming units (PFU) per 10 mL
concentrations of somatic, MSC, and total coliphages were not significantly different from each
other (p ≥ 0.469). Notably, the indicators tested in this study exhibited a geographic stability in
their occurrence, regardless of urban or rural location designation. However, the data collected
represent a three-month monitoring effort during the winter season. This study provides a large,
paired data set consisting of multiple viral and bacterial indicators that can support new or
updated QMRA models (Korajkic et al., 2020a).
Other studies reported on decay characteristics of both coliphage types and adenoviruses in lake
water (McMinn et al., 2020), the effect of various biotic and abiotic environmental parameters on
die-off of coliphages and other fecal microbiota in ambient waters (Korajkic et al., 2019a), as
well as fate and transport of coliphages and other indicators of fecal pollution in a constructed
wetland designed to treat an impaired creek (McMinn et al., 2019).
Boehm et al. (2019) conducted a systematic review and meta-analysis of decay rates of
mammalian viruses and coliphages in surface waters. The decay rate constants of MSC and
somatic coliphages were similar to those of enterovirus, human astrovirus, and rotavirus A.
Norovirus (specifically Norwalk virus), hepatovirus A, and mastadenovirus had smaller decay
rate constants than coliphages. Decay rate constants were higher with increasing water
temperatures. The decay rate constants for coliphages were smaller in fresh water versus
estuarine and marine waters. However, this pattern was not observed for mammalian viruses,
most of which had insufficient data to make a comparison (Boehm et al., 2019).
McMinn et al. (2020) conducted an in situ mesocosm study in a freshwater lake to compare
decay characteristics of MSC and somatic coliphages to human adenovirus 2. Effects of sunlight
and indigenous protozoans on viral decay characteristics were also evaluated. The results
demonstrated that decay of coliphages and adenovirus were similar in the mesocosms and that
the greatest log reductions were observed when viruses were exposed to both sunlight and
predation. Somatic coliphages were the most affected by sunlight (McMinn et al., 2020).
Korajkic et al. (2019a) summarized the most recent published literature on the decay of fecal
microorganisms in the aquatic environment. Studies included in the review demonstrated longer
survival of coliphages in waters < 20℃, and faster decay of coliphages, FIB, and viral pathogens
(measured by culture-based techniques) in marine waters relative to freshwaters (Korajkic et al.,
2019a).
31
McMinn et al. (2019) monitored levels of culture-enumerated FIB, MSC, somatic coliphages,
and clostridia as well as genetic markers for Campylobacter, E. coli and enterococci, human-
associated (HF183) and bird-associated (GFD) MST markers through a constructed wetland to
evaluate its effectiveness at reducing microbial contamination from sewer overflows in stream
water fed to the wetland. Increases in fecal indicator concentrations in the wetland system were
noted. The increase could be attributed to indicator regrowth within the wetland and authors
noted elevated bird activity in areas corresponding to increases in fecal indicators. Lack of
reduction in fecal indicators due to fecal inputs by wildlife has been noted in other studies.
General fecal indicators, such as enterococci and coliphages, widely occur in warm-blooded
animals so bird deposition in the wetland can lead to false assumptions on the effectiveness of
the constructed wetland. The concentration of MSC and somatic coliphages did not significantly
change through the wetland treatment system. Somatic coliphages were correlated to culture-
enumerated E. coli levels (p = <0.0001, R
2
= 0.486). Some treatment efficacy in the wetland was
noted based on decreases of HF183 through the system, whereas increases in the GFD marker
were observed due to the elevated bird activity.
Korajkic et al., (2022) evaluated the effectiveness of two wastewater disinfection strategies
(chlorination and UV light exposure) on removal of coliphages, FIB, and viral pathogens.
Somatic, MSC, and total (CB-390 host strain) coliphages were measured from influent, and 20–
40 L final effluent samples concentrated using dead-end hollow-fiber ultrafiltration (D-HFUF).
Regardless of the effluent disinfection strategy, FIB were generally more sensitive to chlorine
and UV disinfection compared to coliphages. Both MSC and somatic coliphages were equally
susceptible to chlorination, but MSC were more resistant to UV light. Comparisons of coliphage
removal to that of viral pathogens was rarely feasible due to many samples with no detectable
pathogens, but removal of MSC correlated to that of adenovirus removal when chlorination was
the treatment strategy. MSC, GB-124 bacteriophage, and crAssphages were the most resistant to
UV disinfection and performed similarly to infectious enteric viruses and adenoviruses.
Li et al. (2021) evaluated paired measurements of coliphages (MSC and somatic), FIB, and FSI
genetic markers for human, ruminant, canine, and avian feces at beach and river sites in the
Great Lakes region to study the co-occurrence of fecal indicators and host-associated genetic
markers. Study findings demonstrated that general fecal indicators used for monitoring water
quality can influence the interpretation of paired FSI measurements and potentially lead to
prioritization of different pollutant sources for remediation. Routine monitoring with somatic
coliphages or MSC often led to contradictory fecal pollution source trends compared to bacterial
indicators. Both FIB and coliphages can originate from multiple animal sources. Knowledge of
the sources of contamination contributing to a waterbody would improve interpretation of the
general fecal indicator monitoring results.
Ryu et al. (2021) characterized the occurrence of MSC and somatic coliphages in primary
influent and their associated LRVs after secondary treatment and chlorine or UV disinfection.
Coliphages were detected in all influent samples (range 20 to 11,700 PFU/100 mL) but had low
detection rates in effluent samples (7% of effluent samples had detectable MSC and 14% of
effluent samples had detectable somatic coliphages). For both coliphage types, LRVs were
32
similar (approximately 5-log
10
reduction) for both chlorine and UV disinfection. LRVs for
coliphages were greater than the LRV for adenovirus, suggesting less stability than adenovirus.
Fitzmorris et al. (2022) conducted a narrative review using the One Water framework to assess
the strengths and weaknesses of using bacteriophages as viral indicators in wastewater, biosolids,
reclaimed water, recreational water, and shellfish for public health purposes. This review
indicated that scientists are reaching a consensus that bacterial indicators do not account or
represent the risk that viral pathogens pose to public health. Fitzmorris et al. (2022) suggested
that the fate and transport phenomena of bacterial pathogens is different for viral pathogens
present in surface water, groundwater, and wastewater (affected by, for example, treatment
processes, size differences, and lack of metabolic overhead for biological survival). Therefore,
monitoring for fecal indicator viruses, specifically coliphages, could support bacterial fecal
indicator monitoring as part of site-specific health-protection criteria.
2. New Epidemiology Studies Including Coliphages
Epidemiological studies evaluating the association between coliphages measured in waterbodies
and GI illness have been conducted since the first five-year review (Griffith et al., 2016;
Benjamin-Chung et al., 2017). The interest in understanding this association is because enteric
viral pathogens have been recognized as the leading causative agents of recreational water
illnesses in the United States (Graciaa et al., 2018; Vanden Esschert et al., 2020). As a result,
coliphages have also been considered in the context of QMRA to integrate monitoring efforts
with risk assessment modeling to estimate potential health impacts (Boehm, 2019).
Griffith et al. (2016) combined results across three epidemiological prospective cohort studies at
three California beaches to compare culturable MSC with culturable enterococci in relationships
with illness among swimmers. Culturable MSC measured by EPA Method 1602 had a stronger
association with GI illness than culturable enterococci enumerated by EPA Method 1600, at
Avalon and Doheny, where human fecal pollution was known to be affecting the beaches.
Human source markers performed as well or better compared to culturable enterococci at the site
with known human sewage inputs from faulty infrastructure. Overall, site-specific conditions at
each beach determined which indicator best predicted GI illness.
Benjamin-Chung et al. (2017) conducted a pooled analysis of six prospective cohort studies at
coastal beaches in California, Alabama, and Rhode Island. Water quality was measured using
enterococci and coliphages. Under all conditions combined, there was no association between GI
illness and levels of enterococci or coliphages. Associations between coliphage levels and GI
illness were observed only when human fecal pollution was likely present. Additionally, when
human fecal contamination was present, culturable enterococci only performed well when
coliphages were also detected. When enterococci levels were <35 CFU per 100 mL, coliphages
were detected in 72 percent (somatic) and 79 percent (male-specific) of samples taken, indicating
that viral pathogens may be present in waters receiving human fecal contamination even when
water quality is below the EPA criteria value. The study findings included similar associations
between somatic coliphages or enterococci with gastrointestinal illness. Additionally, in human
33
fecal-contaminated marine waters, there was some indication of MSC having a stronger
association with illness than enterococci.
3. QMRA
Boehm (2019) used a QMRA framework to estimate RBTs associated with the illness
benchmark of 32 NGI per 1,000 recreators for MSC and somatic coliphages in ambient waters
receiving untreated wastewater inputs. The framework considers recreational exposure to various
ages of wastewater and the associated coliphages and enteric pathogens to account for the
microbial decay over time. The effect of temperature difference on decay rates were also
considered. Exposure to fresh, unaged wastewater contamination resulted in estimated RBTs for
somatic (60 PFU/100 mL) and MSC (30 PFU/100 mL) coliphages. The estimated RBT generally
decreased as the wastewater ages because coliphages decay more quickly compared to norovirus,
which was used as a reference pathogen in the QMRA, effectively indicating the estimated risk
increases per indicator over time. Estimated RBTs decrease more quickly at higher temperatures
(25°C vs. 15°C). When the age of contamination is unknown, estimated RBTs for both
coliphages ranged between 1 and 10 PFU per 100 mL.
4. Studies of Wastewater Treatment Efficacy
The fate and persistence of norovirus, MSC, and somatic coliphages were evaluated throughout
the treatment process at nine water resource recovery facilities (WRRFs) that utilized various
types of secondary and tertiary treatment and disinfection (Worley-Morse et al., 2019).
Reduction of norovirus genogroups I and II was more similar to reduction of coliphages than FIB
(Worley-Morse et al., 2019). Reduction of MSC and somatic coliphages was variable and
dependent on the specific unit processes employed by a given WRRF (Worley-Morse et al.,
2019). Facilities with non‐biological nutrient removal (BNR) activated sludge and chlorine (de
facto chloramine) disinfection had less reduction of coliphages (1–3 log
10
) compared to FIB
(5 log
10
) (Worley-Morse et al., 2019). Greater than a 4 log
10
reduction was reported for both FIB
and coliphages at facilities that employed BNR processes and UV or ozone disinfection (Worley-
Morse et al., 2019). Although a 4 log
10
reduction was reported for FIB at a facility using
peracetic acid for disinfection, only a 2–3 log
10
reduction was reported for coliphages (Worley-
Morse et al., 2019). Worley-Morse et al. (2019) conclude that many WRRFs in the United States,
in particular those with disinfection steps using peracetic acid and chlorine in the presence of
ammonia (or chloramines), have greater reduction of FIB than coliphages. Thus, viral indicators
may serve as better predictors of the fate of enteric viruses, including norovirus (Mann et al.,
2019; Worley-Morse et al., 2019).
Jones et al. (2022) conducted a systematic review and meta-analysis of occurrence of coliphages
in effluent. Jones et al. found somatic coliphages and MSC density data from WWTP effluent
that had undergone primary, secondary, or tertiary treatment, or disinfection. The densities of
MSC and somatic coliphages in WWTP effluent were significantly lower than the densities in
the influent after receiving secondary biological treatment (with and without nutrient removal) or
tertiary treatment (e.g., treatment ponds or phosphate removal), or disinfection through
chlorination, UV irradiation, or both processes. This systematic review suggested that the

34
combination of tertiary treatment processes may result in greater viral inactivation in WWTP
effluent compared to a single effluent disinfection step. Jones et al. (2022) also concluded that
considering somatic coliphages and MSC as indicators of fecal contamination of sewage and
treated effluent continues to be useful to assess its potential impact on public health and
wastewater utilities.
5. Summary of New Information on Coliphages
• Both somatic and MSC consistently occur in raw wastewater across the United States.
Somatic coliphages are generally more numerous than MSC, and thus have potential to
be used as indicators of fecal contamination in waters affected by sewage and
wastewater treatment (particularly disinfected effluent).
• Viral indicators, such as coliphages, may perform better as surrogates of the fate of
human enteric viruses compared to culture-enumerated FIB during wastewater
treatment.
• Coliphages can exhibit a higher decay rate relative to viral pathogens in ambient
waters.
• Because coliphages have been shown to have a stronger relationship to illness than FIB
where human fecal sources were present, knowledge of the fecal sources affecting a
particular waterbody can improve interpretation of coliphage monitoring data.
C. New Information on Cyanotoxins
While cyanotoxins are not subject to the five-year review since they do not meet the criteria of
pathogens and pathogen indicators, EPA assessed the new information on health effects after
cyanotoxin exposure from literature searches conducted in 2021 by EPA (ORD and Office of
Science and Technology [OST]; see Appendix A for more information on methods). The 2021
literature search focused on six cyanotoxins: anatoxins, β-methylamino-L-alanine (BMAA),
cylindrospermopsin, microcystins, nodularins, and saxitoxins. From the relevant articles
identified in the 2021 literature search, new (defined as published since 2016) studies of health
effects after exposure to cyanotoxins are described here.
Cyanobacteria, also called blue-green algae, are naturally occurring photosynthetic bacteria
found in freshwater and brackish waters. Under certain environmental conditions, such as
elevated levels of nutrients, warmer temperatures, still water, and plentiful sunlight,
cyanobacteria can rapidly multiply to form HABs. Some species of cyanobacteria are able to
produce toxic compounds, known as cyanotoxins, which can be harmful to human and animal
health (U.S. EPA, 2019). EPA developed a public HAB web portal that contains information on
cyanobacteria and managing cyanotoxins in drinking and recreational waters
(https://www.epa.gov/cyanohabs).

35
U.S. exposure information indicates that cyanobacterial HABs have been reported in ambient
waters in all states and occasionally in marine waters (U.S. EPA, 2019; Woods Hole
Oceanographic Institute [WHOI], 2016). During a cyanobacterial HAB, the toxin concentration
can rapidly increase and may become elevated before a visible bloom is observed (Chorus et al.,
2000; WHO, 2021b; U.S. EPA, 2019) and persist after the bloom fades. Therefore, human
exposures can occur before and after the visible signs of a bloom. The most common
cyanotoxins found in ambient waters in the United States are microcystins, cylindrospermopsin,
anatoxin-a, and saxitoxins (U.S. EPA, 2009a; U.S. EPA, 2019). Nodularins are typically
produced by HABs in brackish waters and share similarities to microcystins in both structure and
adverse human health effects resulting from exposure. Exposure to elevated levels of
cyanobacteria and the toxins they produce during recreational activities could lead to adverse
health effects ranging from a skin rash, fever-like symptoms, body aches, respiratory irritation,
and gastrointestinal symptoms to more serious adverse health effects associated with organ
damage or neurological effects (U.S. EPA, 2019).
1. RWQC Guidance Values and Health Advisories for Cyanotoxins
In 2019, EPA published recreational water quality criteria and/or swimming advisories for two
cyanotoxins, microcystins and cylindrospermopsin, based on the best available science (U.S.
EPA, 2019). EPA recommended criteria for these two cyanotoxins include a magnitude (8 μg/L
microcystins or 15 μg/L cylindrospermopsin) and duration (not to be exceeded in more than
three 10-day assessment periods over the course of a recreational season) for freshwaters with a
recreational designated use (Table 2). The recommended magnitude represents the concentration
of microcystins or cylindrospermopsin at or below that which is not expected to result in adverse
human health effects from short-term recreational exposure to the toxins via incidental ingestion
by children while swimming in freshwaters. The recommended values are based on critical
studies identified from the available toxicity information described in the Health Effects Support
Document for the Cyanobacterial Toxin Microcystins (U.S. EPA, 2015a) and Health Effects
Support Document for the Cyanobacterial Toxin Cylindrospermopsin (U.S. EPA, 2015b). These
recommendations are intended as guidance to states, territories, and authorized tribes to consider
when developing WQS. Alternatively, these recommendations can be used as the basis of
swimming advisories for notification purposes in recreational waters to protect the public.
36
Table 2. Recreational Criteria or Swimming Advisory Recommendations for Microcystins
and Cylindrospermopsin
a
Application of
Recommended
Values
Microcystins
Cylindrospermopsin
Magnitude
(µg/L)
Duration Frequency
Magnitude
(µg/L)
Duration Frequency
Recreational
Water Quality
Criteria
8
One in 10-day
assessment
period across a
recreational
season
More than three
excursions in a
recreational
season, not to be
exceeded in more
than 1 year
b
15
One in 10-day
assessment
period across a
recreational
season
More than three
excursions in a
recreational
season, not to be
exceeded in more
than 1 year
b
Swimming
Advisory
1 day
Not to be
exceeded
1 day
Not to be
exceeded
a
These recommendations can apply independently within an advisory program or in water quality standards. States
can choose to apply either or both toxin recommendations when evaluating excursions within and across recreational
seasons.
b
An excursion is defined as a 10-day assessment period with any toxin concentration higher than the criteria
magnitude. When more than three excursions occur within a recreational season, and that pattern reoccurs in more
than 1 year, it is an indication the water quality has been or is becoming degraded and is not supporting its
recreational use. Units are micrograms (μg) per L.
The WHO Guidelines on Recreational Water Quality - Volume 1: Coastal and Fresh Waters
(WHO, 2021a) derived guideline values for four cyanotoxins: anatoxin-a and analogs (60 µg/L),
cylindrospermopsins (6 µg/L), microcystins (24 µg/L), and saxitoxins (30 µg/L). Differences
between EPA’s and WHO’s values for microcystins and cylindrospermopsin are due to
differences in input values such as children’s body weights and incidental ingestion. For
microcystins, WHO selected a different critical study compared to EPA for the basis of
developing recreational values. Additional information for these four cyanotoxins can be found
in WHO’s Toxic Cyanobacteria in Water, 2nd Ed (Chorus and Welker, 2021).
2. Health Studies
Since the last five-year review, new studies of health effects after exposure to cyanotoxins have
been published. Two single-dose, acute toxicity studies (Chernoff et al., 2020; Chernoff et al.,
2021) and three repeat-dose, chronic toxicity studies (Li et al., 2016a; Wang et al., 2016b; Zhou
et al., 2020) of health effects following oral exposure to microcystin were identified. One repeat-
dose, chronic toxicity study of health effects following oral exposure to cylindrospermopsin
(Chernoff et al., 2018) was published. Review of new data from new studies on
cylindrospermopsin and microcystins does not suggest changes to the reference doses (RfDs).
Further details of the studies for microcystins and cylindrospermopsin are described below.
Microcystins
Acute Studies
When EPA developed the Health Advisories in 2015 and the RWQC for Microcystins in 2019,
EPA derived an RfD for all microcystins using microcystin-LR (MC-LR) as a surrogate based on
MC-LR having a robust toxicological data set and relatively high potency among the microcystin
congeners (U.S. EPA, 2015a). Lethal dose 50 percent (LD
50
) studies of the most common

37
microcystin congeners identified a relationship between the congeners with hydrophobic L-
amino acids (-LA, -LR, and -YR) and the highest toxicity, and between hydrophilic amino acids,
such as microcystin-RR, and lower toxicity (Stoner et al., 1989; Gupta et al., 2003).
New studies on microcystin have been published since 2016. Chernoff et al. (2020, 2021)
characterized the acute toxicity of multiple congeners of microcystin. Chernoff et al. (2020)
reported acute toxicity for oral exposure of BALB/c mice to 10 different microcystin congeners
(MC-LR, MC-LA, MC-LF, MC-LW, MC-LY, MC-RR, [Asp3]MC-RR, [Asp3,Dhb7]MC-RR,
MC-WR, and MC-YR) by gavage as single 7 mg/kg doses. Significant differences in the single-
dose acute toxicity of microcystin congeners were observed, with MC-LR, MC-LA, MC-LY, and
MC-YR having effects on ≥ seven toxicity indicators e.g., moribundity, increases in relative liver
weight, and changes in clinical chemistry parameters. Noticeably, MC-LA and MC-LR showed
the greatest toxicity, including significant increases in moribundity. Consistent with previously
published studies, hydrophobic congeners of microcystin (e.g., MC-LR) were more toxic than
the hydrophilic congeners (e.g., MC-RR) (U.S. EPA, 2015a). Of the hydrophobic congeners
tested, MC-LR was more toxic than MC-LW, MC-LF, and MC-WR.
In a follow up study, Chernoff et al. (2021) administered single doses of several microcystin
congeners (LA, LR, LY, and RR) by gavage to BALB/c mice at levels ranging from 0.5 to
11 mg/kg, depending on the congener (Chernoff et al., 2021). All animals were euthanized
24 hours post-dosing. In this study, MC-LA was the most toxic congener among the four with
toxicity. MC-LA induced significant toxic effects in the liver that led to increases in total serum
bilirubin at 3 mg/kg, which was identified as the lowest-observed-adverse-effect level (LOAEL).
In a 28-day exposure of MC-LR, Heinze (1999) identified a LOAEL of 50 μg per kilograms per
day (μg/kg-d), 60 times lower than the acute LOAEL identified by Chernoff et al. (2021) for
MC-LA. The Heinze (1999) LOAEL for MC-LR was used for the derivation of the EPA’s
recreational criteria. Therefore, microcystin recreational criteria based on MC-LR is protective of
short-term, 1- to 28-day exposures to all four microcystin congeners. This newly identified study
(Chernoff et al., 2021) does not change the microcystin recreational criteria as the LOAEL from
an acute exposure study of MC-LR is protective of primary contact exposures to other
hydrophobic microcystins.
Chronic Studies
Three new studies of health effects after chronic exposure to microcystin were identified. Wang
et al. (2016b) administered 1, 10, or 40 μg/L of MC-LR in drinking water for 6 months to female
BALB/C mice to determine the impact of chronic, low-dose exposure to MC-LR on the lungs
(Wang et al., 2016b). The authors observed alveolar collapse and lung cell apoptosis with altered
cell junction integrity at all doses. The authors were not able to detect the presence of MC-LR in
lungs by immunoblot analysis and quantitative dose-response data were not provided.
Li et al. (2016a) characterized the chronic toxicity of MC-LR per L in adult male C57BL/6 mice
treated with 1, 5, 10, 20, and 40 µg MC-LR per L in drinking water for 12 months. Statistically
significant differences in lung/body weight ratios, caused by an increase in the level of lung
inflammatory cytokines that resulted in thickening of the alveolar septa, were observed (Li et al.,
38
2016a). The authors did not provide quantitative data, however, so a dose-response curve could
not be developed.
Zhou et al. (2020) evaluated the effects of chronic exposure to MC-LR on mice testes. Male mice
(age and strain not specified) were exposed to 0, 1, 10, or 100 μg/L MC-LR (purity >96%;
dissolved in 0.1% methanol) in drinking water for 90 or 180 consecutive days. Mice were noted
to be 15 to 25 grams (g), but body weights in Figure 1 presented in the study appear to be more
than 25 g on day 0. Therefore, it is not clear if the mice used were adults. Average intake was
estimated to be 0.15, 1.5, and 15 μg/kg-d by the study authors based on an assumed, rather than
measured, water intake of 1.5 mL per 10 g body weight and presumably the average biweekly
body weights. Body weight was significantly decreased in 10 and 100 μg/L groups beginning at
154 and 126 days, respectively. There are methodological issues with the lack of measured water
intake, the lack of reporting about the age of the animals, and the uncertainties with body weight
measurements at the start of the study. No significant change or trend in relative testes weight at
either 90 or 180 days was observed. However, absolute testes weights were not reported. Based
on decreases in body weights, it would be expected that there would be a change in absolute
testes weights. The lack of these data is a limitation in assessing effects on the testes.
Histological evaluation indicated an increase in abnormal seminiferous tubules in the 100 μg/L
group at both 90 and 180 days and in the 10 μg/L group at 180 days. Zhou et al. (2020) cite
methods used by Chen et al. (2011). As noted in section 7.4.2 of EPA (2015a), peer reviewers
identified concerns with the histological procedure used by Chen et al. (2011) and the issues do
not appear to have addressed in Zhou et al. (2020). Of 4,950 proteins quantified by isobaric tags
for relative and absolute quantitation (iTRAQ)-based proteomics, 20 proteins (8 upregulated and
12 downregulated) were significantly different between the 10 μg/L and control group. The
altered protein expression corresponded to 15 pathways, including renin-angiotensin system,
extracellular matrix-receptor interaction, phosphatidylinositol 3 kinase/protein kinase B
(PI3K/AKT) signaling pathway, focal adhesion, tight junction (TJs), and gap junction (GJs). The
histological analysis found that the blood-testes barrier (BTB) was significantly more permeable
in the two higher dose groups compared to the control group. The results suggest that MC-LR
causes dysfunction of the BTB through affecting TJs and GJs. Adverse effects in the Zhou et al.
(2020) study occurred at the two higher dose groups, with an estimated intake of 1.5 and 15
µg/kg-d, which are lower than the LOAEL of 50 µg/kg-d identified in the Heinze (1999) study
used to develop the EPA’s recreational criteria recommendation for microcystins. However, the
multiple methodological concerns, described above, of the Zhou et al. (2020) study precludes
EPA from considering the data to develop revised criteria.
The three chronic effects studies identified as part of EPA’s literature review assessed the
potential adverse respiratory (Li et al., 2016a; Wang et al., 2016b) and male reproductive (Zhou
et al., 2020) effects after long-term exposure to MC-LR. However, limitations associated with
the study designs and reporting prevented the quantitative use of the information and would not
support revising the RfD used to derive the 2019 recreational criteria recommendation for MC.
39
Cylindrospermopsin
One new oral toxicity study of cylindrospermopsin was identified. The study exposed adult male
and female mice to cylindrospermopsin (75 to 300 μg/kg-d) by gavage for 90 days (Chernoff et
al., 2018). Health effects observed were elevated organ to body weight ratios of the liver and
kidney at all dose levels, increase in serum alanine aminotransferase (ALT) activity, decreases of
blood urea nitrogen (BUN) and serum cholesterol concentrations in males, plus high monocyte
counts in both genders compared to the negative control. The study identified a LOAEL of
75 μg/kg-d based on significant effects in liver and kidney/body weight ratios, reduced BUN,
increased serum monocytes, and multiple signs of histopathology. The health effects as well as
the histopathological findings are consistent with the toxicological findings found in the earlier
studies of Humpage and Falconer (2002, 2003) that were used for the derivation of EPA’s RfD
for cylindrospermopsin. Although a comparison of no-observed-adverse-effect levels (NOAELs)
between the Chernoff et al. (2018) and Humpage and Falconer (2002, 2003) studies is not
possible, the higher LOAEL (75 μg/kg-d) reported in the Chernoff study is higher than the
60 μg/kg-d dose reported in the Humpage and Falconer study, so the new information would not
support revising the RfD used to derive EPA’s 2019 recreational criteria recommendation for
cylindrospermopsin.
Other Cyanotoxins
EPA identified new toxicity studies for anatoxin-a and saxitoxins but not for nodularins. There is
not enough information to support the derivation of RfDs for either anatoxin-a or nodularins. The
EPA literature search did identify a number of new studies, including dose-response studies, for
saxitoxins.
Anatoxin-a
One new study was identified on the lethal dose of anatoxin-a (ATX) and its congener
dihydroanatoxin-a (dhATX) (Puddick, et al., 2021). Purified anatoxin-a and dhATX from the
benthic cyanobacterium Microcoleus autumnalis were administered to female Swiss albino mice
by intraperitoneal (i.p.) injection and by oral ingestion (gavage and feeding) for 14 days to
determine the LD
50
. The researchers observed a difference in lethal doses for each exposure
route, with i.p. injection dhATX was less toxic than anatoxin-a (0.73 mg/kg for dhATX and
0.23 mg/kg for anatoxin-a), but dhATX was more toxic than anatoxin-a with gavage (2.5 mg/kg
for dhATX and 10.6 mg/kg for anatoxin-a) and feeding (8 mg/kg for dhATX and 25 mg/kg for
anatoxin-a). The study design focused on identifying lethality only and did not analyze for
nonlethal health effects such as neurotoxicity, histopathology, hematology, and serum chemistry,
and therefore, this study could not be used to derive a noncancer RfD for ATX or dhATX.
Zhong et al. (2020) is an in vitro study that evaluated the immunotoxicity of anatoxin-a using
Carassius auratus lymphocytes. Non-adherent lymphocytes isolated from the kidneys of C.
auratus fish (6–10 months old) were cultured in Roswell Park Memorial Institute (RPMI)-1640
medium with 5 percent fetal bovine serum, free from antibiotics, and exposed to 0, 0.01, 0.1, 1,
and 10 mg/L for 12 hours. In vitro immunotoxicity from exposure to anatoxin-a was evidenced
by increases in apoptosis of lymphocytes, which increased in a dose-dependent manner, based on

40
both deoxyribonucleic acid (DNA) fragmentation and flow cytometry. Flow cytometry results
indicated that the percentage of apoptotic lymphocytes exposed to 0.01, 0.1, 1, and 10 mg/L of
anatoxin-a reached 18.89, 22.89, 39.23, and 35.58 percent, respectively, with less than 15 percent
observed in controls. Ultrastructural changes associated with anatoxin-a exposure identified
using transmission electron microscopy included cytoplasmic condensation, vacuolation, and
swollen mitochondria in C. auratus lymphocytes. Oxidative stress also increased in a dose-
dependent manner as measured by increases in reactive oxygen species (ROS) and
malonaldehyde, and decreases in superoxide dismutase, glutathione, catalase, glutathione
reductase, glutathione peroxidase, and glutathione-s-transferase. In vivo studies are needed to
confirm the immunotoxicity findings of Zhong et al. (2020).
No human epidemiological data were identified that assessed health effects after exposure to
anatoxin-a. One new human case report of food poisoning following exposure to sea figs
contaminated with anatoxin-a was identified but the study did not measure or report dose
information (Biré et al., 2020).
Studies assessing adverse effects from either the inhalation or dermal route of exposure were not
identified for anatoxin-a. Anatoxin-a was detected on glass fiber filters using in field-deployed
air sampler during an active harmful algal bloom in Massachusetts in 2019 (Sutherland et al.,
2021), but the study was not designed to quantify or characterize the human health risk from
inhalation of anatoxin-a in water droplets.
For anatoxin-a, the current available information on health effects falls short for determining an
RfD. In the absence of EPA recreational water recommendations for anatoxin-a, some states
have adapted the WHO health-based reference values for acute anatoxin-a exposure (WHO,
2021b). EPA will continue to evaluate new scientific data on anatoxin-a and its effects as they
become available.
Saxitoxins
Saxitoxins are a group of 57 analogs grouped by structural similarity, including the parent
compound (STX), neosaxitoxin (neoSTX), gonyautoxins (GTXs), C-toxins,
decarbamoylsaxitoxins, and lyngbyatoxins (LWTXs). STX equivalents (STXeq) are often
reported in studies as the total concentration of STX variants, or they may represent
concentrations adjusted for toxicity (WHO, 2020). New studies on health effects after exposure
to saxitoxin have been published since 2016. EPA has initiated the development of a Health
Effects Support Document (HESD) for STXs. Health effects studies, both human studies and
toxicological studies, were identified and reviewed through EPA’s literature search. The
available human studies include case reports of morbidity and mortality resulting from paralytic
shellfish poisonings (PSPs) in adults and children, as well as epidemiologic and case-series
studies that assessed human health effects after exposure to saxitoxins. Development of this
HESD will continue in 2023.

41
Human Case Reports
A case of PSP poisoning in the United States was reported in 2016 when an elderly female who
ate clams harvested in Roslyn Beach in Kodiak, Alaska, reported symptoms of nausea with dry
heaving, weakness, respiratory depression, and shock (Coleman et al., 2018). PSP testing of the
clams from the suspected meal determined a saxitoxin concentration of 277 µg per 100 g, 309 µg
per 100 g of neosaxitoxin, 576 to 2,490 µg per 100 g of multiple GTX, 7.52 to 11.3 µg per 100 g
of decarbamoyl, and 10.8 to 221 µg per 100 g of C-toxins. Saxitoxins, neosaxitoxins, and GTX1-
4 were also found in the urine of the patient (64.0 µg/g-creatinine, 60 µg/g-creatinine, and 492-
4,780 µg/g-creatinine, respectively).
Knaack et al. (2016) evaluated 11 patients with suspected PSP. Of the 11 patients, four were
confirmed to have STX-PSP by urine testing (24–364 ng STX/g-creatinine) and five patients had
clinical manifestations of PSP. Results revealed that dysphagia and dysarthria appeared to be
stronger indicators of PSP than paresthesia and nausea, which are commonly used to clinically
diagnose patients with PSP. Meal remnants obtained from six presumptive PSP cases were
analyzed and all six samples tested positive for PSP toxins. The results of this report are limited
because only 4 of 11 patients were confirmed to have PSP.
Animal Studies
Diehl et al. (2016) conducted a toxicological study in rats exposed to two doses of saxitoxin
equivalents orally via drinking water for 30 days and then assessed for behavioral effects. The
results of the behavioral test battery indicate that the STX-exposed rats had decreases in learning
and memory processes compared to negative controls (Diehl et al., 2016).
Selwood et al. (2017) conducted a series of studies to determine the acute toxicity of STX and
multiple STX analogs administered by i.p. injection, as compared to oral gavage or consumption.
Exposure data were used to identify the LD
50
and NOAEL. Mice were first dosed a step below
the best preliminary estimates of the LD
50
, and subsequent animals receive a lower dose (if the
previous animal dies) or a higher dose (if the previous animal survives). In general, the LD
50
values for each STX analog were lower following i.p. injection than they were with oral
administration. This was attributed to slower absorption of the toxins via the oral route.
Nonlethal health effects of exploratory behavior, grip strength, changes in abdominal breathing,
and lethargy were assessed and used as the basis for the NOAEL values for oral gavage (544
nanomoles per kilogram [nmol/kg] for STX; 276 nmol/kg [neoSTX] to 25,500 nmol/kg [C3&4]
for STX analogs) and dietary exposures (4,360 nmol/kg [decarbamoyl neosaxitoxin
(dcNEOSTX)] to 17,400 nmol/kg [C1&2] for STX analogs; not determined for STX). The
symptoms of toxicity manifested by the STX analogs following the oral gavage and feeding
routes of exposure were proportional to those for i.p. injection except that the onset of toxicity,
recovery from sublethal doses, and time to death were delayed for the oral exposure routes. The
recovery and time to death results for the different exposure routes (i.p. injection < gavage <
feeding) indicate the toxin has to be delivered to systemic circulation before the effects are
manifested (Selwood et al., 2017).

42
In Vitro Studies
Peripheral blood mononuclear cells obtained from wild-captured harbor seals were used to study
the effects of STX on immune cell modulation and phocine distemper virus (PDV) replication
(Bogomolni et al., 2016). Exposure to 10 ppb STX led to a 78 percent increase in lymphocyte
proliferation compared to the control. In STX-exposed cultures, there was an 8-fold increase in
lymphocyte fraction PDV loads on average at day 5 post-infection, and a 2.5-fold increase in the
supernatant fraction PDV load at day 9 post-infection. Given these findings, the authors suggest
that exposure to STX could increase systemic virus dissemination upon in vivo infection in
marine mammals.
D'Mello et al. (2017) investigated the cytotoxicity of STX and other cyanotoxins on primary
human astrocytes using the lactate dehydrogenase (LDH) release assay. LDH activity was
significantly increased with 0.1 nmol STX, and inhibitory concentration (IC) IC
20
and IC
50
(concentration giving 20% or 50% of the maximum inhibitory response) values were determined
to be 0.65 and 0.95 nmol, respectively. Cell proliferation was significantly decreased with STX
exposure at both the IC
20
and IC
50
doses, with proliferation at the IC
50
dose at 46.4 ± 2.7 percent
of the control.
Two mammalian neuronal cell lines were used to study effects of extended, low-dose exposure
to STX on neuronal development (O'Neill et al., 2017). STX exposure prevented normal
morphological changes in neuronal morphology even with the lowest concentration tested. The
effects observed appeared to be dose dependent. The results support a conclusion that STX
exposure can cause a change in neuronal cell morphologies in both the central and autonomic
nervous system.
The interactions between pristine single-walled carbon nanotubes (SWCNTs) or carboxylated
single-walled carbon nanotubes (SWCNT-COOH) and STX were evaluated to understand the
potential effects of those interactions on cell toxicity (Ramos et al., 2017). No effects on cell
viability or cell proliferation were reported with any treatment at 30 minutes. At 24 hours,
exposure to STX with SWCNT led to a significant decrease in cell viability compared to
controls. No other statistically significant effects at 24 hours were found. These data suggest a
weak interaction between STXs and SWCNTs that could alter the effect of STX on mammalian
neuronal cells.
Abi-Khalil et al. (2017) investigated the immunotoxicity and localization of STX in hemocytes
from a species of oyster. Fluorescent coumarin-coupled STX (STX-Cou) was detected in the
cytoplasm of oyster hemocytes but did not co-localize with the mitochondria. The negative
control, Gua-Cou, showed no specific localization of fluorescent coumarin labeling, suggesting a
STX-specific accumulation in the cytoplasm of oyster hemocytes. Upon evaluation of
phosphatidylserine translocation and membrane permeability, a strong green-fluorescent signal
indicated the presence of phosphatidylserine on the cell surface in both Etoposide and STX-
treated hemocytes. Additionally, the signal depended on dose; it was detected after treatment
with 3.3 µM STX but not with 0.8 µM STX (Abi-Khalil et al., 2017).
43
The apoptotic potential of STX was determined as the imaging of cells exposed to 0.8 µM STX
showed marked chromatin condensation at the nucleus periphery, a hallmark of apoptosis. A
similar effect was noted in the positive control (50 µM of Etoposide) but not the sterile sea water
negative control. DNA fragmentation and apoptosis were determined in cells exposed to 0.8 µM
STX for 3.5 hours. Tetramethylrhodamine nick end labeling (TMRNEL) revealed that
hemocytes exposed to 0.8 µM STX exhibited a greater proportion of cells with fragmented
nuclear DNA compared to controls. 3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyltetrazolium
bromide (MTT) assay results showed that exposure to 3.3 µM STX resulted in a decrease (34% ±
8%) in living cells similar to that of 50 µM Etoposide (49% ± 8%), however, no effect was
observed after exposure to 0.8 µM STX. Over a 2-hour period, exposure to STX did not exhibit a
detectable effect on ROS concentrations, suggesting that cell death observed in other assays is
not due to ROS (Abi-Khalil et al., 2017).
Some state and international agencies have developed health-based values for saxitoxins in
drinking and recreational waters. The Ohio Environmental Protection Agency (OHEPA) issues
Recreational Public Health Advisories at 0.8 μg/L (OHEPA, 2022). The Ohio Department of
Health (ODH) also issues “Do Not Drink” advisories for saxitoxins in drinking water: 0.3 μg/L
for bottle-fed infants and children younger than school age, pregnant women, nursing mothers,
individuals with pre-existing liver conditions or who are immunocompromised, and individuals
receiving dialysis treatment; and 1.6 μg/L for all people of all ages as well as for pets and
livestock (ODH, 2020). WHO (WHO, 2020) estimated an acute oral RfD for saxitoxins of
0.5 µg/kg-body weight (bw) based on mild shellfish poisoning symptoms from 500 case reports
exposed to PSP, as reported by the European Food Safety Authority (EFSA, 2009). EFSA
estimated a NOAEL of 0.5 μg STXeq kg-bw based on human PSP symptoms and supported by
animal studies: based on the point of departure of the lowest acute NOAEL for neoSTX of
87 µg/kg-bw (Munday et al., 2013) from a gavage toxicology study, an acute oral RfD for
neoSTX of 0.87 µg/kg-bw (applying an uncertainty factor of 100) was derived. This value is of
the same order of magnitude as the reference values obtained with human data (WHO, 2020).
Nodularins
No new oral toxicity studies of nodularins, published since 2016, were identified in EPA’s 2021
literature search. EPA has previously reviewed the available toxicity data for nodularins and
found that the previously published (before 2016) data are inadequate to derive an RfD to use to
support developing RWQC and/or Swimming Advisories.
3. Summary of New Information on Cyanotoxins
• EPA has identified several repeat-dose, acute, and chronic toxicity studies of health
effects of cyanotoxins published in the last 5 years.
• For cylindrospermopsin and microcystins, these new studies either supported the existing
reference doses identified in EPA’s HESDs or did not provide new information to
support derivation of a revised RfDs.
• EPA evaluated several toxicity studies characterizing effects from exposure to anatoxin-
a, saxitoxins, and nodularins.

44
o Work is underway at EPA to evaluate data to support development of an HESD
for saxitoxins.
o The limited available toxicity information available for anatoxin-a and nodularins
does not support development of an HESD at this time.
D. New Information on Antimicrobial Resistance
To identify new literature on AMR and antimicrobial resistant genes (ARGs), EPA reviewed
three relevant review articles that were published since the last five-year review (Nappier et al.,
2020; Korajkic et al., 2020b; Franklin et al., 2021).
1. EPA AMR Research
The review article, Korajkic et al. (2020b), on AMR in Enterococcus species in marine and
estuarine environments indicates that E. faecium and E. faecalis exhibit resistance to the highest
number of antibiotics, followed by E. casseliflavus, E. hirae, and E. durans. Further, studies
found that all Enterococcus species were resistant to erythromycin. The highest proportion of
resistant enterococci were measured in the water column (>18%), with other important reservoirs
of resistant enterococci being feces and tissues of animals such as seabirds, whales, and clams,
and to a lesser degree sediments and sands. Regarding methodology, the review identified a lack
of standardized procedures to study, detect, and classify antibiotic resistant Enterococcus in the
environment and recommended the establishment a standardized framework for studying
antibiotic resistance in the environment.
The review article by Franklin et al. (2021) on the available molecular methods to analyze AMR
in surface waters was conducted to support One Health Assessments
(https://www.epa.gov/healthresearch/one-health). This article described three state-of-the-art
methodologies that can provide information about the presence, diversity, and dynamics of ARG
targets in the environment including surface waters (Franklin et al., 2021). The three techniques,
high-throughput qPCR, metagenomics, and whole-genome sequencing, are all culture-
independent and can target a high diversity of genes simultaneously. The article noted that there
is value in combining molecular information with culture-based approaches because molecular
methods can identify most, if not all, genetic elements that may confer AMR in a single
environmental isolate and/or a microbial community, while culture-based methods can provide
phenotypic confirmation of AMR in environmental isolates. Used together, the methods can
more fully characterize AMR in surface waters.
The state-of-the-science review by Nappier et al. (2020) of AMR bacteria and ARGs in
recreational waters reported that new studies found that ARGs can be acquired through
spontaneous mutations and can persist in microbial populations via selective pressure. For
example, AMR and heavy metal resistance genes often co-occur on the same mobile genetic
elements (e.g., plasmids), resulting in a co-selection and propagation of mobile genetic elements
carrying ARGs, even in the absence of antibiotics. Since resistant genes can move between
pathogenic and nonpathogenic microorganisms, a better mechanistic understanding of horizontal
gene transfer and AMR selection is warranted to improve AMR monitoring. Nappier et al.
(2020) also reported that studies indicate that untreated wastewater, treated WWTP effluent,
45
medical waste, pharmaceutical production, biosolids, agriculture, aquaculture, plant, and animal
agriculture (e.g., concentrated animal feeding operations [CAFOs]), and the feces of birds and
other wildlife can be sources of AMR bacteria and ARGs. Such observations are consistent with
prior studies that show differential effects of different treatment processes on AMR bacteria and
ARGs.
2. Summary of New Information on AMR
• Treatment processes commonly used at WWTPs are not typically calibrated to target
removal of AMR bacteria or ARGs.
• An increased understanding of horizontal gene transfer mechanisms and AMR
selection can be used to improve monitoring for AMR in ambient waters.
• A combination of techniques (including both molecular- and culture-based) are
necessary to fully understand AMR in surface waters.
E. New Information on Human and Non-Human FSI
FSI techniques are used to characterize fecal sources potentially present in polluted waters.
These methods rely on the detection and/or quantification of host-identifiers such as chemical or
microbial targets closely associated with a particular pollution source (Hagedorn et al., 2011).
Accurate and reliable FSI technologies have the potential to improve future water quality
management in the United States. The 2012 RWQC (U.S. EPA, 2012a) has provisions that
recommend these FSI methods for use as a sanitary characterization tool and has published
information to support alternative criteria eligibility of FSI (U.S. EPA, 2014a).
EPA has invested considerable resources to develop, validate, standardize, and implement FSI
technologies. High-priority research areas include completion and publication of standardized
EPA methods for human-associated qPCR methods, development and public release of a
companion standard reference material, further investigation of data interpretation approaches,
and science to advance virus-based human FSI method development.
Since 2017, EPA researchers have published 15 peer-reviewed studies, released two nationally
validated human-associated FSI qPCR methods, and codeveloped a standard reference material
in collaboration with the National Institute of Standards and Technology (NIST). In addition to
EPA activities, there are numerous significant contributions by other researchers that have
advanced FSI recreational water science.
The systematic literature review identified 85 relevant articles (Figure 1) discussing advances in
MST or fecal source identification techniques (Appendix B). Additionally, 22 relevant references
were identified via an ad hoc process (Blatchley et al., 2007; Shanks et al., 2009, 2010, 2011,
2014; Sinclair et al., 2009; Okoh et al., 2010; Soller et al., 2010a,b; Hagedorn et al., 2011;
Boehm et al., 2013; Ervin et al., 2013; Dutilh et al., 2014; U.S. EPA, 2014a; Qiu et al, 2015;
46
Stachler et al., 2017; Korajkic et al., 2020a; McKee et al, 2020; Shrestha et al., 2020; Ahmed et
al., 2021; Kralj et al., 2021; Sivaganesan et al., 2022).
1. Human FSI Research
Advances in Bacterial-Based FSI
Researchers have advanced methods for bacterial-based identification of human fecal sources in
recreational water. In particular, the Bacteroides HF183 genetic marker is well characterized
(Ahmed et al., 2016b; Holcomb and Stewart, 2020; Jiang et al., 2018; Somnark et al., 2018;
Staley and Edge, 2016; Vadde et al., 2019) and routinely used for qPCR studies due to its strong
human-host association (Devane et al., 2019; Staley and Edge, 2016; Symonds et al., 2017).
HF183 was found to be a more specific marker for combined sewage overflows than human
polyomavirus, pepper mild mottle virus, and six other human pathogens in Cobbs and Tacony
Creeks in Philadelphia (McGinnis et al., 2018). HF183 was also readily detected at Lake
Pontchartrain beach and was used to determine that rainfall events introduce large amounts of
human fecal bacteria into lake waters (Xue et al., 2018). Green et al. (2019) assessed HF183 and
ruminant-associated markers during dry weather in Onondaga Creek, New York. They found that
the upstream ruminant inputs decayed and/or were diluted out and that the high levels of urban
bacterial contamination were human associated and most likely due to failing infrastructure
and/or illicit discharges independent of rain events. In addition to HF183, other human-
associated genetic markers have been used for identifying human sources in recreational waters,
such as HumM2, HumM3, NifH, and BifHM (Devane et al., 2019; Zeki et al., 2021; Zhang et al.,
2020; Ballesté et al., 2018; Shanks et al., 2009).
Napier at al. (2017) assessed the presence of four human-associated Bacteroides markers
(HF183, BsteriF1, BuniF2, and HumM2) with self-reported gastrointestinal illness, diarrhea, and
respiratory illnesses. The analysis used data from 12,060 adult visitors who enrolled in the
NEEAR studies at six beaches from 2003 to 2007. The HF183/HumM2 detection rate at NEEAR
marine and freshwater beaches were 26 percent and 28 percent, respectively. Overall, findings
suggest inconsistent associations between illness and the detection of human-associated
Bacteroides markers. The authors concluded that quantitative measures of human fecal pollution,
rather than presence-absence, may be necessary to improve assessment of potential links
between public health risk and the occurrence of human fecal pollution.
Mattioli et al. (2020) used HF183 as a microbial tracer to evaluate potential sources of
contamination of a drinking water well linked to multiple outbreaks of norovirus documented
over a month-long period. This is a new application for human-associated FSI genetic markers,
used to mitigate exposure.
Advances in Virus-Based FSI
Enteric viruses, such as noroviruses, are reported to be the dominant etiological agents of
recreational waterborne disease (Sinclair et al., 2009; Soller et al., 2010b). These enteric viruses
47
can react to waste treatment and environment stressors in different ways compared to bacterial
fecal indicators (Blatchley et al., 2007; Okoh et al., 2010; Qiu et al., 2015). Viral FSI
technologies offer an alternative to current bacterial-based methods; however, many are either
not sensitive enough or lack sufficient host specificity (Boehm et al., 2013). An ideal viral FSI
method would target a virus that is both closely associated with a particular animal host and is
highly abundant. There is a growing body of evidence suggesting that some virus-based FSI
genetic targets are highly abundant in sewage, are closely associated with human waste, and
persist for longer periods of time in wastewater and environmental settings compared to many
bacterial fecal indicators.
Since 2017, there have been significant advances in the performance characterization of recently
reported crAssphage-like human-associated genetic markers (Stachler et al., 2017) identified
from a metagenome cross-assembly (Dutilh et al., 2014). EPA scientists and external partners
conducted studies demonstrating a uniform distribution of crAssphage-like sequences in
untreated wastewater across the contiguous United States (Korajkic et al., 2020a). Notable
efforts include evidence that crAssphage-like sequences are highly abundant in sewage occurring
at similar concentrations to HF183 genetic markers (Ahmed et al., 2018b), correlate with some
enteric viral pathogens (Crank et al., 2020), more closely mimic enteric virus decay rates
compared to bacterial-based FSI targets (Ahmed et al., 2021; Crank et al., 2019), and can be
recovered in multiple ambient surface water types (Petcharat et al., 2020). However, research
also suggests, like any other methodology, that standardized protocols are important for
generating consistent and reliable measurements within and between laboratories (Ahmed et al.,
2020).
Advances in Anthropogenic Chemical-Based FSI
Napier et al. (2018) used data from seven NEEAR study beaches impacted by WWTP discharges
for Enterococcus spp. measured by qPCR and 62 potential anthropogenic chemical markers. The
markers were assessed for potential associations with self-reported GI illness, diarrhea, and
respiratory illnesses. Across all beaches, no individual chemical marker or chemical category
showed strong or consistent association between Enterococcus spp. measured by qPCR and
health outcomes.
New Tools for Human-Associated FSI
EPA developed and published two methods (Method 1696.1 and 1697.1; U.S. EPA, 2022a,b)
and collaborated with NIST to develop a standard reference material (SRM 2917) that functions
with 11 qPCR FSI protocols, including human-associated HF183/BacR287 and HumM2
protocols (Kralj et al. 2021). These accomplishments represent two key advances for FSI science
since 2017 and are discussed in more detail in the methods section (Section III.F).
A series of studies were conducted to support the implementation of EPA Methods 1696.1 and
1697.1. Notable contributions included the demonstration of genetic target occurrence uniformity
in untreated wastewater across the contiguous United States (Korajkic et al., 2020a), a large-
scale field demonstration at 29 freshwater sites (Li et al., 2019), and use of QMRA modeling to
48
investigate links to public health risk in raw sewage (Schoen et al., 2020), as well as the use of a
new censored data analysis approach to rank recreational water sites based on human fecal
pollution levels (Cao et al., 2018a) to investigate potential links with rainfall (Shrestha et al.,
2020) and describe human fecal pollution source trends in waters routinely monitored for
bacterial and viral fecal indicators (Li et al., 2021).
2. Non-Human FSI Advances
Since 2017, there has been a growing interest in the use of non-human FSI methods to
characterize wildlife, pet, and agricultural fecal sources potentially present in recreational waters,
especially using qPCR-based methods. Non-human fecal source information can help prioritize
impaired sites, focus mitigation resources, and manage public health risk. Most research
activities focused on avian-, ruminant-, and swine-associated genetic markers.
Ruminant (i.e., cattle and deer), avian (i.e., gull and Canada goose), and domestic pets (i.e., dogs)
can contribute to elevated FIB levels in ambient surface waters (Ervin et al., 2013). In response,
EPA conducted a series of studies to explore the implementation of non-human FSI methods.
Notable contributions include the large-scale implementation of ruminant-, cattle-, avian-, swine-
and dog-associated qPCR methods at over 30 freshwater sites (Li et al., 2019, McKee et al.,
2020), development of SRM 2917 in collaboration with NIST, which functions with avian,
ruminant, dog, and swine FSI qPCR methods ( Sivaganesan et al., 2022; Willis et al., 2022), and
characterization of decay trends in fresh and marine waters (Korajkic et al., 2019b).
A variety of host-associated avian genetic markers have been investigated, including the general
avian marker GFD targeting Helicobacter spp. (Ahmed et al., 2016a; Symonds et al., 2017;
Vadde et al., 2019; Zhang et al., 2020), the poultry-associated markers LA35 and CL targeting
Brevibacterium sp. (Gibson et al., 2017), and the poultry-associated BifPL targeting
Bifidobacterium (Ballesté et al., 2018). Multiple gull-associated markers targeting Catellicoccus
marimammalium were also evaluated including CAT (Wu et al., 2017), Gull2 (Cloutier and
McLellan, 2017; Symonds et al., 2017), Gu112 (Thulsiraj et al., 2017), and qGull4 (Staley et al.,
2018a,b). Overall, findings suggest that these methods can be highly specific for avian targets,
however, most methods exhibit less than ideal sensitivity.
Multiple swine-associated markers were investigated, including Pig2Bac, targeting Bacteroidales
(Ballesté et al., 2018; Derx et al., 2021; He et al., 2016; Kongprajug et al., 2019; Liang et al.,
2021; Somnark et al., 2018; Vadde et al., 2019; Zhang et al., 2020), L.amylovorus, targeting
Lactobacillus amylovorus (He et al., 2016; Zhang et al., 2020), and PF, targeting Bacteroidales
(Symonds et al., 2017). In general, Pig2Bac exhibited a high specificity and sensitivity
suggesting that this methodology may be a reliable method for future recreational water quality
testing.
Ruminant FSI methods are generally grouped into cattle-associated and ruminant-associated
methods. Several studies explored the use of cattle-associated qPCR methods including
Bac3qPCR (Kongprajug et al., 2020) and CowM2/CowM3 (Xue et al., 2018; Xue et al., 2019).
Ruminant-associated methods can identify cattle as well as other animals that share the same
49
digestive physiology such as deer and elk, among others. Numerous ruminant-associated
methods were investigated in field studies targeting BacCow (Seidel et al., 2017; Symonds et al.,
2017); BifCW, targeting Bifidobacterium (Ballesté et al., 2018); and Bacteroidetes 16S
ribosomal RNA (rRNA) genes, including BoBac (Bushon et al., 2017) and BacR (Derx et al.,
2021; Kolm et al., 2019). Some ruminant sources such as cattle may under some circumstances
represent a similar health risk to humans (Soller et al., 2010a). In addition, cattle-associated
genetic marker shedding patterns are linked to animal diet (Shanks et al., 2011, Shanks et al.,
2010) and age (Shanks et al., 2014) resulting in potentially different occurrence expectations
from one monitoring site to another.
Feng and McLellan (2019) analyzed Bacteroides populations in sewage and non-human animal
hosts by targeting the V4–V5 and V6 16S rRNA gene hypervariable genome regions. Findings
suggest that the most abundant Bacteroides in untreated sewage were not human fecal associated
but sewage infrastructure derived. A qPCR assay was developed targeting an abundant sewage
infrastructure-associated Bacteroides. The authors assert that a qPCR assay that targets
organisms closely associated with the sewer pipe habitat can potentially provide source
information that is independent of the human fecal microbiome and could be useful for
identifying sewage pollution in water. Beyond microbial populations, Staley et al. (2018a)
evaluated environmental DNA (eDNA) utilizing the mitochondrial cytochrome oxidase I genetic
marker for DNA barcoding, which identifies DNA from various animal species. eDNA from cow
and chicken were only detected in creek and beach surface waters in Lake Ontario following an
extreme rain event. The authors note that caution should be used when interpreting eDNA results
because these sequences may not be of fecal origin. Advances in community-based FSI science
suggest that these approaches can complement FIB and quantitative FSI methods such as qPCR,
as well as identify new infrastructure-associated genetic targets. Additional research is warranted
to further characterize the utility of these methodologies.
3. Community-based FSI
Microbial community-based FSI methods are an active area of research for characterizing
recreational waters. Characterizing the occurrence of microbial groups utilizing sequencing for
identification can provide information about fecal sources and other microbial inputs. In addition
to genomics (nucleic acid sequencing), metabolomics (characterization of metabolites) has been
explored for characterizing recreational waters. For example, Beale et al. (2017) utilized
metagenomics sequencing (hypervariable V5 and V6 regions of the 16S rRNA gene) to report
the most abundant metabolically active bacteria in water samples from five sites along the
Brisbane River, Queensland, Australia. They also conducted gas chromatography mass
spectrophotometry analysis of water samples to characterize metabolites, such as sugars, fatty
acids, among others. Additional research is warranted to investigate the impacts of agricultural
practices, sewage treatment, and environmental endpoints using community-based approaches
(Beale et al., 2017). Another study utilized amplicon sequencing of the V3–V4 region of the
bacterial 16S rRNA gene to determine human sewage, avian, and horse fecal sources in the
Navesink River in New Jersey (Phelan et al., 2019).
50
4. Summary of New Information on FSI
• EPA Methods 1696.1 and 1697.1 are now available and facilitate the use of human-
associated qPCR technologies in recreational settings (see more details in Section III.F,
Analytical Methods).
• Field demonstrations have been published that use EPA Methods 1696.1 and 1697.1 and
establish their performance in wastewater and surface waters.
• Release of SRM 2917, a high-quality standard control material that functions with 11 FSI
qPCR methods indicative of human (HF183/BacR287, HumM2, CPQ_056, CPQ_064),
cattle (CowM2, CowM3), ruminant (Rum2Bac), canine (DG3, DG37), swine (Pig2Bac),
and avian (GFD) fecal sources, facilitates implementation of FSI qPCR methods.
• A new censored data analysis approach has been published that allows for quantitative
analyses of FSI qPCR data sets consisting of high proportions of non-detections and
detections below the limit of quantification.
• As the use of FSI tools for human fecal pollution characterization becomes a more
common practice, practitioners are recognizing that the contribution of non-human fecal
pollution sources plays an integral role in water quality management.
• Recent studies on microbial community-based FSI offer new strategies and tools to
characterize fecal pollution sources; however, additional studies are needed to evaluate
performance and demonstrate utility for recreational water quality monitoring.
• Understanding the decay of microorganisms associated with specific fecal pollution
sources is important for many FSI applications. Unlike culture-based FIB methods, FSI
genetic methods target nucleic acids instead of cells capable of growing and reproducing
on selective and differential media. This fundamental difference can contribute to
variable persistence behaviors in ambient surface water settings.
• Potential surrogates for enteric viral pathogens, such as enterococci qPCR, culturable
MSC and somatic coliphages, and human-associated genetic markers (HF183 and Hum
M2), have been included in health study evaluations and provide additional information
for assessing potential risk of illness (Griffith et al., 2016; Benjamin-Chung et al., 2017;
Ahmed et al., 2018a; Boehm et al., 2018; Boehm and Soller, 2020; Schoen et al., 2020;
Wade et al., 2022).
• Advances have been made in establishing potential links between public health risk and
human-associated FSI marker measurements using QMRA (see Section III.A, QMRA,
for details).

51
F. New Information on Analytical Methods for Recreational Waters
As discussed above, EPA has identified five types of indicators of risk in recreational waters:
FIB, coliphages, cyanotoxins, antimicrobial resistant bacteria and genes, and human and non-
human FSI genetic markers. Following is a discussion of the new research and scientific
information on the analytical methods for identifying and quantifying these potential risk
indicators.
The systematic literature search identified 80 articles (Figure 1) relevant to analytical methods
(Appendix B). Additional relevant articles were identified via an ad hoc process (U.S. EPA,
2012b, 2015c, 2020; Sivaganesan et al., 2018, 2022; Steele et al., 2018; Nshimyimana et al.,
2019; Lane et al., 2020a; Kralj et. al., 2021; Bone et al., 2022).
1. FIB
The 2012 RWQC protect human health from exposure to fecal contamination by detecting and
enumerating FIB. There are many analytical methods to enumerate E. coli and enterococci in
recreational waters. These methods either enumerate by growing the bacteria on selective and
differential media (culture methods) or by quantifying specific sequences of DNA from the FIB
(molecular methods). Currently, there are more than a dozen microbiological methods approved
for use under the CWA to monitor ambient water (see Code of Federal Regulations (CFR) 40
CFR 136.3.IH) and each method is a culture-based method.
Culture methods have been used to monitor water quality for decades. In May of 2021, EPA
approved a culture method for E. coli in ambient water that requires only 10 hours to provide a
result (May 19, 2021, Federal Register (FR) 86 FR 27235 and 40 CFR136.3.IH.3) and
https://www.epa.gov/cwa-methods). This incubation time is the shortest of the culture methods.
The length of time culture methods take to provide results means that people may have already
been exposed to fecally contaminated water, and water quality may have changed by the time the
result is available. Lag time between sampling and results may lead to unnecessary or delayed
action.
Since 2016, significant work has been conducted to update existing microbial methods, or add
methods with a faster turnaround time, applicable to the FIB E. coli and enterococci including
updated molecular-based qPCR methods and draft digital PCR (dPCR) methods. Molecular
methods can provide results faster than culture methods. EPA Method 1609.1 produces
enterococci results in about 4 hours, allowing monitoring in the morning and the ability to take
action to close a beach by noon, if necessary, and thereby reducing the number of swimmers
exposed to fecal contamination as indicated by elevated levels of FIB (EPA Method 1609.1).
This method is based on detecting specific sequences of enterococci DNA using qPCR
technology. Though faster than culture methods, molecular methods are generally more
expensive and technically more difficult to perform than culture methods. The target sequence
can be detected in viable but non-culturable, and metabolically inactive, cells and it is possible to
52
detect the target sequence associated with free DNA from cells that have lysed. Research in
molecular water monitoring methods is advancing rapidly and there are additional molecular
technologies under development or that are being deployed in the field in addition to qPCR.
PCR-Based Methods for FIB
EPA has made scientific advancements in qPCR methods, including the development and
publication of new methods, the development of standard control materials, the completion of
multiple laboratory performance studies, and studies characterizing linkages between FIB qPCR
and culture-based measurements in recreational waters.
A qPCR approach offers the advantage over culture methods of providing rapid results (2–6
hours versus 10–48 hours), allowing managers to make same-day decisions to protect
beachgoers. Since the initial public release of the Enterococcus qPCR method (EPA Method
1611; U.S. EPA, 2012b), EPA has made significant advances, including addressing potential
matrix interference issues in water types other than those studied at the NEEAR effluent-affected
beach sites culminating in the publication of an improved qPCR-based method for enterococci
enumeration (EPA Method 1609.1; U.S. EPA, 2015c) and the development of a draft E. coli
qPCR protocol (Haugland, et al., 2021). Despite the advantages of a qPCR water quality
monitoring approach, the use of these methods by state water quality programs has been slow
(Shrestha and Dorevitch, 2020).
Since 2017, EPA has published 18 manuscripts, developed standard control materials, and
released updated automated data analysis tools to help address research and implementation
priorities. Recent studies are summarized below.
Implementation of qPCR methods requires the generation of a standard curve to interpret results.
Quality and consistency of standard curves strongly influences the precision and reproducibility
of qPCR measurements. To address this need, EPA researchers developed a reference material
(IDTSMART-KAN_Std1_Xho), using droplet digital PCR (ddPCR) and advanced statistics, for
both the Enterococcus and E. coli qPCR methods in the Great Lakes region (Sivaganesan et al.,
2018). To help facilitate qPCR method implementation on a national scale, EPA researchers
recently collaborated with NIST to codevelop SRM 2917, which is expected to replace the initial
reference material in future recreational water analyses (Kralj et al., 2021; Sivaganesan et al.,
2022; Willis et al., 2022). EPA also reviewed qPCR methods for microbial water quality
monitoring (Nappier et al., 2019b).
To evaluate the performance of the E. coli qPCR method and establish custom data acceptance
metrics for future practitioners, EPA helped organize two ad hoc multi-laboratory studies. The
first effort consisted of 21 participating laboratories, primarily from the Great Lakes region using
a standardized protocol with the same set of reagents and consumables (Sivaganesan et al.,
2019). Findings suggested that a qPCR-based methodology can be successfully implemented
across a large network of laboratories and led to the development of custom data acceptance
metrics. In the second study, the feasibility of multiple laboratories to meet newly developed data
acceptance metrics was evaluated with most participants, both new and experienced,
53
demonstrating acceptable qPCR measurements (Aw et al., 2019). Given these findings, a
companion data analysis workbook was customized to incorporate new data acceptance metrics
and streamline calculations (Lane et al., 2020a).
Several studies were conducted to characterize linkages between FIB qPCR and culture-based
measurements in recreational waters. One effort investigated the temporal stability of enterococci
culture and qPCR paired measurements at freshwater recreational beaches over a 24-hour period
(Wymer et al., 2021). Findings reinforced the concept that same-day qPCR results are more
indicative of current water quality conditions compared to culture results obtained 24 hours after
sample collection. Another study generated nearly 7,000 E. coli culture and qPCR paired
measurements from 101 Lake Michigan recreational beach sites (Haugland et al., 2021). This
data set provided the State of Michigan with the scientific basis for the adoption of a statewide
Beach Notification Value for the E. coli qPCR method (Lane et al., 2020b) and could serve as a
blueprint for other states, territories, and authorities.
In 2020, EPA developed and evaluated a dPCR method using 38 recreational fresh and marine
water samples collected from 24 states across the country. The results were used to develop draft
quantitative quality control criteria that could be used to assess laboratory proficiency prior to
conducting a multi-laboratory validation study. The method incorporates elements from ORD’s
qPCR methods as well as a dPCR method being piloted in San Diego, California, recreational
waters (U.S. EPA, 2020). The results from the study indicate that the dPCR procedure is suitable
for multi-laboratory validation in both fresh and marine waters for E. coli and/or enterococci
(Bone et al., 2022).
2. Coliphage Methods
As discussed in Section III.B, coliphages are better indicators of some enteric viruses in
recreational waters compared to traditional methods measuring FIB. In 2018, EPA published
updated culture-based single-agar layer (SAL) methods to enumerate MSC and somatic
coliphages in recreational waters and wastewater (Method 1642) and in secondary wastewater
with no disinfection (Method 1643) (U.S. EPA, 2018ab). EPA Method 1642 includes
ultrafiltration to concentrate larger sample volumes (2 L), needed for recreational water
monitoring to address potential low coliphage densities in ambient waters downstream of human
fecal inputs. EPA conducted a multi-laboratory validation study (U.S. EPA, 2018c) of Method
1642 for MSC and somatic coliphages in fresh and marine recreational waters and secondary
wastewater effluents with disinfection. EPA Method 1643 reflects the results of a multi-
laboratory validation study of EPA Method 1602 for 100 mL secondary (no disinfection)
wastewater samples for MSC and somatic coliphages and is used for monitoring secondary (no
disinfection) wastewater matrices under the CWA. For both methods, the multi-laboratory
validation results allowed for the development of initial precision and recovery and ongoing
precision and recovery and matrix spike quality control acceptance criteria. Results from both
methods can be obtained in 16 to 24 hours.
EPA researchers published a study comparing performance of EPA Method 1642 to that of two
other alternative methods (modified SAL and direct membrane filtration [DMF]) capable of
54
processing large volumes of surface waters (1 L per coliphage type) (McMinn et al., 2018). All
methods were evaluated for the following metrics: percentage of samples with no detectable
coliphage, concentrations of coliphage, cost, and time required to process a single sample.
Method 1642 outperformed both alternative methods as it generated the least percentage of non-
detects (≤35%), the highest coliphage concentrations (0.30–1.65 log
10
PFU/L) and required the
least amount of time, while cost per sample was comparable to that of DMF.
An additional study evaluated performance of Method 1642 on freshwater and marine ambient
water samples collected nationwide and assessed the effect of spike titer protocols (i.e., SAL
versus double agar layer [DAL]), which are required for initial and ongoing precision recovery
tests (Korajkic et al., 2021). Study findings indicated that average percent recovery (53%–72%)
was comparable to previous reports, confirming a consistent performance of Method 1642 across
a wide variety of water types. Somatic coliphage recovery was not affected by water type, but
MSC results were significantly lower in marine waters compared to freshwater. Similarly,
somatic coliphage were not affected by spike titer protocol, but utilization of DAL significantly
underestimated MSC recovery compared to SAL.
Currently, there are E. coli host strains under evaluation that detect both MSC and somatic
coliphages. These host strains include CB-390 and C-3000 (Rose et al., 2004; Guzmán et al.,
2008; Agulló-Barceló et al., 2016; Korajkic et al., 2020a). A single host strain could simplify
Methods 1642 and 1643 by allowing the enumeration of both MSC and somatic coliphages in a
SAL assay.
Other New Studies for FIB Using PCR
Some notable studies compared EPA methods to other methods. For example, Byappanahalli et
al. (2018) compared culture methods (EPA Method 1603 for E. coli and Method 1600 for
enterococci) to qPCR-based methods (modified EPA Method 1611 qPCR for Enterococcus) at
coastal beaches and rivers. Overall, the culture-based and qPCR-based results for both indicators
were correlated in this study. While the qPCR methods would have resulted in fewer water
quality advisories or notifications, the authors concluded that the increased benefit of same-day
results provided by qPCR provides better protection overall.
Dorevitch et al., (2017) analyzed nine Chicago beaches by E. coli culture (Colilert method) and
Enterococcus spp. qPCR (EPA Methods 1609 and 1609.1). This study looked at the two pieces
of information available to beach managers on a given day: Enterococcus qPCR results of
samples collected that morning and E. coli culture results of samples collected the previous day.
The study found that if the same-day qPCR results had not been available, culture-based results
from water samples collected the day before would have triggered unnecessary, belated beach
advisories 24 percent of the time. And, on 4.7 percent of the beach-days, culture results would
have resulted in a “failure to act” (meaning, failure to trigger advisories) when advisories were
needed (based on exceedance of the same-day qPCR results). Furthermore, the 71.3 percent
concordance of beach actions between same-day qPCR and culture results from water samples
collected the day before can be explained entirely by chance. The authors concluded that there is

55
little scientific rationale for continued E. coli culture testing in Chicago beaches waters for the
purposes of public notification.
Digital PCR
Detection of enterococci in water samples was compared using ddPCR, qPCR (EPA Method
1609.1), and culture-based Enterolert (Crain et al., 2021). A comparison of enterococci qPCR
and ddPCR monitoring data demonstrated an Index of Agreement of 0.89 and a significant
Pearson’s correlation coefficient of r = 0.87 (p < 0.001). A subset of data used for the ddPCR and
qPCR comparison also included Enterolert data, which was evaluated using linear regression to
develop an intrinsic copy number equation that was then used to adjust the direct comparison
between ddPCR and Enterolert monitoring data. A ddPCR advisory threshold of 1,413 gene
copies per 100 mL was derived from this analysis and determined to be equivalent to the BAV of
104 MPN per 100 mL used in California (Crain et al., 2021). The ddPCR threshold value was
implemented as a BAV to monitor coastal beach water quality at San Diego County beaches in
2022 (County of San Diego Department of Environmental Health, http://www.sdbeachinfo.com).
Other FIB Method Technologies
Multiple methods for the real-time measurement of E. coli and enterococci have been evaluated,
including culture-based methods (chromogenic and fluorogenic substrates), cultivation-
independent detection methods (i.e., flow cytometry, biosensors), and microbial identification by
mass spectrometry (matrix-assisted laser desorption ionization time-of-flight, as reviewed by
Bonadonna et al., 2019). A critical review suggests that none of these technologies are currently
able to achieve real-time analysis. Additional research is warranted to develop real-time
approaches for monitoring FIB.
Kolm et al. (2017, 2019) developed helicase-dependent amplification (HDA) assays to detect
different fecal targets in surface water including fecal indicators. Only a heating block is required
for use of the assay, and the sample is applied directly to a test strip that detects and displays the
amplification products by marker-specific hybridization probes using a colorimetric reaction in 2
hours. The HDA assay yielded comparable results in sensitivity, specificity, and limits of
detection to qPCR. The authors suggest that this low-cost simple assay that requires minimal
training could be used instead of qPCR to yield comparable results (Kolm et al., 2019).
Loop-mediated isothermal amplification (LAMP) is a nucleic acid diagnostic method that is
potentially useful for testing of water samples in low-resource settings lacking sophisticated
laboratory equipment and highly trained personnel (Martzy et al., 2017; Nieuwkerk et al., 2020).
Oliveira et al. (2020) used LAMP to detect E. coli (uidA) in polluted lake and river samples.
Quantification of Enterococcus spp. and E. coli using an MPN-LAMP approach and qPCR
protocol were correlated, demonstrating that the MPN-LAMP method could be used for water
quality testing in areas that have limited resources (Fu et al., 2021).
3. Cyanotoxin Methods
As discussed in Section III.C, under certain environmental conditions cyanobacteria can rapidly
multiply and form HABs and some species may produce cyanotoxins. EPA has developed

56
several methods for the analysis of various cyanotoxins in ambient water, including Method 546:
Determination of Total Microcystins and Nodularins in Drinking and Ambient Waters (U.S.
EPA, 2016); Single Laboratory Validated Method for Determination of Cylindrospermopsin and
Anatoxin-a in Ambient Freshwaters (U.S. EPA, 2017b); and Single Laboratory Validated
Method for Determination of Microcystins and Nodularin in Ambient Freshwaters (U.S. EPA,
2017c).
Since 2017, EPA has published seven molecular technology-related peer-reviewed studies on
employing molecular tools for the detection of toxigenic cyanobacteria and cyanotoxins (Chen et
al., 2017; Zhu et al., 2019; Lu et al., 2019, 2020; Li et al., 2020; Wang et al., 2021; Tanvir et al.,
2021; Duan et al., 2022). EPA’s research found that there are high correlations between
microcystin and the mcy gene specific qPCR/reverse transcriptase (RT)-qPCR signals, while mcy
specific RT-qPCR can signal activities of toxin-producing genes and toxigenic species and
indicate the initiation of bloom-formation and toxin production and the subsequent subsidence of
toxin production. An early warning signal of one-week prior to microcystin production using
qPCR/RT-qPCR methods was identified (Lu et al., 2020). An additional study developed early
warning threshold values of mcy gene copy or transcript numbers corresponding to the
recommended swimming advisories for microcystins (Duan et al., 2022). The qPCR and RT-
qPCR threshold values provided here are specific for Harsha Lake, but they have been supported
by the data from lab culture experiments and current research collaborations with regions/states
(Duan et al., 2022).
In 2020, EPA developed qPCR detection methods for the detection of microcystin producers (Lu
et al., 2020), and filed a patent (U.S. Serial Number 16/142,319) on CyanoHAB qPCR assays.
This patent is for detecting genus-specific toxigenic cyanobacteria, providing a standardized
qPCR protocol, and the early warning process for cyanotoxin production. EPA is presently
developing qPCR detection methods for anatoxin, saxitoxin, and cylindrospermopsin producers
in lakes and rivers and qPCR methods for the detection of toxic/nontoxic benthic cyanobacteria.
4. AMR Bacteria
As discussed above in Section III.D, AMR bacteria can be produced by untreated wastewater and
many other sources and pose a risk to human health. No standard methods exist for measuring
AMR in recreational waters. Researchers are using a variety of different methods to study
environmental AMR with variations in the bacteria or genes targeted, the antibiotics tested, and
type of method (culture or molecular methods). This variation hinders cross comparison of data
sets. WHO has selected E. coli, specifically extended spectrum beta-lactamase (ESBL)-E. coli,
for global surveillance spanning human, animal, and environmental samples (Matheu et al.,
2017; WHO, 2018, 2019).
Human and Non-Human FSI
As described in Section III.C, the source of fecal contamination provides important information
needed to fully estimate the health risk and remediation options for any fecally contaminated
waters. Since 2017, EPA researchers have published 15 peer-reviewed studies on FSI, released

57
two nationally validated human-associated microbial source tracking qPCR methods, and
codeveloped a standard control material in collaboration with NIST.
5. Human FSI
EPA and numerous external partners collaborated to publish nationally validated EPA Methods
1696.1 and 1697.1 for human-associated qPCR assays HF183/BacR287 and HumM2,
respectively (U.S. EPA, 2022a,b). These methods include standardized protocols, custom data
acceptance metrics, a self-administered method proficiency testing protocol, and an automated
data analysis tool (https://www.epa.gov/cwa-methods/other-clean-water-act-test-methods-
microbiological).
EPA Method 1696.1 and 1697.1 implementation requires the use of a standard control material
to interpret results. In response, EPA designed a control material that functions with 11 qPCR
FSI protocols, including HF183/BacR287 and HumM2, and partnered with NIST to develop a
large-scale preparation for mass distribution on a national scale. The result is SRM 2617. This
material was subject to a rigorous set of certification experiments by NIST using dPCR (Kralj et
al., 2021); performance was confirmed by EPA using qPCR (Sivaganesan et al., 2022; Willis et
al., 2022) and it was made available to the public in April 2022.
Adaptation of dPCR for FSI
Since 2017, research focusing on the development and implementation of dPCR protocols for
FSI applications has increased (Pendergraph et al., 2021; Staley et al., 2018b; Steele et al., 2018;
Zimmer-Faust et al., 2021). dPCR can offer greater precision, superior trace level detection, and
is less prone to amplification inhibition compared to qPCR (Cao et al., 2016) . However, the
conversion of well-characterized qPCR protocols to a dPCR platform is not always
straightforward. For example, some researchers have observed a decrease in specificity with
dPCR protocols (Nshimyimana et al., 2019; Staley et al., 2018b), while others report the opposite
compared to a respective qPCR method (Nshimyimana et al., 2019). In addition, dPCR is ideal
for multiplex applications when the goal is to simultaneously amplify two or more targets in the
same reaction. Multiplexing can streamline laboratory testing workflow, reduce costs, and offer
increased certainty in fecal pollution characterization. Duplex dPCR-based FSI protocols have
been developed by combining the widely used HF183 16S rRNA human-associated genetic
marker with a crAssphage-like method (CPQ_056) (Ahmed et al., 2019) and an enterococci
assay (Cao et al., 2018b).
Emerging Technologies for FSI
Emerging methods are also being tested for utility in FSI. Three methods, a test strip (Kolm et
al., 2017, 2019), inversely coupled immunomagnetic separation/adenosine triphosphate (Inv-
IMS/ATP) (Zimmer-Faust et al., 2018), and LAMP (Jiang et al., 2018), are discussed in more
detail above. Briefly, a test strip was used to differentiate between spring/groundwater, surface
water, and wastewater exhibiting a high degree of agreement with paired qPCR measurements
(Kolm et al., 2017). A ruminant-associated version of the test strip correctly detected six
ruminant sources but did not react with human and 15 other non-ruminant sources (Kolm et al.,

58
2019). LAMP was used to identify human fecal contamination in water by detecting as few as
17 copies per mL of human-associated Bacteroides HF183 sequence with no false positives from
dog or cat feces (Jiang et al., 2018).
6. Summary of New Findings on Analytical Methods
• Analytical methods continued to advance over the previous 5 years, providing more
precise and rapid tools to assess the quality of recreational waters.
• Development of dPCR methods (e.g., Crain et al., 2021) offers the promise of rapid
results as compared to bacterial culture methods.
• Publication of EPA Methods 1642 and 1643 for culture-based coliphage methods allows
enumeration of MSC and somatic coliphage types in recreational waters.
• Development of FSI methods (1696.1 and 1697.1) and standard control material (SRM
2617) advances useful tools to mitigate and further inform the human health risk once
fecal contamination has been identified.
G. New Implementation Tools
This section discusses the development of implementation tools over the past 5 years, including
those for the 2012 RWQC and 2019 Cyanotoxins AWQC; advances in both predictive and
process modeling tools; and an update on the status of outreach and training efforts, as well as
information on BEACH Act grant funding.
1. Implementation of the 2012 RWQC
Site-Specific Alternative Recreational Criteria
As scientific advancements lead to new technologies for quantifying indicators of fecal
contamination in terms of improvements in rapidity, sensitivity, specificity, and method
performance for site-specific applications, states and authorized tribes could consider using these
methods to develop site-specific water quality criteria. Information demonstrating that these new
site-specific alternative criteria developed by states and authorized tribes are scientifically
defensible and protective of the recreational use is necessary to support new or revised water
quality standards.
To help states and authorized tribes in this effort, EPA recently developed and made public an
Excel spreadsheet tool (AltCalc Tool; U.S. EPA, 2021a,b,c) that complements EPA’s Site-
Specific Alternative Recreational Criteria Technical Support Materials for Alternative Indicators
and Methods (Alternative Indicators TSM; U.S. EPA, 2014a; available online at:
https://www.epa.gov/wqc/recreational-water-quality-criteria-and-methods). The Alternative
Indicators TSM outlines the scientific information needed before an alternative indicator/method
can replace the use of a recommended or approved method on a site-specific basis and describes
how to compare indicators after users have identified a candidate site and an alternative
indicator/method that has desirable attributes. The AltCalc Tool facilitates this comparison by

59
providing a user-friendly way to compute the index of agreement and Pearson's correlation
coefficient squared (R-squared) statistics for microbial water quality data sets from two different
microbial water quality methods. The index of agreement and R-squared values are used to
assess whether the alternative water quality method is appropriate for replacing an existing water
quality method using the steps in EPA’s Alternative Methods TSM (pages 16–19).
Several states have taken advantage of the information in the Alternative Indicators TSM
(available online at: https://www.epa.gov/wqc/recreational-water-quality-criteria-and-methods).
In Michigan, the state and EPA collaborated on a large-scale project to evaluate EPA Draft
Method C for enumerating E. coli with qPCR compared to previously approved EPA culture-
based methods (Best et al., 2018; Lane et al., 2020b; Haugland et al., 2021). Results from this
study were used to facilitate efforts to implement qPCR-based E. coli detection for rapid
recreational water quality monitoring in the State of Michigan. See the Outreach, Training, and
Grants section below for more information. In California, EPA Region 9 approved a pilot
program for the rapid ddPCR test to assess beach water quality to provide same-day notices in
San Diego County. (Wang et al., 2016a; California Water Boards Water Quality Coordinating
Committee, 2017; U.S. EPA, 2020; Crain et al., 2021). Other examples include Illinois
(Shrestha and Dorovitch, 2019), Hawaii (Fujioka et al., 2015; Esgaib Vaz Guimarães, 2017),
and Wisconsin (Hernandez, 2018).
Sanitary Surveys
In July 2020, EPA released its updated Sanitary Survey App for Marine and Fresh Waters with
enhanced features to help states, territories, and tribes gather sanitary survey data to identify
sources of fecal contamination and potential HAB events affecting coastal recreation waters. In
2021, EPA was able to allow increased access to the Sanitary Survey App for use by local
governments, participatory scientists, non-governmental organizations, and the public. The
surveys allow users to collect and share data on potential sources of fecal pollution and
information on potential HAB events in local surface waters, including designated recreational
waters. The data from the Sanitary Survey App can be exported for use in predictive models and
for sharing within or between groups.
The Sanitary Survey App is now a mobile web application and can be used on any device (i.e.,
phone, tablet, computer). Special features include photo storage, real-time geolocation, links to
websites such as the National Weather Service to access data, and free data storage. Additional
information is located on the Sanitary Surveys for Recreational Waters web page.
The EPA Sanitary Survey App is available for use by states, territories, tribes, local
governments, citizen science and environmental groups, and the public. EPA conducted
extensive outreach since the release of the app in July 2020. Stakeholders were notified of the
availability of the app by email, listserv announcements, social media posts, press releases, and
presentations at four national conferences, two state beach conferences, and several state and
tribal meetings. EPA also provided more than 15 virtual training webinars that included
demonstrations of the freshwater and marine routine surveys. EPA plans to continue its outreach
efforts to raise awareness and encourage use of the app by all stakeholders.

60
2. Cyanotoxins
EPA published materials to support implementation of the 2019 recommended recreational water
quality criteria and/or swimming advisories for microcystins and cylindrospermopsin. These
materials include the Final Technical Support Document: Implementing the 2019 Recommended
Human Health Recreational Ambient Water Quality Criteria or Swimming Advisories for
Microcystins and Cylindrospermopsin (U.S. EPA, 2021d), and guidance on EPA’s Monitoring
and Responding to Cyanobacteria and Cyanotoxins in Recreational Waters web page.
3
The
technical support document contains several questions and answers relating to the recommended
recreational water quality criteria and/or swimming advisories for microcystins and
cylindrospermopsin. The information in this document is primarily intended to support states,
authorized tribes, and territories interested in adopting the recommended criteria into their state
or tribal WQS or using the recommended values as the basis for swimming advisories and
related public notification purposes. The document provides recommendations on how to
monitor for cyanobacteria and two of their known cyanotoxins (microcystins and
cylindrospermopsin) in waterbodies and information on how to complete assessments, list
impaired or threatened waters, and develop TMDLs. EPA’s Monitoring and Responding to
Cyanobacteria and Cyanotoxins in Recreational Waters web page and materials found there
provide detailed information to support states and authorized tribes in their efforts to monitor and
respond to cyanobacterial blooms and cyanotoxins in recreational waters. EPA also developed
HABs infographics that can be used by federal agency, state, territorial, tribal, and local
government partners to help them educate recreators about the potential dangers of HABs in both
marine and freshwaters.
To aid in tracking the occurrence of HABs and their toxins in the nation’s freshwaters, EPA
developed the Tracking CyanoHABs Storymap. This user-friendly, interactive resource compiles
state-issued recreational waterbody and drinking water health advisories for HABs from across
the country. Each month, EPA collects available information on HAB advisories in drinking and
recreational freshwater systems that state health and/or environmental agencies publicly report
online. This information is displayed in two interactive maps that illustrate current advisories and
advisories since 2015.
In addition, EPA facilitated a series of workshops across the country to build relationships and
identify shared HABs-related goals, needs, and barriers among federal, state, and tribal CWA
and Safe Drinking Water Act (SDWA) programs. The workshops, held from 2015 to 2018 across
the country, provided information on the health outcomes associated with exposure to HABs,
strategies for the prevention and management of HABs in surface waters, and effective treatment
techniques for HABs-related cyanotoxins in drinking water. In addition, the workshops provided
a forum for state and tribal health and environmental agencies to exchange information on their
HABs programs, experiences, and needs. EPA also held a series of webinars specific to tribal
waters in 2021. Information on these and other webinars can be found on EPA Newsletter and
Collaboration and Outreach on HABs website.
3
https://www.epa.gov/cyanohabs/monitoring-and-responding-cyanobacteria-and-cyanotoxins-recreational-waters
61
3. Predictive Modeling Research
The 2012 RWQC encourage the use of predictive models to supplement water quality
monitoring to enable more timely beach notification decisions. Predictive modeling uses past
water quality data and current observed hydrometeorological measurements to estimate water
quality at a given time. Accurate and reliable predictive models can improve water quality
management.
EPA maintains an active research program focusing on priority research areas identified in the
2017 Five-Year Review of the 2012 Recreational Water Quality Criteria (U.S. EPA, 2018d)
including development of predictive models and technology transfer to stakeholders. EPA has
organized and participated in multiple workshops and training sessions on the use of Virtual
Beach across the United States, including in-person workshops in Ohio, South Carolina, and
New England. Additionally, EPA has held over a dozen online training sessions and webinars in
the last 5 years. Together, these online and in-person training opportunities reached
approximately four hundred participants.
4. Process Modeling Research
Process or deterministic models are valuable tools for the simulation of contaminant sources,
concentrations, transport, and exploration of mitigation scenarios in recreational waters. Single
models and integrated environmental modeling (IEM) are two types of approaches that can be
applied when simulating the fate and transport of microbial contaminants at the watershed scale.
IEM consists of a construct of modules and/or models that simulate individual processes where
data is exchanged seamlessly to produce an output that targets the problem of interest (Kim et al.,
2018; Whelan et al., 2018). Over the past 5 years, EPA has published multiple QMRA software
modules and tutorials. The workflow was developed to estimate risk associated with inputs of
microbial contaminants from a variety of land uses at the watershed scale (Whelan et al.,
2017a,b). Data collection has been automated for watershed analysis, allowing Hydrologic Unit
Code (HUC)-8 or Pour Point analyses (Whelan et al., 2015). The IEM automatically delineates
watersheds and sub-watersheds and the system accounts for minimum stream lengths, minimum
subwatershed sizes, and minimum land use type sizes. Local data files contain a Microbial
Properties Database that provides physico-microbial properties relevant to release, fate,
transport, exposure, and effects modeling (Whelan et al., 2017c). Precipitation data can be
entered from either monitored gauges or radar/remote sensing precipitation sources. The system
automatically estimates microbial loads on all sub-watersheds based on the number of animals
reported per land use patterns by calculating manure-based source terms and processes point and
non-point sources of fecal microbial contaminants. The information is used to prepopulate a
watershed model, the Hydrological Simulation Program-Fortran (HSPF), with microbial decay
and transport constants (Kim et al., 2017). The system is compatible with both graphical and
tabular viewers in HSPF and Better Assessment Science Integrating Point and Nonpoint Sources
(BASINS) and allows users to investigate water flows and microbial concentration time series at
multiple locations throughout the watershed. Finally, the system is designed to be linked to a
pathogen risk model, the microbial risk assessment-interface tool (MRA-IT). Supporting

62
information for the use, training, and application of the QMRA software can be found in the
Quantitative Microbial Risk Assessment Tutorial – Primer.
Modeling the fate and transport of MSC and somatic coliphages was another example of an IEM
application where information produced in laboratory studies contributed with critical data inputs
for the development of a hydrodynamic coliphage model in the Great Lakes. EPA evaluated the
light-induced inactivation of MSC and somatic coliphages using both laboratory and wastewater
treatment plant-isolated strains (Zepp et al., 2018; Nelson et al., 2018). Solar simulators were
used to develop biological weighing functions and ultraviolet inactivation constants that, in turn,
were used to model the inactivation of coliphages over a range of conditions in aquatic
environments. A three-dimensional model of coliphage inactivation and transport was developed
for Washington Park Beach in Lake Michigan using the organism-specific action spectra
developed in the laboratory studies (Safaie et al., 2020). Results indicated that coliphage models
outperformed a previously tested E. coli model suggesting that coliphages may serve as
conservative indicators of microbiological contamination.
EPA published a study that evaluated the importance of sediment resuspension in FIB loading
calculations in watersheds of various sizes (Bradshaw et al., 2021). While suspended transport
was the dominant mechanism for FIB movement regardless of stream size, FIB loads from
overland and bedload transport should be considered when performing modeling and microbial
assessments at the watershed scale.
EPA also modeled FIB using a high-frequency, multi-year microbial data set with the Soil and
Water Assessment Tool (SWAT) (Sowah et al., 2020). SWAT was applied to understand the
sources and parameters impacting microbial water quality in a mixed-used watershed in
southeastern United States. A key scientific advancement of this work was the development of a
criterion to evaluate the performance of bacterial models in SWAT.
5. Outreach and Grants
EPA Stakeholder Technology Transfer
An essential step in the successful implementation of recreational water quality, laboratory-based
methods is technology transfer. Molecular and newly developed coliphage culture methods can
be technically demanding, requiring specialized equipment, detailed procedures, and specialized
training making it challenging to implement on a national scale. To help address this need, EPA
ensures public access to standardized protocols and companion implementation tools, as well as
provides technical support and hands-on training opportunities. Since 2017, EPA has provided
support to 56 different federal, state, territory, or local laboratories, as well as to more than 20
academic research groups. These activities help facilitate the proper use of newly developed
recreational water quality laboratory methods.
6. BEACH Act Grant Funding
Under the BEACH Act, EPA awards grants to eligible state, territorial and tribal applicants to
help them and their local government partners monitor water quality at coastal and Great Lakes
beaches. When bacteria levels are too high for safe swimming, these agencies notify the public

63
by posting beach warnings or closing the beach. Since 2001, state, and local governments,
territories and tribes have been awarded nearly $206 million in EPA BEACH Act grants to
monitor beaches for FIB, maintain and operate public notification systems, and report results of
monitoring and notification activities to EPA. While beach grant funding totals allocated by
Congress were $9.2 million in 2019 and 2020, $10.1 million has been awarded in 2022. See
https://www.epa.gov/beach-tech/beach-grants for detailed information on BEACH Act
grant awards.
64
IV. Summary of Major Findings and Priorities for Further Work
A. Summary of Major Findings
1. Summary of Findings Identified in New Epidemiological Studies
• Recent publications have advanced understanding and further confirm that
epidemiological and outbreak data demonstrate that young children (i.e., 10 years old and
under) have a higher risk of illness compared to adults when exposed to fecal
contamination in recreational waters.
• Young children’s (i.e., 10 years old and under) behavior, such as increased time spent in
water, length of time spent playing in the sand, and more vigorous activity, can increase
their potential exposure to fecal contamination and is associated with greater health risk
compared to other age groups, especially those 18 and over.
• Children’s recreational exposure is greater than adolescents and adults because they
ingest more total volume of water, independent of body weight, than do the other age
groups.
• Studies demonstrate that human fecal contamination can pose higher risks of illness in
recreators relative to some non-human fecal sources.
• While FIB can be a useful indicator where raw and poorly treated sewage dominates
water quality, multiple studies discuss the inconsistent performance of culture-
enumerated FIB related to health and point to other indicators to better represent the
potential risk of illness (e.g., Enterococcus measured by qPCR).
• Waters receiving human fecal contamination can have viral pathogens present at health-
relevant levels and yet also be below recommended water quality levels for culturable
FIB.
• The health risk of recreating during or following wet weather compared to dry weather
can be different due to changes in fecal loading dynamics to a waterbody.
• Recent QMRA studies corroborate past findings that waters affected by human fecal
inputs can pose higher health risks compared to non-human fecal sources.
• Several QMRAs utilized norovirus as a primary reference pathogen to characterize risk
from human enteric viruses in waters affected by human fecal pollution.
• Across QMRA studies, the age of fecal contamination was identified as an important
factor affecting risk estimates and calculation of RBT values for alternative indicators.
65
• Outbreak surveillance in the United States and elsewhere continues to indicate the
importance of human enteric viruses as a leading etiologic agent of illness in ambient
recreational waters, even where low densities of FIB occur.
• Multiple outbreak investigations demonstrate the increased health burden children
experience when exposed to fecal pollution in ambient waters.
2. Summary of New Information on Coliphages
• Both somatic and MSC consistently occur in raw wastewater across the United States,
with somatic coliphages more numerous than MSC, and thus have potential to be used as
indicators of fecal contamination in waters affected by sewage.
• Viral surrogates, such as coliphages, may perform as better surrogates of the fate of
human enteric viruses compared to culture-enumerated FIB during wastewater treatment.
• Coliphages can exhibit a higher decay rate relative to viral pathogens in ambient waters.
• When human fecal sources are suspected based on location but not water quality
measures, MSC had a stronger relationship to illness. Knowledge of the fecal sources
affecting a particular waterbody, especially human contamination sources, can improve
interpretation of monitoring data.
3. Summary of New Information on Cyanotoxins
• New data were identified for saxitoxins and work is underway at EPA to evaluate data to
support development of an HESD.
• For cylindrospermopsin and microcystins, new studies reviewed did not provide new
information to support derivation of a revised RfDs.
• Limited new toxicity information is available for anatoxin-a and available literature
reviewed does not support development of an HESD at this time.
• No new toxicity information was identified on nodularins and available literature
reviewed does not support development of an HESD at this time.
4. Summary of New Information on AMR
• An increased understanding of horizontal gene transfer mechanisms and AMR selection
can be used to improve monitoring for AMR in ambient waters.
• A combination of techniques (including both molecular- and culture-based) are necessary
to fully understand AMR in surface waters.
66
5. Summary of New Information on FSI
• Release of SRM 2917, a high-quality standard control material that functions with 11 FSI
qPCR methods indicative of human (HF183/BacR287, HumM2, CPQ_056, CPQ_064),
cattle (CowM2, CowM3), ruminant (Rum2Bac), canine (DG3, DG37), swine (Pig2Bac),
and avian (GFD) fecal sources, facilitates implementation of FSI qPCR methods.
• Large-scale field demonstrations were conducted using new human-associated EPA
Methods 1696.1 and 1697.1 to establish performance in wastewater and surface waters.
• As the use of FSI tools for human fecal pollution characterization becomes a more
common practice, practitioners are recognizing that the contribution of non-human fecal
pollution sources plays an integral role in water quality management.
• Understanding the decay of microorganisms associated with specific fecal pollution
sources is important for many FSI applications.
6. Summary of New Findings on Analytical Methods
• Analytical methods continued to advance over the previous 5 years, providing more
precise and rapid tools to assess the quality of recreational waters.
• Development of dPCR methods (e.g., Crain et a., 2021) offers the promise of rapid
results as compared to bacterial culture methods and also easier implementation as
compared to qPCR methods.
• Publication of EPA Methods 1642 and 1643 for culture-based coliphage methods allows
identification of human health risk from viral pathogens.
• Development of FSI methods (1696.1 and 1697.1) and standard control materials (SRM
2617) advances useful tools to mitigate and further inform the human health risk once
fecal contamination has been identified.
• The exposure-response relationship was more consistently monotonic for Enterococcus
measured using qPCR methods versus culture methods across different analyses, even
when restricted to beaches without a known point source of pollution.
B. Assessment of the Need to Revise the 2012 RWQC
Based on a review of the latest scientific knowledge EPA has determined that the 2012 RWQC
need to be revised and that there are several additional implementation tools that EPA can make
available to manage recreational waters.
The available science demonstrates an increased health risk for children compared to adults
when recreationally exposed to fecal contamination. Since 2017, EPA has published nationally
67
validated protocols for qPCR methods and codeveloped a standard reference material for
national use that supports the use of qPCR technologies in recreational settings.
Based on the findings in this review:
1. EPA plans to develop additional criteria recommendations for qPCR-enumerated
enterococci protective of children, which would be protective of all recreators.
2. EPA plans to continue to develop recommendations for coliphages to help address
potential risks from human enteric viruses in ambient waters.
3. Finally, EPA plans to explore how best to use human fecal source identifiers, such as
HF183, for water quality management. Being able to demonstrate that a waterbody has
been impacted by human fecal contamination will enable risk managers to use the
appropriate tools to evaluate and manage risks for waters impacted by human sources.
C. Priorities for Further Work
Health Studies
The analysis of risks to children during recreation is a significant driver for future research.
Further analysis of Enterococcus spp. qPCR data for children is needed for consideration in
criteria development. An evaluation of how QMRA can be used to address risk to children from
swimming exposure is needed as is guidance on QMRA. Additional health studies would be
valuable to assess the risks to children during recreation. While studies have shown that children
have greater exposure to recreational water, which could explain greater illness rates, it is
important to conduct studies to understand whether the children’s developing immune system is
a biological factor that increases their susceptibility to illness.
Coliphages
Based on the findings of studies conducted to date, future research efforts should focus on
improving and refining virus detection methodologies in estuarine and marine waters, as well as
on determining coliphage occurrence trends in these water types and across seasons through
measurements. It is also important to further understand the treatment efficacies of different
combinations of traditional and advanced treatment processes by comparing levels of multiple
enteric viruses and coliphages. Studies to investigate the relationship between viral surrogates
and human fecal pollution may be useful to support the development of risk-based standards to
protect public health from pathogenic viruses in ambient waters. Finally, research is needed to
understand the decay patterns of coliphages and viral pathogen in recreational waters and
wastewater.
Cyanotoxins
EPA has reviewed the available toxicity data for saxitoxin and is preparing an HESD to
determine whether there is adequate toxicity data to derive an oral RfD.
There is a need to synthesize the information on human health risk from exposure to cyanotoxins
and to conduct additional studies to support health assessments for individual and groups of

68
cyanotoxins. EPA’s ORD is working with other EPA offices and regions to develop a systematic
literature review and evidence map to characterize the human health effects of exposure
cyanotoxins from any route of exposure. The scope of the review includes available
epidemiological and toxicological literature for anatoxins, BMAA, cylindrospermopsin,
microcystins, nodularins, and saxitoxins.
Future research needs include research on the effects of cyanotoxins on both aquatic life and
human health. There is a need for research on the toxicity of HABs on aquatic life and aquatic-
dependent wildlife and the development and validation of analytical methods for cyanotoxins in
diverse sample matrices in order to evaluate cyanotoxins accumulation in aquatic species and the
food web. Research needs on the toxicity of HABs to humans include short-term, sub-chronic,
and chronic toxicity and epidemiology studies of anatoxin-a and derivatives and nodularins,
research to evaluate risk associated with dermal exposure to algal toxins, and research to
evaluate risk associated with inhalation of algal toxins, including long-term exposure. In
addition, a systematic literature review of the impact of climate change on HAB formation is
needed.
Antimicrobial Resistance
Future research needs include evaluation of best management practices for AMR attenuation,
characterization of AMR occurrence in human stool, characterization of environmental
reservoirs of AMR, characterization of AMR targets at subtropical beaches, and application of
QMRA to investigate the risk of recreational exposure to AMR in surface waters. Specific
research needs include data on exposure pathways and dose-response relationships for various
health outcomes. More data are needed on the variability and load contributions of potential
sources (i.e., WWTPs, animal feeding operations [AFOs], CAFOs, medical/health care facilities,
wildlife, slaughterhouse waste streams, landfills, septic tanks, cesspools, and cemeteries) of
AMR bacteria and ARGs in surface waters to better understand their behavior in the environment
and across wastewater treatment processes. Further, active surveillance in surface waters of
clinically relevant AMR bacteria strains is needed to provide exposure information for protection
of human health. Standardized methods for culturable and gene targets are needed to study,
detect, and classify AMR bacteria in surface waters.
Human and Non-Human Fecal Source Identification
Further advances in avian FSI applications are needed, especially the development of methods
with improved host distribution. Further research is needed to develop a toolbox approach for
ruminant fecal source characterization in recreational waters. Additional research is warranted to
further characterize the utility of these methodologies.
Considering findings from EPA and others, monitoring of crAssphage-like human-associated
genetic markers could offer substantial advantages for future water quality management and
warrant further research.
69
Further advances in avian FSI applications are needed, especially the development of methods
with improved host distribution.
Further research is needed to develop a toolbox approach for ruminant fecal source
characterization in recreational waters.
The EPA publication of nationally validated protocols and the public release of SRM 2917
combined with key scientific studies provides necessary tools and information for the use of
human-associated qPCR technologies in recreational settings. Future research needs to support
national implementation of EPA Methods 1696.1 and 1697.1 include interlaboratory
performance assessment of SRM 2917, method performance assessment in subtropical marine
waters, further science to support data interpretation, and adaptation to a digital PCR platform.
Finally, additional efforts are needed toward the development of human-associated virus-based
microbial source tracking methods to complement bacterial HF183/BacR287 (U.S. EPA, 2022a)
and HumM2 (U.S. EPA, 2022b) protocols.
Non-Human Fecal Sources Technical Support Materials
EPA is developing a TSM to support derivation of site-specific alternative water quality criteria
for ambient recreational waters where the predominant contamination is from non-human fecal
sources. The TSM describes the use of QMRA to derive site-specific alternative water quality
criteria for ambient recreational waters. Specifically, this TSM provides information on how to
use a risk-based approach to develop water quality criteria that are equally health protective as
EPA’s 2012 RWQC for waterbodies predominantly affected by non-human fecal contamination.
The QMRA framework discussed in the TSM is based on the current science, EPA’s FIB
recommendations for enterococci or E. coli, and associated enumeration methods (EPA Methods
1600, 1603; U.S. EPA, 2009b, 2014b). Additional information includes characterization of the
exposure scenario, selection the QMRA parameters, information on dose-response, information
on risk characterization, descriptions of the reference pathogens, and examples of the types of
supporting evidence that are useful.
Analytical Methods
Several scientific advances in methods have been made in the past 5 years. The use of dPCR
platform should continue to be explored in the future. A large-scale performance assessment of
FIB qPCR methods in marine waters is needed.
Implementation Tools
Predictive Modeling
Future research needs for predictive modeling approaches to improve recreational water quality
management include the development of a web-based Virtual Beach solution providing an easy-
to-use interface with more powerful analytics and the completion of current Great Lakes and
Boston Harbor predictive modeling efforts. There is also a need to assess predictive model
performance in subtropical recreational marine waters.
70
Process Modeling
Multiple research activities are needed to augment the development of site-specific criteria using
process models; these include improving modeling of human versus non-human sources at the
watershed scale. It will also be important to enhance the understanding of watershed connectivity
as well as potential impacts on marine recreational coastal environments from mixed-used
watersheds. Process modeling can also be improved by implementing novel tools for calibration
of bacterial models such as global sensitivity analysis. IEM future research should also address
developing a link between process modeling approaches and the new EPA Sanitary Survey App.
Information gathered by the app could be automatically imported into watershed microbial
models, thus speeding up the process of parameterization and model calibration.
71
V. References
Abia, A.L.K., Ubomba-Jaswa, E., Genthe, B., Momba, M.N.B. 2016. Quantitative microbial risk
assessment (QMRA) shows increased public health risk associated with exposure to river
water under conditions of riverbed sediment resuspension. Science of the Total Environment,
566: 1143-1151.
Abi-Khalil, C., Finkelstein, D.S.; Conejero, G., Du Bois, J., Destoumieux-Garzon, D., Rolland,
J.L. 2017. The paralytic shellfish toxin, saxitoxin, enters the cytoplasm and induces apoptosis
of oyster immune cells through a caspase-dependent pathway. Aquatic Toxicology. 190: 133-
141.
Agulló-Barceló, M., Galofré, B., Sala, L., García-Aljaro, C., Lucena, F., Jofre, J. 2016.
Simultaneous detection of somatic and F-specific coliphages in different settings by
Escherichia coli strain CB390. FEMS Microbiology Letters, 363(17):fnw180.
Ahmed, W., Harwood, V.J., Nguyen, K., Young, S., Hamilton, K., Toze, S. 2016a. Utility of
Helicobacter spp. associated GFD markers for detecting avian fecal pollution in natural
waters of two continents. Water Research, 88: 613-622.
Ahmed, W., Hughes, B., Harwood, V.J. 2016b. Current status of marker genes of Bacteroides
and related taxa for identifying sewage pollution in environmental waters. Water, 8(6): 231.
Ahmed, W., Hamilton, K.A., Lobos, A., Hughes, B., Staley, C., Sadowsky, M.J., Harwood, V.J.
2018a. Quantitative microbial risk assessment of microbial source tracking markers in
recreational water contaminated with fresh untreated and secondary treated sewage.
Environmental International, 117: 243-249.
Ahmed, W., Lobos, A., Senkbeil, J., Peraud, J., Gallard, J., Harwood, V.J. 2018b. Evaluation of
the novel crAssphage marker for sewage pollution tracking in storm drain outfalls in Tampa,
Florida. Water Research, 131: 142-150.
Ahmed, W., Payyappat, S., Cassidy, M., Besley, C. 2019. A duplex PCR assay for the
simultaneous quantification of Bacteroides HF183 and crAssphage CPQ_056 marker genes
in untreated sewage and stormwater. Environmental International, 126: 252-259.
Ahmed, W., Payyappat, S., Cassidy, M., Harrison, N., Besley, C. 2020. Interlaboratory accuracy
and precision among results of three sewage-associated marker genes in urban environmental
estuarine waters and freshwater streams. Science of the Total Environment, 741: 140071.
Ahmed, A., Toze, S., Veal, C., Fisher, P., Zhang, Q., Zhu, Z., Staley, C., Sadowsky, M.J. 2021.
Comparative decay of culturable faecal indicator bacteria, microbial source tracking genes,
and enteric pathogens in laboratory microcosms that mimic a sub-tropical environment.
Science of the Total Environment, 751: 141475.

72
Arnold, B.F., Schiff, K.C., Ercumen, A., Benjamin-Chung, J., Steele, J.A., Griffith, J.F.,
Steinberg, S.J., Smith, P., McGee, C.D., Wilson, R., Nelsen, C., Weisberg, S.B., Colford,
J.M., Jr. 2017. Acute illness among surfers after exposure to seawater in dry- and wet-
weather conditions. American Journal of Epidemiology, 186: 866-875.
Arnold, B.F., Wade, T.J., Benjamin-Chung, J.S., Schiff, K.C., Griffith, J.F., Dufour, A.P.,
Weisberg, S.B., Colford, J.M., Jr. 2016. Acute gastroenteritis and recreational water: Highest
burden among young U.S. children. American Journal of Public Health, 106: 1690-1697.
Augustine, S.A.J., Eason, T.N., Simmons, K.J., Griffin, S.M., Curioso, C.L., Ramudit, M.K.D.,
Sams, E.A., Oshima, K.H., Dufour, A., Wade, T.J. 2020. Rapid salivary IgG antibody
screening for hepatitis A. Journal of Clinical Microbiology, 58(10): e00358-00320.
Aw, T.G., Sivaganesan, M., Briggs, S., Dreelin, E., Aslan, A., Dorevitch, S., Shrestha, A., Isaacs,
N., Kinzelman, J., Kleinheinz, G., Noble, R., Rediske, R., Scull, B., Rosenberg, S.,
Weberman, B., Sivy, T., Southwell, B., Siefring, S., Oshima, K., Haugland, R. 2019.
Evaluation of multiple laboratory performance and variability in analysis of recreational
freshwaters by a rapid E. coli qPCR method (Draft Method C). Water Research, 156: 465-
474.
Ballesté, E., García-Aljaro, C., Blanch, A.R. 2018. Assessment of the decay rates of microbial
source tracking molecular markers and faecal indicator bacteria from different sources.
Journal of Applied Microbiology, 125(6): 1938-1949.
Beale, D.J., Karpe, A.V., Ahmed, W., Cook, S., Morrison, P.D., Staley, C., Sadowsky, M.J.,
Palombo, E.A. 2017. A community multi-omics approach towards the assessment of surface
water quality in an urban river system. International Journal of Environmental Research and
Public Health, 14(3): 303.
Benjamin-Chung, J., Arnold, B.F., Wade, T.J., Schiff, K., Griffith, J.F., Dufour, A.P., Weisberg,
S.B., Colford, J.M., Jr. 2017. Coliphages and gastrointestinal illness in recreational waters:
Pooled analysis of six coastal beach cohorts. Epidemiology, 28(5): 644-652.
Best, S, Rediske, R, Lane, M. 2018. A comparison study of Colilert and qPCR methods at Pere
Marquette Beach, Muskegon County, MI. Student Summer Scholars Manuscripts. 203.
http://scholarworks.gvsu.edu/sss/203
Biré, R., Bertin, R., Dom, I., Hort, V., Schmitt, C., Diogene, J., Lemée, R., De Haro, L., Nicolas,
M. 2020. First Evidence of the Presence of Anatoxin-A in Sea Figs Associated with Human
Food Poisonings in France. Marine Drugs, 18(6): 285.
Blatchley, E.R., Gong, W.L., Alleman, J.E., Rose, J.B., Huffman, D.E., Otaki, M., Lisle, J.T.
2007. Effects of wastewater disinfection on waterborne bacteria and viruses. Water
Environmental Research, 79: 81-92.
73
Boehm, A.B., Van De Werfhorst, L.C., Griffith, J.F., Holden, P.A., Jay, J.A., Shanks, O.C.,
Wang, D., Weisberg, S.B. 2013. Performance of forty-one microbial source tracking
methods: A twenty-seven lab evaluation study. Water Research, 47: 6812-6828.
Boehm, A.B. 2019. Risk-based water quality thresholds for coliphages in surface waters: effect
of temperature and contamination aging. Environmental Science Process & Impacts, 21(12):
2031-2041.
Boehm, A.B., Soller, J.A., Shanks, O.C. 2015. Human-associated fecal quantitative polymerase
chain reaction measurements and simulated risk of gastrointestinal illness in recreational
waters contaminated with raw sewage. Environmental Science & Technology Letters, 2(10):
270-275.
Boehm, A.B., Graham, K.E., Jennings, W.C. 2018. Can we swim yet? Systematic review, meta-
analysis, and risk assessment of aging sewage in surface waters. Environmental Science &
Technology, 52: 9634-9645.
Boehm, A.B., Silverman, A.I, Schriewer, A., Goodwin, K. 2019. Systematic review and meta-
analysis of decay rates of waterborne mammalian viruses and coliphages in surface waters.
Water Research, 164: 114898.
Boehm, A.B., Soller, J.A. 2020. Refined ambient water quality thresholds for human-associated
fecal indicator HF183 for recreational waters with and without co-occurring gull fecal
contamination. Microbial Risk Analysis, 16.
Bogomolni, A.L., Bass, A.L., Fire, S., Jasperse, L., Levin, M., Nielsen, O., Waring, G., De
Guise, S. 2016. Saxitoxin increases phocine distemper virus replication upon in-vitro
infection in harbor seal immune cells. Harmful Algae, 51: 89-96.
Bonadonna, L., Briancesco, R., La Rosa, G. 2019. Innovative analytical methods for monitoring
microbiological and virological water quality. Microchemical Journal, 150: 104160.
Bone, T., Chambers-Velarde, Y., Ghei R., Miller, K., Teachey, M. 2022. Single-Laboratory
Validation of Enterococci and E. coli by Droplet Digital Polymerase Chain Reaction in
Ambient Waters Used for Recreation. 2022 American Society for Microbiology conference
in Washington D.C.
Bortagaray, V., Girardi, V., Pou, S., Lizasoain, A., Tort, L.F.L., Spilki, F.R., Colina, R., Victoria,
M. 2020. Detection, quantification, and microbial risk assessment of group A rotavirus in
rivers from Uruguay. Food and Environmental Virology, 12(2): 89-98.
Bradshaw, K., Snyder, B. Spidle, D., Sidle, R., Sullivan, K., Molina, M. 2021. Sediment and
fecal indicator bacteria loading in a mixed land use watershed: Contributions from suspended
sediment and bedload transport. Journal of Environmental Quality, 50(3): 598-611.

74
Brown, K.I., Graham, K.E., Boehm, A.B. 2017. Risk-based threshold of gull-associated fecal
marker concentrations for recreational water. Environmental Science & Technology Letters,
4(2): 44-48.
Bushon, R.N., Brady, A.M., Christensen, E.D., Stelzer, E.A. 2017. Multi-year microbial source
tracking study characterizing fecal contamination in an urban watershed. Water Environment
Research, 89: 127-143.
Byappanahalli, M.N., Nevers, M.B., Shively, D.A., Spoljaric, A., Otto, C. 2018. Real-time water
quality monitoring at a Great Lakes National Park. Journal of Environmental Quality, 47:
1086-1093.
California Water Boards Water Quality Coordinating Committee (WQCC). 2017. Development
of an Early Warning System for Cross-Border Sewage Flows in the Tijuana River Valley.
Background Information for 2017 WQCC Meeting October 25–26, 2017. Sacramento, CA:
CalEPA.
California Water Boards. 2022. Water boards structure.
https://www.waterboards.ca.gov/santaana/about_us/water_boards_structure/regional_board_
overview.html, Last Accessed: 03/24/2023.
Cao, Y., Raith, M.R., Griffith, J.F. 2016. A duplex digital PCR assay for simultaneous
quantification of the Enterococcus spp. And the human fecal-associated HF183 marker in
waters. Journal of Visualized Experiments, 109: 53611.
Cao, Y., Sivaganesan, M., Kelty, C.A., Wang, D., Boehm, A.B., Griffith, J.F., Weisberg, S.B.,
Shanks, O.C. 2018a. A human fecal contamination score for ranking recreational sites using
the HF183/BacR287 quantitative real-time PCR method. Water Research, 128: 148-156.
Cao, Y., Raith, M.R., Griffith, J.F. 2018b. Testing of general and human-associated fecal
contamination in waters. Methods Molecular Biology, 1768: 127-140.
Chen, Y., Xu, J., Li, Y., Han, X. 2011. Decline of sperm quality and testicular function in male
mice during chronic low-dose exposure to microcystin-LR. Reproductive Toxicology, 31(4):
551-557.
Chen, K., Allen, J., Lu, J.R. 2017. Community Structures of Phytoplankton with Emphasis on
Toxic Cyanobacteria in an Ohio Inland Lake during Bloom Season. Journal of Water
Resource and Protection, 9(11): 1-29.
Chernoff, N., Hill, D.J., Chorus, I., Diggs, D.L., Huang, H., King, D., Lang, J.R., Le, T.T.,
Schmid, J.E., Travlos, G.S., Whitley, E.M., Wilson, R.E., Wood, C.R. 2018.
Cylindrospermopsin toxicity in mice following a 90-d oral exposure. Journal of Toxicology
and Environmental Health Part A, 81(13): 549-566.
75
Chernoff, N., Hill, D., Lang, J., Schmid, J., Farthing, A., Huang, H. 2021. Dose-Response Study
of Microcystin Congeners MCLA, MCLR, MCLY, MCRR, and MCYR Administered Orally
to Mice. Toxins (Basel), 13(2): 86.
Chernoff, N., Hill, D., Lang, J., Schmid, J., Le, T., Farthing, A., Huang, H. 2020. The
Comparative Toxicity of 10 Microcystin Congeners Administered Orally to Mice: Clinical
Effects and Organ Toxicity. Toxins (Basel), 12(6): 403.
Chorus, I., Falconer, I.R., Salas, H.J., Bartram, J. 2000. Health risks caused by freshwater
cyanobacteria in recreational waters. Journal of Toxicology and Environmental Health – Part
B: Critical Reviews, 3(4): 323-347.
Chorus, I, Welker M; eds. 2021. Toxic Cyanobacteria in Water, 2
nd
edition. CRC Press,
Boca Raton (FL), on behalf of the World Health Organization, Geneva, CH.
Cloutier, D.D., McLellan, S.L. 2017. Distribution and differential survival of traditional and
alternative indicators of fecal pollution at freshwater beaches. Applied and Environmental
Microbiology, 83(4): e02881-02816.
Coleman, R.M., Ojeda-Torres, G., Bragg, W., Fearey, D., McKinney, P., Castrodale, L.,
Verbrugge, D., Stryker, K., DeHart, E., Cooper, M., Hamelin, E., Thomas, J., Johnson, R.C.
2018. Saxitoxin Exposure Confirmed by Human Urine and Food Analysis, Journal of
Analytical Toxicology, 42(7): e61 -e64.
Crain, C., Kezer, K., Steele, S., Owiti, J., Rao, S., Victorio, M., Austin, B., Volner, A., Draper,
W., Griffith, J., Steele, J., Seifert, M. 2021. Application of ddPCR for detection of
Enterococcus spp. In coastal water quality monitoring. Journal of Microbiological Methods,
184: 106206.
Crank, K., Li, X., North, D., Ferraro, G.B., Iaconelli, M., Mancini, P., La Rosa, G., Bibby, K.
2020. CrAssphage abundance and correlation with molecular viral markers in Italian
wastewater. Water Research, 184: 116161.
DeFlorio-Barker, S., Arnold, B.F., Sams, E.A., Dufour, A.P., Colford, J.M., Jr Weisberg, S.B.,
Schiff, K.C., Wade, T.J. 2018. Child environmental exposures to water and sand at the beach:
Findings from studies of over 68,000 subjects at 12 beaches. Journal of Exposure Science
and Environmental Epidemiology, 28: 93-100.
Derx, J., Demeter, K., Linke, R., Cervero-Aragó, S., Lindner, G., Stalder, G., Schijven, J.,
Sommer, R., Walochnik, J., Kirschner, A., Komma, J., Blaschke, A.P., Farnleitner, A.H.
2021. Genetic microbial source tracking support QMRA modeling for a riverine wetland
drinking water resource. Frontiers in Microbiology, 12: 668778.
Devane, M.L., Moriarty, E.M., Robson, B., Lin, S., Wood, D., Webster-Brown, J., Gilpin, B.J.
2019. Relationships between chemical and microbial faecal source tracking markers in urban
76
river water and sediments during and post-discharge of human sewage. Science of the Total
Environment, 651: 1588-1604.
Dias, E., Ebdon, J., Taylor, H. 2018. The application of bacteriophages as novel indicators of
viral pathogens in wastewater treatment systems. Water Research, 129: 172-179.
Diehl, F., Ramos, P.B., Dos Santos, J.M., Barros, D.M., Yunes, J.S. 2016. Behavioral alterations
induced by repeated saxitoxin exposure in drinking water. Journal of Venomous Animals and
Toxins Including Tropical Diseases, 22: 18.
D’Mello, F., Braidy, N., Marçal, H., Guillemin, G., Rossi, F., Chinian, M., Laurent, D., Teo, C.,
Neilan, B.A. 2017. Cytotoxic effects of environmental toxins on human glial cells.
Neurotoxicity Research, 31: 245-258.
Dorevitch, S., Shrestha, A., DeFlorio-Barker, S., Breitenbach, C., Heimler, I. 2017. Monitoring
urban beaches with qPCR vs. culture measures of fecal indicator bacteria: Implications for
public notification. Environmental Health, 16: 45.
Duan, X., Zhang, C., Struewing, I., Li, X., Allen, J., Lu, J. 2022. Cyanotoxin-encoding genes as
powerful predictors of cyanotoxin production during harmful cyanobacterial blooms in an
inland freshwater lake: Evaluating a novel early-warning system. Science of The Total
Environment, 830: 154568.
Dufour, A.P., Behymer, T.D., Cantú, R., Magnuson, M., Wymer, L.J. 2017. Ingestion of
swimming pool water by recreational swimmers. Journal of Water and Health, 15(3): 429-
437.
Dutilh, B.E., Cassman, N., McNair, K., Sanchez, S.E., Silva, G.G., Boling, L., Barr, J.J., Speth,
D.R., Seguritan, V., Aziz, R.K., Felts, B., Dinsdale, E.A., Mokili, J.L., Edwards, R.A. 2014.
A highly abundant bacteriophage discovered in the unknown sequences of human faecal
metagenomes. Nature Communications, 5: 4498.
Egorov, A.I., Griffin, S.M., Ward, H.D., Reilly, K., Fout, G.S., Wade, T.J. 2018. Application of a
salivary immunoassay in a prospective community study of waterborne infections. Water
Research, 142: 289-300.
Egorov, A.I., Converse, R., Griffin, S.M., Bonasso, R., Wickersham, L., Klein, E., Kobylanski,
J., Ritter, R., Styles, J.N., Ward, H., Sams, E., Hudgens, E., Dufour, A., Wade, T.J. 2021.
Recreational water exposure and waterborne infections in a prospective salivary antibody
study at a Lake Michigan beach. Scientific Reports, 11(1): 20540.
Eregno, F.E., Tryland, I., Tjomsland, T., Myrmel, M., Robertson, L., Heistad, A. 2016.
Quantitative microbial risk assessment combined with hydrodynamic modelling to estimate
the public health risk associated with bathing after rainfall events. Science of the Total
Environment, 548-549: 270-279.

77
Esgaib Vaz Guimarães, E. 2017. Rapid Response: Application of a qPCR-Based test for
enterococci and human-associated Bacteroides as a rapid beach management tool in Hawai‘i.
(University of Hawaiʻi at Mānoa Thesis, Esgaib Vaz Guimarães, E).
http://hdl.handle.net/10125/62257. Last Accessed: 03/24/2023
Ervin, J.S., Russell, T.L., Layton, B.A., Yamahara, K.M., Wang, D., Sassoubre, L.M., Cao, Y.,
Kelty, C.A., Sivaganesan, M., Boehm, A.B., Holden, P.A., Weisberg, S.B., Shanks, O.C.
2013. Characterization of fecal concentrations in human and other animal sources by
physical, culture-based, and quantitative real-time PCR methods. Water Research, 47: 6873-
6882.
EFSA (European Food Safety Authority). 2009. Scientific opinion: Marine biotoxins in shellfish
– Saxitoxin group1. Scientific Opinion of the Panel on Contaminants in the Food Chain.
(Question No EFSA-Q-2006-065E). The EFSA Journal, 1019: 1-76.
Farkas, K., Walker, D.I., Adriaenssens, E.M., McDonald, J.E., Hillary, L.S., Malham, S.K.,
Jones, D.L. 2020. Viral indicators for tracking domestic wastewater contamination in the
aquatic environment. Water Research, 181: 115926.
Federigi, I., Verani, M., Donzelli, G., Cioni, L., Carducci, A. 2019. The application of
quantitative microbial risk assessment to natural recreational waters: A review. Marine
Pollution Bulletin, 144: 334-350.
Feng, S., McLellan, S.L. 2019. Highly specific sewage-derived Bacteroides quantitative PCR
assays target sewage-polluted waters. Applied and Environmental Microbiology, 85: e02696-
02618.
Ferguson, A., Del Donno, C., Obeng-Gyasi, E., Mena, K., Altomare, T.K., Guerrero, R., Gidley,
M., Montas, L., Solo-Gabriele, H.M. 2019. Children exposure-related behavior patterns and
risk perception associated with recreational beach use. International Journal of
Environmental Research and Public Health, 16(15): 2783.
Ferguson, A., Dwivedi, A., Adelabu, F., Ehindero, E., Lamssali, M., Obeng-Gyasi, E., Mena, K.,
Solo-Gabriele, H. 2021. Quantified activity patterns for young children in beach
environments relevant for exposure to contaminants. International Journal of Environmental
Research and Public Health, 18(6): 3274.
Fitzmorris-Brisolara, K., Maal-Bared, R., Worley-Morse, T., Danley-Thomson, A., Sobsey, M.
2022. Monitoring coliphages to reduce waterborne infectious disease transmission in the One
Water framework. International Journal of Hygiene and Environmental Health, 240: 113921.
Franklin, A.M., Brinkman, N.E., Jahne, M.A., Keely, S.P. 2021. Twenty-first century molecular
methods for analyzing antimicrobial resistance in surface waters to support One Health
assessments. Journal of Microbiological Methods, 184: 106174.
78
Fu, J., Chiang, E.L.C., Medriano, C.A.D., Li, L., Bae, S. 2021. Rapid quantification of fecal
indicator bacteria in water using the most probable number – loop-mediated isothermal
amplification (MPN-LAMP) approach on a polymethyl methacrylate (PMMA) microchip.
Water Research, 199: 117172.
Fujioka, R.S., Solo-Gabriele, H.M., Byappanahalli, M.N., Kirs, M. 2015. U.S. Recreational
Water Quality Criteria: A Vision for the Future. 12: 7752-7776.
Gibson, K.E., Lee, J.A., Jackson, J.M., Smith, L.N., Almeida, G. 2017. Identification of factors
affecting fecal pollution in Beaver Lake Reservoir. Journal of Environmental Quality, 46:
1048-1056.
Gitter, A., Mena, K.D., Wagner, K.L., Boellstorff, D.E., Borel, K.E., Gregory, L.F., Gentry, T.J.,
Karthikeyan, R. 2020. Human health risks associated with recreational waters: Preliminary
approach of integrating quantitative microbial risk assessment with microbial source
tracking. Water, 12(2): 327.
Goh, S.G., Liang, L., Gin, K.Y.H. 2021. Assessment of Human Health Risks in Tropical
Environmental Waters with Microbial Source Tracking Markers. Water Research, 207:
117748.
Graciaa, D.S., Cope, J.R., Roberts, V.A., Cikesh, B.L., Kahler, A.M., Vigar, M., Hilborn, E.D.,
Wade, T.J., Backer, L.C., Montgomery, S.P., Secor, W.E., Hill, V.R., Beach, M.J., Fullerton,
K.E., Yoder, J.S., Hlavsa, M.C. 2018. Outbreaks associated with untreated recreational water
– United States, 2000-2014. MMWR: Morbidity and Mortality Weekly Report, 67(25): 701-
706.
Green, H., Weller, D., Johnson, S., Michalenko, E. 2019. Microbial source-tracking reveals
origins of fecal contamination in a recovering watershed. Water, 11(10): 2162.
Griffith, J.F., Weisberg, S.B., Arnold, B.F., Cao, Y., Schiff, K.C., Colford, J.M., Jr. 2016.
Epidemiologic evaluation of multiple alternate microbial water quality monitoring indicators
at three California beaches. Water Research, 94: 371-381.
Gupta, N., Pant, S.C., Vijayaraghavan, R., Rao, P.V. 2003. Comparative toxicity evaluation of
cyanobacterial cyclic peptide toxin microcystin variants (LR, RR, YR) in mice. Toxicology,
188(2-3): 285-96.
Guzmán, C., Mocé-Llivina, L., Lucena, F., Jofre, J. 2008. Evaluation of Escherichia coli host
strain CB390 for simultaneous detection of somatic and F-specific coliphages. Applied
Environmental Microbiology, 74(2): 531-4.
Hagedorn, C., Blanch, A.R., Harwood, V.J. 2011. Microbial Source Tracking: Methods,
Applications, and Case Studies. Publisher: Springer Press: New York, NY.

79
Haugland, R.A., Siefring, S., Varma, M., Oshima, K.H., Sivaganesan, M., Cao, Y., Raith, M.,
Griffith, J., Weisberg, S.B., Noble, R.T., Blackwood, A.D., Kinzelman, J., Anan’eva, T.,
Bushon, R.N., Harwood, V. J., Gordon, K.V., Sinigalliano, C., 2016. Multi-laboratory survey
of qPCR enterococci analysis method performance in U.S. coastal and inland surface waters.
Journal of Microbiological Methods, 123: 114–125.
Haugland, R., Oshima, K., Sivaganesan, M., Dufour, A., Varma, M., Siefring, S., Nappier, S.,
Schnitker, B., Briggs, S. 2021. Large-scale comparison of E. coli levels determined by
culture and a qPCR method (EPA Draft Method C) in Michigan towards the implementation
of rapid, multi-site beach testing. Journal of Microbiological Methods, 184: 106186.
He, X., Liu, P. Zheng, G., Chen, H., Shi, W., Cui, Y., Ren, H., Zhang, X.X. 2016. Evaluation of
five microbial and four mitochondrial DNA markers for tracking human and pig fecal
pollution in freshwater. Scientific Reports, 6: 35311.
Heinze, R. 1999. Toxicity of the cyanobacterial toxin microcystin-LR to rats after 28 days intake
with the drinking water. Environmental Toxicology, 14(1):57-60.
Hernandez Jr, R. 2018. Detection of E. coli in northern Lake Michigan waters using qPCR
method c. (Hernandez Jr, R. Thesis University of Wisconsin,
https://minds.wisconsin.edu/handle/1793/78721). Last Accessed: 03/24/2023
Holcomb, D.A., Stewart, J.R. 2020. Microbial indicators of fecal pollution: Recent progress and
challenges in assessing water quality. Current Environmental Health Reports, 7: 311-324.
Humpage, A.R. and I.R. Falconer. 2002. Oral Toxicity of Cylindrospermopsin: No Observed
Adverse Effect Level Determination in Male Swiss Albino Mice. The Cooperative Research
Centre for Water Quality and Treatment, Salisbury, South Australia. Research Report No. 13.
(93 pages).
Humpage, A.R., Falconer, I.R. 2003. Oral toxicity of the cyanobacterial toxin
cylindrospermopsin in male Swiss albino mice: Determination of no observed adverse effect
level for deriving a drinking water guideline value. Environmental Toxicology, 18(2): 94-
103.
Jiang, Y.S., Riedel, T.E., Popoola, J.A., Morrow, B.R., Cai, S., Ellington, A.D., Bhadra, S. 2018.
Portable platform for rapid in-field identification of human fecal pollution in water. Water
Research, 131: 186-195.
Jones, K.R., Eftim, S., Lindahl, A.J., Black, S., Nappier, S.P. 2022. Occurrence of coliphage in
effluent: A systematic literature review and meta-analysis. Hygiene and Environmental
Health Advances, 3: 100014.
Joosten, R., Sonder, G., Parkkali, S., Brandwagt, D., Fanoy, E., Mughini-Gras, L., Lodder, W.,
Ruland, E., Siedenburg, E., Kliffen, S., van Pelt, W. 2017. Risk factors for gastroenteritis
80
associated with canal swimming in two cities in the Netherlands during the summer of 2015:
A prospective study. PloS One, 12(4): e0174732.
Kim, K., Whelan, G., Molina, M., Parmar, R., Wolfe, K., Galvin, M., Duda, P., Zepp, R.,
Kinzelman, J., Kleinheinz, G., Borchardt, M. 2018. Using Integrated Environmental
Modeling to Assess Sources of Microbial Contamination in Mixed-Use Watersheds. Journal
of Environmental Quality, 47(5): 1103-1114.
Knaack, J.S., Porter, K.A., Jacob, J.T., Sullivan, K., Forester, M., Wang, R.Y., Trainer, V.L.,
Morton, S., Eckert, G., McGahee, E., Thomas, J., McLaughlin, J., Johnson, R.C. 2016. Case
diagnosis and characterization of suspected paralytic shellfish poisoning in Alaska. Harmful
Algae, 57: 45-50.
Kolm, C., Martzy, R., Brunner, K., Mach, R.L., Krska, R., Heinze, G., Sommer, R., Reischer,
G.H., Farnleitner, A.H. 2017. A complementary isothermal amplification method to the U.S.
EPA quantitative polymerase chain reaction approach for the detection of Enterococci in
environmental waters. Environmental Science & Technology, 51: 7028-7035.
Kolm, C., Martzy, R., Führer, M., Mach, R.L., Krska, R., Baumgartner, S., Farnleitner, A.H.,
Reischer, G.H. 2019. Detection of a microbial source tracking marker by isothermal helicase-
dependent amplification and a nucleic acid lateral-flow strip test. Scientific Reports, 9: 393.
Kongprajug, A., Chyerochana, N., Somnark, P., Leelapanang Kampaengthong, P., Mongkolsuk,
S., Sirikanchana, K. 2019. Human and animal microbial source tracking in a tropical river
with multiple land use activities. International Journal of Hygiene and Environmental Health,
222: 645-654.
Kongprajug, A., Chyerochana, N, Mongkolsuk, S, Sirikanchana, K. 2020. Effect of quantitative
polymerase chain reaction data analysis using sample amplification efficiency on microbial
source tracking assay performance and source attribution. Environmental Science &
Technology, 54: 8232-8244.
Korajkic, A., McMinn, B.R., Harwood, V.J. 2018. Relationships between Microbial Indicators
and Pathogens in Recreational Water Settings. International Journal Environmental Research
and Public Health, 15(12): 2842.
Korajkic, A., Wanjugi, P., Brooks, L., Cao, Y., Harwood, V.J. 2019a. Persistence and Decay of
Fecal Microbiota in Aquatic Habitats. Microbiology Molecular Biology Reviews, 83(4):
e00005-19.
Korajkic, A., McMinn, B.R., Ashbolt, N.J., Sivaganesan, M., Harwood, V.J., Shanks, O.C.
2019b. Extended persistence of general and cattle-associated fecal indicators in marine and
freshwater environment. Science of the Total Environment, 650: 1292-1302.
81
Korajkic, A., McMinn, B., Herrmann, M.P., Sivaganesan, M., Kelty, C.A., Clinton, P., Nash,
M.S., Shanks, O.C. 2020a. Viral and Bacterial Fecal Indicators in Untreated Wastewater
across the Contiguous United States Exhibit Geospatial Trends. Applied and Environmental
Microbiology, 86(8): e02967-19.
Korajkic, A., McMinn, B.R., Staley, Z.S., Ahmed, W., Harwood, V. 2020b. Antibiotic-resistant
Enterococcus species in marine habitats: A review. Current Opinion in Environmental
Science & Health, 16:92-100.
Korajkic, A., McMinn, B.R., Herrmann, M.P., Pemberton, A.C., Kelleher, J., Oshima, K.,
Villegas, E.N. 2021. Performance evaluation of a dead-end hollowfiber ultrafiltration method
for enumeration of somatic and F+ coliphage from recreational waters. Journal of Virological
Methods, 296: 114245.
Korajkic, A., Kelleher, J., Shanks, O.C., Herrmann, M.P., McMinn, B.R. 2022. Effectiveness of
two wastewater disinfection strategies for the removal of fecal indicator bacteria,
bacteriophage, and enteric viral pathogens concentrated using dead-end hollow fiber
ultrafiltration (D-HFUF). Science of the Total Environment, 831: 154861.
Kralj, J., Servetas, S., Hunter, M., Toman, B., Jackson, S. 2021. Certification of standard
reference material 2917 plasmid DNA for fecal indicator detection and identification. NIST
Special Publication, 1-41.
Kuhn, K. G., Nielsen, E.M., Mølbak, K., Ethelberg, S. 2018. Determinants of sporadic
Campylobacter infections in Denmark: A nationwide case-control study among children and
young adults. Clinical Epidemiology, 10: 1695-1707.
Lane, M.J., McNair, J.N., Rediske, R.R., Briggs, S., Sivaganesan, M., Haugland, R., 2020a.
Simplified analysis of measurement data from a rapid E. coli qPCR method (EPA Draft
Method C) using a standardized Excel workbook. Water, 12: 775.
Lane, M.J., Rediske, R., McNair, J., Briggs, S., Rhodes, G., Dreelin, E., Sivy, T., Flood, M.,
Scull, B., Szlag, D., Southwell, B., Isaacs, N., Pike, S., 2020b. A comparison of E. coli
concentration estimates quantified by the U.S. EPA and a Michigan laboratory network using
U.S. EPA Draft Method C. Journal of Microbiological Methods, 179: 106086.
Lapen, D.R., Schmidt, P.J., Thomas, J.L., Edge, T.A., Flemming, C., Keithlin, J., Neumann, N.,
Pollari, F., Ruecker, N., Simhon, A., Topp, E., Wilkes, G., Pintar, K.D.M. 2016. Towards a
more accurate quantitative assessment of seasonal Cryptosporidium infection risks in surface
waters using species and genotype information. Water Research, 105: 625-637.
Li, X., Xu, L., Zhou, W., Zhao, Q., Wang, Y. 2016a. Chronic exposure to microcystin-LR
affected mitochondrial DNA maintenance and caused pathological changes of lung tissue in
mice. Environmental Pollution, 210: 48-56.

82
Li, X., Sivaganesan, M., Kelty, C.A., Zimmer-Faust, A., Clinton, P., Reichman, J.R., Johnson,
Y., Matthews, W., Bailey, S., Shanks, O.C. 2019. Large-scale implementation of
standardized quantitative real-time PCR fecal source identification procedures in the
Tillamook Bay Watershed. PloS One, 14: e0216827.
Li, H., Barber, M., Lu, J., Goel, R. 2020. Microbial community successions and their dynamic
functions during harmful cyanobacterial blooms in a freshwater lake. Water Research, 185:
116292.
Li, X., Kelty, C.A., Sivaganesan, M., Shanks, O.C. 2021. Variable fecal source prioritization in
recreational waters routinely monitored with viral and bacterial general indicators. Water
Research, 192: 116845.
Liang, H., Yu, Z., Wang, B., Ndayisenga, F., Liu, R., Zhang, H., Wu, G. 2021. Synergistic
application of molecular markers and community-based microbial source tracking methods
for identification of fecal pollution in river water during dry and wet seasons. Frontiers in
Microbiology, 12: 660368.
Liao, H., Krometis, L.A., Kline, K. 2016. Coupling a continuous watershed-scale microbial fate
and transport model with a stochastic dose-response model to estimate risk of illness in an
urban watershed. Science of the Total Environment, 551-552: 668-675.
Lim, K.Y., Shao, S., Peng, J., Grant, S.B., Jiang, S.C. 2017. Evaluation of the dry and wet
weather recreational health risks in a semi-enclosed marine embayment in Southern
California. Water Research, 111: 318-329.
Lu, J., Struewing, I., Wymer, L., Tettenhorst, D.R., Shoemaker, J., Allen, J. 2020. Use of qPCR
and RT-qPCR for monitoring variations of microcystin producers and as an early warning
system to predict toxin production in an Ohio inland lake. Water research, 170: 115262.
Lu, J., Zhu, B., Struewing, I., Xu, N., Duan, S. 2019. Nitrogen–phosphorus-associated metabolic
activities during the development of a cyanobacterial bloom revealed by
metatranscriptomics. Scientific Reports, 9(1): 1-1.
Martzy, R., Kolm, C., Brunner, K., Mach, R.L., Krska, R., Šinkovec, H., Sommer, R.,
Farnleitner, A.H., Reischer, G.H. 2017. A loop-mediated isothermal amplification (LAMP)
assay for the rapid detection of Enterococcus spp. in water. Water Research, 122: 62-69.
Matheu, J., Aidara-Kane, A., Andremont, A. 2017. The ESBL Tricycle AMR Surveillance
Project: A Simple, One Health Approach to Global Surveillance. One Health.
http://resistancecontrol.info/2017/the-esbl-tricycle-amr-surveillance-project-a-simple-one-
health-approach-to-global-surveillance/. Last Accessed: 03/24/2023.
Mattioli, M.C., Benedict, K.M., Murphy, J., Kahler, A., Kline, K.E., Longenberger, A., Mitchell,
P. K., Watkins, S., Berger, P., Shanks, O.C., Barrett, C. E., Barclay, L., Hall, A. J., Hill, V.,
83
Weltman, A. 2021. Identifying septic pollution exposure routes during a waterborne
norovirus outbreak – A new application for human-associated microbial source tracking
qPCR. Journal of Microbiological Methods, 180: 106091.
McGinnis, S., Spencer, S., Firnstahl, A., Stokdyk, J., Borchardt, M., McCarthy, D.T., Murphy,
H.M. 2018. Human Bacteroides and total coliforms as indicators of recent combined sewer
overflows and rain events in urban creeks. Science of the Total Environment, 630: 967-976.
McKee, A., Molina, M., Cyterski, M., Couch, A. 2020. Microbial source tracking (MST) in
Chattahoochee River National Recreational Area: seasonal and precipitation trends in MST
marker concentrations, and associations with E. coli levels, pathogenic marker presence, and
land use. Water Research, 171:115435.
McMinn, B.R., Rhodes, E.R., Huff, E.M., Wanjugi, P., Ware, M.M., Nappier, S.P., Cyterski, M.,
Shanks, O.C., Oshima, K., Korajkic, A. 2018. Comparison of somatic and F+ coliphage
enumeration methods with large volume surface water samples. Journal of Virological
Methods, 261: 63-66.
McMinn, B.R., Klemm, S., Korajkic, A., Wyatt, K.M., Herrmann, M.P., Haugland, R.A., Lu, J.,
Villegas, E.N. and Frye, C. 2019. A Constructed Wetland for Treatment of an Impacted
Waterway and the Influence of Native Waterfowl on its Perceived Effectiveness. Ecological
Engineering, 128: 48-56.
McMinn, B.R., Rhodes, E.R., Huff, E.M. and Korajkic, A. 2020. Decay of infectious adenovirus
and coliphages in freshwater habitats is differentially affected by ambient sunlight and the
presence of indigenous protozoa communities. Virology Journal, 17(1): 1.
Mosnier, E., Martin, N., Razakandrainibe, R., Dalle, F., Roux, G., Buteux, A., Favennec, L.,
Brousse, P., Guarmit, B., Blanchet, D., Epelboin, L., Girouin, C., Martin, E., Djossou, F.,
Nacher, M., Demar, M. 2018. Cryptosporidiosis outbreak in immunocompetent children from
a remote area of French Guiana. American Journal of Tropical Medicine and Hygiene, 98:
1727-1732.
Munday, R., Thomas, K., Gibbs, R., Murphy, C., Quilliam, M.A. 2013. Acute toxicities of
saxitoxin, neosaxitoxin, decarbamoyl saxitoxin and gonyautoxins 1&4 and 2&3 to mice by
various routes of administration. Toxicon, 76: 77-83.
Napier, M.D., Haugland, R., Poole, C., Dufour, A.P., Stewart, J.R., Weber, D.J., Varma, M.,
Lavender, J.S., Wade, T.J. 2017. Exposure to human-associated fecal indicators and self-
reported illness among swimmers at recreational beaches: A cohort study. Environmental
Health, 16(1): 103.
Napier, M.D., Poole, C., Stewart, J.R., Weber, D.J., Glassmeyer, S.T., Kolpin, D.W., Furlong,
E.T., Dufour, A.P., Wade, T.J. 2018. Exposure to human-associated chemical markers of

84
fecal contamination and self-reported illness among swimmers at recreational beaches.
Environmental Science & Technology, 52: 7513-7523.
Nappier, S.P., Hong, T., Ichida, A., Goldstone, A., Eftim, S.E. 2019. Occurrence of coliphage in
raw wastewater and in ambient water: A meta-analysis. Water Research, 153: 263-273.
Nappier, S.P., Ichida, A., Jaglo, K., Haugland, R., Jones, K.R. 2019. Advancements in mitigating
interference in quantitative polymerase chain reaction (qPCR) for microbial water quality
monitoring. Science of the Total Environment, 671: 732-740.
Nappier, S.P., Liguori, K., Ichida, A.M., Stewart, J.R., Jones, K.R. 2020. Antibiotic Resistance in
Recreational Waters: State of the Science. International Journal of Environmental Research
and Public Health, 17(21): 8034.
Nelson, K.L., Boehm, A.B., Davies-Colley, R.J., Dodd, M.C., Kohn, T., Linden, K.G., Liu, Y.,
Maraccini, P.A., McNeill, K., Mitch, W.A., Nguyen, T.H., Parker, K.M., Rodriguez, R.A.,
Sassoubre, L.M., Silverman, A.I., Wigginton, K.R., Zepp, R.G. 2018. Sunlight-mediated
inactivation of health-relevant microorganisms in water: a review of mechanisms and
modeling approaches. Environmental Science Processes and Impacts, 20(8): 1089-1122.
Nieuwkerk, D.M., Korajkic, A., Valdespino, E.L., Herrmann, M.P., Harwood, V.J. 2020. Critical
review of methods for isothermal amplification of nucleic acids for environmental analysis.
Journal of Microbiological Methods, 179: 106099.
Nshimyimana, J.P., Cruz, M.C., Wuertz, S., Thompson, J.R. 2019. Variably improved microbial
source tracking with digital droplet PCR. Water Research, 159: 192-202.
ODH (Ohio Department of Health). 2020. State of Ohio harmful algal bloom response strategy
for recreational waters. Ohio Department of Health, Ohio Environmental Protection Agency,
Ohio Department of Natural Resources. August 2020.
https://epa.ohio.gov/static/Portals/35/hab/HABResponseStrategy.pdf?ver=2020-10-28-
164629-413. Last Accessed: 03/24/2023.
OHEPA (Ohio Environmental Protection Agency). 2022. Public water system harmful algal
bloom response strategy. Ohio Environmental Protection Agency, Division of Drinking and
Ground Waters. November 2022.
https://epa.ohio.gov/static/Portals/28/documents/habs/PWS-HAB-Strategy.pdf. Last
Accessed: 3/26/2023
Okoh, A.I., Sibanda, T., Gusha, S.S. 2010. Inadequately treated wastewater as a source of human
enteric viruses in the environment. International Journal of Environmental Research and
Public Health, 7: 2620-2637.
85
Oliveira, B.B., Veigas, B., Carlos, F.F., Sánchez-Melsió, A., Balcázar, J.L., Borrego, C.M.,
Baptista, P.V. 2020. Water safety screening via multiplex LAMP-Au-nanoprobe integrated
approach. Science of the Total Environment, 741: 140447.
O’Neill, K., Musgrave, I.F., Humpage, A. 2017. Extended low-dose exposure to saxitoxin
inhibits neurite outgrowth in model neuronal cells. Basic & Clinical Pharmacology &
Toxicology, 120: 390-397.
Pendergraph, D., Ranieri, J., Ermatinger, L., Baumann, A., Metcalf, A.L., DeLuca, T.H., Church,
M.J. 2021. Differentiating sources of fecal contamination to wilderness waters using droplet
digital PCR and fecal indicator bacteria methods. Wilderness Environmental Medicine, 32:
332-339.
Petcharat, T., Kongprajug, A., Chyerochana, N., Sangkaew, W., Mongkolsuk, S., Sirikanchana,
K. 2020. Assessing human-specific crAssphage recovery after acidification-filtration
concentrating method in environmental water. Water Environmental Research, 92: 35-41.
Phelan, S., Soni, D., Medina, W.R.M., Fahrenfeld, N.L. 2019. Comparison of qPCR and
amplicon sequencing based methods for fecal source tracking in a mixed land use estuarine
watershed. Environmental Science: Water Research Technology, 5: 2108-2123.
Polkowska, A., Räsänen, S., Al-Hello, H., Bojang, M. Lyytikäinen, O., Nuorti, J.P., Jalava, K.
2018. An outbreak of norovirus infections associated with recreational lake water in Western
Finland, 2014. Epidemiology and Infection, 146: 544-550.
Poma, H.R., Kundu, A., Wuertz, S., Rajal, V.B. 2019. Data fitting approach more critical than
exposure scenarios and treatment of censored data for quantitative microbial risk assessment.
Water Research, 154: 45-53.
Puddick, J., van Ginkel, R., Page, C.D., Murray, J.S., Greenhough, H.E., Bowater, J., Selwood,
A.I., Wood, S.A., Prinsep, M.R., Truman, P., Munday, R., and Finch, S.C. 2021. Acute
toxicity of dihydroanatoxin-a from Microcoleus autumnalis in comparison to anatoxin-a.
Chemosphere, 263: 127937.
Qiu, Y., Lee, B.E., Neumann, N., Ashbolt, N.J., Craik, S., Maal-Bared, R., Pang, X. 2015.
Assessment of human virus removal during municipal wastewater treatment in Edmonton,
Canada. Journal of Applied Microbiology, 119: 1729-1739.
Ramos, P., Schmitz, M., Filgueira, D., Votto, A.P., Durruthy, M., Gelesky, M., Ruas, C., Yunes,
J., Tonel, M., Fagan, S., Monserrat, J. 2017. Interaction of single-walled carbon nanotubes
and saxitoxin: Ab initio simulations and biological responses in hippocampal cell line HT-22.
Environmental Toxicology and Chemistry, 36: 1728-1737.
Rose, J.B., Farrah, S.R., Harwood, V.J., Levine, A.D., Lukasik, J., Menendez, P., Scott, T.M.
2004. Reduction of pathogens, indicator bacteria, and alternative indicators by wastewater
86
treatment and reclamation processes. Water Environment Research Foundation (WERF)
Report 00-PUM-2T.
Ryu, H., Addor, Y., Brinkman, N.E., Ware, M.W., Boczek, L., Hoelle, J., Mistry, J.H., Keely,
S.P., Villegas, E.N. 2021. Understanding Microbial Loads in Wastewater Treatment Works
as Source Water for Water Reuse. Water (Basel), 13(11): 10.3390/w13111452.
Safaie, A., Nguyen, T., Whelan, G., Molina, M., Cyterski, M., Zepp, R., Acrey, B., Pachepsky,
Y.,Phanikumar, M. 2020. Modeling the photoinactivation and transport of somatic and F-
specific coliphages at a Great Lakes beach. Journal of Environmental Quality, 49(6): 1612-
1623.
Schets, F.M., De Roda Husman, A.M., Havelaar, A.H. 2011. Disease outbreaks associated with
untreated recreational water use. Epidemiology and Infection, 139(7): 1114-1125.
Schets, F.M., van den Berg, H., Vennema, H., Pelgrim, M.T.M., Collé, C., Rutjes, S.A., Lodder,
W.J. 2018. Norovirus outbreak associated with swimming in a recreational lake not
influenced by external human fecal sources in the Netherlands, August 2012. International
Journal of Environmental Research and Public Health, 15(11): 2550.
Schoen, M.E., Boehm, A.B., Soller, J., Shanks, O.C. 2020. Contamination scenario matters when
using viral and bacterial human-associated genetic markers as indicators of a health risk in
untreated sewage-impacted recreational waters. Environmental Science & Technology, 54:
13101-13109.
Seidel, L., Strathmann, M., Nocker, A. 2017. The feasibility of improved live-dead distinction in
qPCR-based microbial source tracking. Journal of Microbiological Methods, 140: 23-31.
Selwood, A.I., Waugh, C., Harwood, D.T., Rhodes, L.L., Reeve, J., Sim, J., Munday, R. 2017.
Acute toxicities of the saxitoxin congeners gonyautoxin 5, gonyautoxin 6, decarbamoyl
gonyautoxin 2&3, decarbamoyl neosaxitoxin, C-1&2 and C-3&4 to mice by various routes of
administration. Toxins (Basel), 9.
Shanks, O.C., Kelty, C.A., Sivaganesan, M., Varma, M., Haugland, R.A. 2009. Quantitative PCR
for genetic markers of human fecal pollution. Applied and Environmental Microbiology, 75:
5507–5513.
Shanks, O.C., White, K., Kelty, C.A., Hayes, S., Sivaganesan, M., Jenkins, M., Varma, M.,
Haugland, R.A. 2010. Performance assessment of cattle-associated PCR and quantitative
real-time PCR assays targeting Bacteroidales Genes. Applied and Environmental
Microbiology, 76: 1359-1366.
Shanks, O.C., Kelty, C.A., Archibeque, S.L., Jenkins, M., Newton, R.J., McLellan, S.L., Huse,
S.M., Sogin, M.L. 2011. Community structures of fecal bacteria in cattle from different
animal feeding operations. Applied and Environmental Microbiology, 77: 2992-3001.
87
Shanks, O.C., Kelty, C.A., Peed, L.A., Sivaganesan, M., Mooney, T., Jenkins, M. 2014. Age-
related shifts in density and distribution of genetic marker water quality indicators in cow and
calf feces. Applied and Environmental Microbiology, 80: 1588-1594.
Shoults, D.C., Li, Q.Z., Petterson, S., Rudko, S.P., Dlusskaya, L., Leifels, M., Scott, C.,
Schlosser, C., Ashbolt, N.J. 2021. Pathogen performance testing of a natural swimming pool
using a cocktail of microbiological surrogates and QMRA-derived management goals.
Journal of Water and Health, 19: 629-641.
Shrestha, A., Dorevitch, S. 2019. Evaluation of rapid qPCR method for quantification of E. coli
at non-point source impacted Lake Michigan beaches. Water Research, 156: 395-403.
Shrestha, A., Dorevitch, S. 2020. Slow adoption of rapid testing: Beach monitoring and
notification using qPCR. Journal of Microbiological Methods, 174: 105947.
Shrestha, A., Kelty, C.A., Sivaganesan, M., Shanks, O.C., Dorevich, S. 2020. Fecal pollution
source characterization at non-point source impacted beaches under dry and wet weather
conditions. Water Research, 182: 116014.
Sinclair, R.G., Jones, E.L., Gerba, C.P. 2009. Viruses in recreational water-borne disease
outbreaks: a review. Journal of Applied Microbiology, 107: 1769-1780.
Sips, G.J., Dirven, M.J.G., Donkervoort, J.T., van Kolfschoten, F.M., Schapendonk, C.M.E.,
Phan, M.V.T., Bloem, A., van Leeuwen, A.F., Trompenaars, M.E., Koopmans, M.P.G., van
der Eijk, A.A., de Graaf, M., Fanoy, E.B. 2020. Norovirus outbreak in a natural playground:
A One Health approach. Zoonoses and Public Health, 67: 453-459.
Sivaganesan, M., Varma, M., Siefring, S., Haugland, R., 2018. Quantification of plasmid DNA
standards for U.S. EPA fecal indicator bacteria qPCR methods by droplet digital PCR
analysis. Journal of Microbiological Methods, 152: 135-142.
Sivaganesan, M., Aw, T.G., Briggs, S., Dreelin, E., Aslan, A., Dorevitch, S., Shrestha, A., Isaacs,
N., Kinzelman, J., Kleinheinz, G., Noble, R., Rediske, R., Scull, B., Rosenberg, S.,
Weberman, B., Sivy, T., Southwell, B., Siefring, S., Oshima, K., Haugland, R. 2019.
Standardized data quality acceptance criteria for a rapid E. coli qPCR method (Draft Method
C) for water quality monitoring at recreational beaches. Water Research, 156: 456-464.
Sivaganesan, M., Willis, J.R., Karim, M., Babatola, A., Catoe, D., Boehm, A.B., Wilder, M.,
Green, H., Lobos, A., Harwood, V.J., Hertel, S., Klepikow, R., Howard, M.F., Laksanalamai,
P., Roundtree, A., Mattioli, M., Eytcheson, S., Molina, M., Lane, M., Rediske, R., Ronan, A.,
D’Souza, N., Rose, J.B., Shrestha, A., Hoar, C., Silverman, A.I., Faulkner, W., Wickman, K.,
Kralj, J.G., Servetas, S.L., Hunter, M.E., Jackson, S.A., Shanks, O.C. 2022. Interlaboratory
performance and quantitative PCR data acceptance metrics for NIST SRM® 2917. Water
Research, 225:119162.
88
Soller, J.A., Schoen, M.E., Bartrand, T., Ravenscroft, J.E., Ashbolt, N.J. 2010a. Estimated
human health risks from exposure to recreational waters impacted by human and non-human
sources of faecal contamination. Water Research, 44(16): 4674-4691.
Soller, J.A., Bartrand, T., Ashbolt, N.J., Ravenscroft, J., Wade, T.J. 2010b. Estimating the
primary etiologic agents in recreational freshwaters impacted by human sources of faecal
contamination. Water Research, 44(16): 4736-4747.
Soller, J.A., Eftim, S., Wade, T.J., Ichida, A.M., Clancy, J.L., Johnson, T.B., Schwab, K.,
Ramirez-Toro, G., Nappier, S., Ravenscroft, J.E. 2016. Use of quantitative microbial risk
assessment to improve interpretation of a recreational water epidemiological study. Microbial
Risk Analysis, 1: 44238.
Soller, J.A., Schoen, M., Steele, J.A., Griffith, J.F., Schiff, K.C. 2017. Incidence of
gastrointestinal illness following wet weather recreational exposures: Harmonization of
quantitative microbial risk assessment with an epidemiologic investigation of surfers. Water
Research, 121: 280-289.
Somnark, P., Chyerochana, N., Mongkolsuk, S., Sirikanchana, K. 2018. Performance evaluation
of Bacteroidales genetic markers for human and animal microbial source tracking in tropical
agricultural watersheds. Environmental Pollution, 236: 100-110.
Sowah, R.A., Bradshaw, K., Snyder, B., Spidle, D., Molina, M. 2020. Evaluation of the soil and
water assessment tool (SWAT) for simulating E. coli concentrations at the watershed-scale.
Science of the Total Environment, 746: 140669.
Stachler, E., Kelty, C.A., Sivaganesan, M., Li, X., Bibby, K., Shanks, O.C. 2017. Development
of CrAssphage quantitative real-time PCR assays for human fecal pollution measurement.
Environmental Science & Technology, 51: 9146-9154.
Staley, Z.R., Chuong, J.D., Hill, S.J., Grabuski, J., Shokralla, S., Hajibabaei, M., Edge, T.A.
2018a. Fecal source tracking and eDNA profiling in an urban creek following an extreme
rain event. Scientific Reports, 8(1): 14390.
Staley, Z.R., Boyd, R.J., Shum, P., Edge, T.A. 2018b. Microbial source tracking using
quantitative and digital PCR to identify sources of fecal contamination in stormwater, river
water, and beach water in a Great Lakes area of concern. Applied and Environmental
Microbiology, 84(20): e01634-01618.
Staley, Z.R., Edge, T.A. 2016. Comparative microbial source tracking methods for identification
of fecal contamination sources at Sunnyside Beach in the Toronto region area of concern.
Journal of Water and Health, 14: 839-850.
89
Steele, J.A., Blackwood, A.D., Griffith, J.F., Noble, R.T., Schiff, K.C. 2018. Quantification of
pathogens and markers of fecal contamination during storm events along popular surfing
beaches in San Diego, California. Water Research, 136: 137-149.
Stoner, R.D., Adams, W.H., Slatkin, D.N., Siegelman, H.W. 1989. The effects of single L-amino
acid substitutions on the lethal potencies of microcystins. Toxicon, 27(7): 825- 828.
Sunger, N., Hamilton, K.A., Morgan, P. M., Haas, C. N. 2019. Comparison of pathogen-derived
‘total risk’ with indicator-based correlations for recreational (swimming) exposure.
Environmental Science and Pollution Research International, 26: 30614-30624.
Sutherland, J.W., Turcotte, R.J., Molden, E., Moriarty, V., Kelly, M., Aubel, M., Foss, A. 2021.
The detection of airborne anatoxin-a (ATX) on glass fiber filters during a harmful algal
bloom. Lake and Reservoir Management, 37: 113–119.
Symonds, E.M., Young, S., Verbyla, M.E., McQuaig-Ulrich, S.M., Ross, E., Jimenez, J.A.,
Harwood, V.J., Breitbart, M. 2017. Microbial source tracking in shellfish harvesting waters
in the Gulf of Nicoya, Costa Rica. Water Research, 111: 177-184.
Tanvir, R.U., Hu, Z., Zhang, Y., Lu, J. 2021. Cyanobacterial community succession and
associated cyanotoxin production in hypereutrophic and eutrophic freshwaters.
Environmental Pollution, 290: 118056.
Teunis, P.F., Moe, C.L., Liu, P., Miller, S.E., Lindesmith, L., Baric, R.S., Le Pendu, J., Calderon,
R.L. 2008. Norwalk virus: how infectious is it? Journal of Medical Virology, 80(8): 1468-76.
Teunis, P., Schijven, J., Rutjes, S. 2016. A generalized dose-response relationship for adenovirus
infection and illness by exposure pathway. Epidemiology and Infection, 144(16): 3461-3473.
Teunis, P.F.M., Bonačić Marinović, A., Tribble, D.R., Porter, C.K., Swart, A. 2018. Acute
illness from Campylobacter jejuni may require high doses while infection occurs at low
doses. Epidemics, 24:1-20.
Teunis, P.F.M., Le Guyader, F.S., Liu, P., Ollivier, J., Moe, C.L. 2020. Noroviruses are highly
infectious but there is strong variation in host susceptibility and virus pathogenicity.
Epidemics, 32:100401.
Thulsiraj, V., Zimmer-Faust, A.G., Jay, J.A. 2017. Use of viability-based methods for improved
detection of recent fecal contamination in a microbial source tracking study near Tijuana,
Mexico. Water, Air, and Soil Pollution, 228: 63.
U.S. EPA (United States Environmental Protection Agency). 2009a. National Lakes Assessment:
A Collaborative Survey of the Nation’s Lakes. EPA 841-R-09-001.
90
U.S. EPA. 2009b. Method 1600: Enterococci in Water by Membrane Filtration Using
membrane-Enterococcus Indoxyl-β-D-Glucoside Agar (mEI). EPA-821-R-09-016.
U.S. EPA. 2010. Quantitative microbial risk assessment to estimate illness in freshwater
impacted by agricultural animal sources of fecal contamination. EPA 822-R-10-005.
Washington, DC.
U.S. EPA. 2011. Exposure Factors Handbook: 2011 Edition. EPA/600/R-09/052F.
U.S. EPA. 2012a. Recreational water quality criteria. Office of Water. Washington DC. EPA
820-F-12-058.
U.S. EPA. 2012b. Method 1611: Enterococci in water by TaqMan® quantitative polymerase
chain reaction (qPCR) assay. Office of Water, Washington, D.C. EPA-821-R-12-008.
U.S. EPA. 2014a. Site-Specific Alternative Recreational Criteria Technical Support Materials for
Alternative Indicators and Methods. EPA-820-R-14-011.
U.S. EPA. 2014b. Method 1603: Escherichia coli (E. coli) in Water by Membrane Filtration
Using Modified Membrane-Thermotolerant Escherichia coli Agar (Modified mTEC). EPA-
821-R-14-010.
U.S. EPA. 2015a. Health Effects Support Document for the Cyanobacterial Toxin Microcystins.
Washington, DC. EPA 820R15102.
U.S. EPA. 2015b. Health Effects Support Document for the Cyanobacterial Toxin
Cylindrospermopsin. Washington, DC. EPA 820R15103.
U.S. EPA. 2015c. Method 1609.1: Enterococci in water by TaqMan® quantitative polymerase
chain reaction (qPCR) assay with internal amplification control (IAC) assay. Office of Water,
Washington, D.C. EPA-820-R-15-009.
U.S. EPA. 2016. Method 546. Determination of Total Microcystins and Nodularins in Drinking
Water and Ambient Water by Adda Enzyme-Linked Immunosorbent Assay. Cincinnati, OH,
EPA 815-B-16-011.
U.S. EPA. 2017a. Proceedings from the U.S. Environmental Protection Agency (EPA)
Coliphage Experts Workshop March 1-2, 2016. EPA Office of Water, Office of Science and
Technology, Health and Ecological Criteria Division. EPA 822-R-17-003.
U.S. EPA. 2017b. Single Laboratory Validated Method for Determination of
Cylindrospermopsin and Anatoxin-a in Ambient Freshwaters by Liquid Chromatography/
Tandem Mass Spectrometry (LC/MS/MS). EPA/600/R-17/130.
U.S. EPA. 2017c. Single Laboratory Validated Method for Determination of Microcystins and
Nodularin in Ambient Freshwaters by Solid Phase Extraction and Liquid

91
Chromatography/Tandem Mass Spectrometry (LC/MS/MS) November 2017 U.S.
Environmental Protection Agency Office of Research and Development 1200 Pennsylvania
Avenue, NW, EPA/600/R-17/344.
U.S. EPA. 2018a. Method 1642: Male-specific (F+) and Somatic Coliphage in Recreational
Waters and Wastewater by Ultrafiltration (UF) and Single Agar Layer (SAL) Procedure
(Draft) Office of Water. EPA-820-R-18-001.
U.S. EPA. 2018b. Method 1643: Male-specific (F+) and Somatic Coliphage in Recreational
Waters and Wastewater by Ultrafiltration (UF) and Single Agar Layer (SAL) Procedure
(Draft). Office of Water. EPA-820-R-18-001.
U.S. EPA. 2018c. Results of the Multi-Laboratory Validation of Method 1642 for Coliphage in
Fresh and Marine Recreational Waters and Advanced Treatment Wastewater Effluent
Samples that have been Concentrated using Ultrafiltration and Method 1643 for 100-mL
Secondary Wastewater (No Disinfection) Effluents. Office of Water. EPA 820-R-18-002.
U.S. EPA. 2018d. 2017 Five-Year Review of the 2012 Recreational Water Quality Criteria. EPA
823-R-18-001.
U.S. EPA. 2019. Recommended Human Health Recreational Ambient Water Quality Criteria or
Swimming Advisories for Microcystins and Cylindrospermopsin. EPA 822-R-19-001.
U.S. EPA. 2020. EPA Letter: Approval to Implement ddPCR for Beach Water Quality Rapid
Detection Method for Recreational Beaches in San Diego County. (October 6, 2020)
https://www.epa.gov/sites/default/files/2021-
04/documents/epa_approval_of_ddpcr_beach_pilot-san-diego-c-10-06-2020.pdf
U.S. EPA. 2021a. Spreadsheet: Alternative Methods Calculator Tool. EPA 821-B-21-002.
U.S. EPA. 2021b. User Guide: Alternative Methods Calculator Tool. EPA 821-B-21-001.
U.S. EPA. 2021c. Fact Sheet: Alternative Methods Calculator Tool. EPA 821-F-21-005.
U.S. EPA. 2021d. Final Technical Support Document: Implementing the 2019 National Clean
Water Act Section 304(a) Recommended Human Health Recreational Ambient Water
Quality Criteria or Swimming Advisories for Microcystins and Cylindrospermopsin. EPA-
823-R-21-002.
U.S. EPA. 2022a. Method 1696.1: Characterization of Human Fecal Pollution in Water by
HF183/BacR287 TaqMan® Quantitative Polymerase Chain Reaction (qPCR) Assay. Office
of Water. EPA 821-R-22-001.
92
U.S. EPA. 2022b. Method 1697.1 Characterization of Human Fecal Pollution in Water by
TaqMan Quantitative Polymerase Chain Reaction (qPCR) Assay. Office of Water. EPA-821-
R-22-002.
Vadde, K.K., McCarthy, A.J., Rong, R., Sekar, R. 2019. Quantification of microbial source
tracking and pathogenic bacterial markers in water and sediments of Tiaoxi River (Taihu
Watershed). Frontiers in Microbiology, 10: 699.
Van Abel, N., Mans, J., Taylor, M.B. 2017. Quantitative microbial risk assessment to estimate
the health risk from exposure to noroviruses in polluted surface water in South Africa.
Journal of Water and Health, 15(6): 908-922.
Vanden Esschert, K.L., Mattioli, M.C., Hilborn, E.D., Roberts, V.A., Yu, A.T., Lamba, K.,
Arzaga, G., Zahn, M., Marsh, Z., Combes, S.M., Smith, E.S., Robinson, T.J., Gretsch, S.R.,
Laco, J.P., Wikswo, M.E., Miller, A.D., Tack, D.M., Wade, T.J., Hlavsa, M.C. 2020.
Outbreaks associated with untreated recreational water – California, Maine, and Minnesota,
2018-2019. Morbidity and Mortality Weekly Report, 69(25): 781-783.
Vergara, G., Rose, J.B., Gin, K.Y.H. 2016. Risk assessment of noroviruses and human
adenoviruses in recreational surface waters. Water Research, 103: 276-282.
Verhougstraete, M.P., Pogreba-Brown, K., Reynolds, K.A., Lamparelli, C.C., Zanoli Sato, M.I.,
Wade, T.J., Eisenberg, J.N.S. 2020. A critical analysis of recreational water guidelines
developed from temperate climate data and applied to the tropics. Water Research, 170:
115294.
Wade, T.J., Augustine, S.A.J., Griffin, S.M., Sams, E.A., Oshima, K.H., Egorov, A.I., Simmons,
K.J., Eason, T.N., Dufour, A.P. 2018. Asymptomatic norovirus infection associated with
swimming at a tropical beach: A prospective cohort study. PloS One, 13: e0195056.
Wade, T.J., Griffin, S.M., Egorov, A.I., Sams, E., Hudgens, E., Augustine, S., DeFlorio-Barker,
S., Plunkett, T., Dufour, A.P., Styles, J.N., Oshima, K. 2019. Application of a multiplex
salivary immunoassay to detect sporadic incident norovirus infections. Scientific Reports,
9(1): 1-7.
Wade, T.J., Arnold, B.F., Schiff, K., Colford Jr, J.M., Weisberg, S.B., Griffith, J.F. and Dufour,
A.P. 2022. Health risks to children from exposure to fecally contaminated recreational water.
PloS One 2022, 17(4), p.e0266749.
Wang, D., Yamahara, K.M., Cao, Y., Boehm, A.B. 2016a. Absolute quantification of
Enterococcal 23S rRNA gene using digital PCR. Environmental Science & Technology, 50:
3399-3408.

93
Wang, C., Gu, S., Yin, X., Yuan, M., Xiang, Z., Li, Z., Cao, H., Meng, X., Hu, K., Han, X.
2016b. The toxic effects of microcystin-LR on mouse lungs and alveolar type II epithelial
cells. Toxicon, 115: 81–88.
Wang, K., Mou, X., Cao, H., Struewing, I., Allen, J., Lu, J. 2021. Co-occurring microorganisms
regulate the succession of cyanobacterial harmful algal blooms. Environmental Pollution,
288: 117682.
Wanjugi, P., Sivaganesan, M., Korajkic, A., McMinn, B., Kelty, C.A., Rhodes, E., Cyterski, M.,
Zepp, R., Oshima, K., Stachler, E., Kinzelman, J., Kurdas, S.R., Citriglia, M., Hsu, F.C.,
Acrey, B. and Shanks, O.C. 2018. Incidence of somatic and F+ coliphage in Great Lake
Basin recreational waters. Water Research, 140: 200-210.
Whelan, G., Kim, K., Wolfe, K., Parmar, R., Galvin, M., Molina, M., Zepp, R. 2015.
Quantitative Microbial Risk Assessment Tutorial – Navigate the SDMPB and Identify an 8-
digit HUC of Interest. U.S. EPA Office of Research and Development, EPA/600/B-15/273.
Whelan, G., Kim, K. Wolfe, K., Parmar, R., Galvin, M. 2017a. Quantitative Microbial Risk
Assessment Tutorial Installation of Software for Watershed Modeling in Support of QMRA.
U.S. EPA Office of Research and Development, EPA/600/B-15/276.
Whelan, G., Wolfe, K., Parmar, R., Galvin, M. Molina, M., Zepp, R., Kim, K., Duda, P. 2017b.
Quantitative Microbial Risk Assessment Tutorial – Primer. U.S. EPA Office of Research and
Development, EPA/600/B-17/323.
Whelan, G., Parmar, R., Wolfe, K., Galvin, M., Duda, P., Gray, M. 2017d. Quantitative
Microbial Risk Assessment Tutorial – SDMProjectBuilder: Import Local Data Files to
Identify and Modify Contamination Sources and Input Parameters. U.S. EPA Office of
Research and Development, EPA/600/B-15/316.
Whelan, G., Kim, K., Parmar, R., Laniak, G.F., Wolfe, K., Galvin, M., Molina, M., Pachepsky,
Y.A., Duda, P., Zepp, R., Prieto, L., Kinzelman, J.L., Kleinheinz, G.T., Borchardt, M.A.
2018. Capturing Microbial Sources Distributed in a Mixed-use Watershed within an
Integrated Environmental Modeling Workflow. Environmental Modelling and Software, 99:
126-146.
Willis, J.R., Sivaganesan, M., Haugland. R.A., Kralj, J., Servetas, S., Hunter, M.E., Jackson,
S.A., Shanks, O.C. 2022. Performance of NIST SRM® 2917 with 13 recreational water
quality monitoring qPCR assays. Water Research, 212: 118114.
WHOI (Woods Hole Oceanographic Institution) 2016. Distribution of HABs in the U.S.
https://www.whoi.edu/redtide/regions/us-distribution.

94
WHO (World Health Organization). 2018. Global Antimicrobial Resistance Surveillance System
(GLASS) Report: Early Implementation 2017–2018; World Health Organization: Geneva,
Switzerland, 2018.
WHO. 2019. Antimicrobial Resistance; WHO: Geneva, Switzerland, Available online:
https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance
WHO. 2020. Cyanobacterial toxins: saxitoxins. Background document for development of WHO
Guidelines for drinking-water quality and Guidelines for safe recreational water
environments. Geneva: World Health Organization (WHO/HEP/ECH/WSH/2020.8).
License: CC BY-NC-SA 3.0 IGO.
WHO. 2021a. Guidelines on recreational water quality: Volume 1, coastal and fresh waters.
Geneva: World Health Organization, ISBN 978-92-4-003130-2
WHO. 2021b. Toxic Cyanobacteria in Water: A Guide to their Public Health Consequences,
Monitoring, and Management, Second Edition.
https://www.who.int/publications/m/item/toxic-cyanobacteria-in-water---second-edition.
Last Accessed: 08/31/2022.
Worley-Morse, T., Mann, M., Khunjar, W., Olabode, L., Gonzalez, R. 2019. Evaluating the fate
of bacterial indicators, viral indicators, and viruses in water resource recovery facilities.
Water Environmental Research, 91(9): 830-842.
Wu, B., Wang, X.C., Dzakpasu, M. 2017. Genetic characterization of fecal impacts of seagull
migration on an urban scenery lake. Water Research, 117: 27-36.
Wu, B.L., Wang, C.W., Zhang, C.M., Sadowsky, M.J., Dzakpasu, M., Wang, X.C.C. 2020.
Source-associated gastroenteritis risk from swimming exposure to aging fecal pathogens.
Environmental Science & Technology, 54(2): 921-929.
Wymer, L.J., Wade, T.J., Sams, E., Oshima, K., Dufour, A.P. 2021. Comparative stability of
assay results of enterococci measured by culture and qPCR over time in bathing beach
waters. Journal of Microbiological Methods, 188: 106274.
Xue, J., Lin, S., Lamar, F.G., Lamori, J.G., Sherchan, S. 2018. Assessment of fecal pollution in
Lake Pontchartrain, Louisiana. Marine Pollution Bulletin, 129: 655-663.
Xue, J., Feng, Y. 2019. Comparison of microbial source tracking efficacy for detection of cattle
fecal contamination by quantitative PCR. Science of the Total Environment, 686: 1104-1112.
Young, N. 2016. The association between marine bathing and infectious diseases – a review.
Journal of the Marine Biological Association of the United Kingdom, 96: 93-100.
95
Zeki, S., Aslan, A., Burak, S., Rose, J.B. 2021. Occurrence of a human-associated microbial
source tracking marker and its relationship with faecal indicator bacteria in an urban estuary.
Letters in Applied Microbiology, 72: 167-177.
Zepp, R.G., Cyterski, M., Wong, K., Georgacopoulos, O., Acrey, B., Whelan, G., Parmar, R.,
Molina, M. 2018. Biological Weighting Functions for Evaluating the Role of Sunlight-
Induced Inactivation of Coliphages at Selected Beaches and Nearby Tributaries.
Environmental Science & Technology, 52(22): 13068-13076.
Zhang, Y., Wu, R., Lin, K., Wang, Y., Lu, J. 2020. Performance of host-associated genetic
markers for microbial source tracking in China. Water Research, 175: 115670.
Zhong, Y., Shen, L., Ye, X., Zhou, D., He, Y., Li, Y., Ding, Y., Zhu, W., Ding, J., Zhang, H.
Neurotoxic Anatoxin-a. 2020. Can Also Exert Immunotoxicity by the Induction of Apoptosis
on Carassius auratus Lymphocytes in vitro When Exposed to Environmentally Relevant
Concentrations. Frontiers in Physiology, 11:316.
Zhou, Y., Sun, M., Tang, Y., Chen, Y., Zhu, C., Yang, Y., Wang, C., Yu, G., Tang, Z. 2020.
Responses of the proteome in testis of mice exposed chronically to environmentally relevant
concentrations of Microcystin-LR. Ecotoxicological Environmental Safety, 187: 109824.
Zhu, B., Cao, H., Li, G., Du, W., Xu, G., Santo Domingo, J., Gu, H., Xu, N., Duan, S., Lu, J.
2019. Biodiversity and dynamics of cyanobacterial communities during blooms in temperate
lake (Harsha Lake, Ohio, USA). Harmful Algae, 82: 9-18.
Zimmer-Faust, A.G., Thulsiraj, V., Lee, C.M., Whitener, V., Rugh, M., Mendoza-Espinosa, L.,
Jay, J.A. 2018. Multi-tiered approach utilizing microbial source tracking and human
associated-IMS/ATP for surveillance of human fecal contamination in Baja California,
Mexico. Science of the Total Environment, 640: 475-484.
Zimmer-Faust, A.G., Steele, J.A., Xiong, X., Staley, C., Griffith, M., Sadowsky, M.J., Diaz, M,
Griffith, J.F. 2021. A combined digital PCR and next generation DNA-sequencing based
approach for tracking nearshore pollutant dynamics along the Southwest United
States/Mexico Border. Frontiers in Microbiology, 12: 674214.
A-1
Appendix A. Literature Search and Review Strategies
1. Background
Context
Under the Beaches Environmental Assessment and Coastal Health (BEACH) Act amendments to
the Clean Water Act (CWA) Section 304(a)(9)(B) EPA is required to complete a review of its
recommended recreational water quality criteria every 5 years. In 2018, EPA published a review
of the 2012 304(a) recommended recreational water quality criteria (RWQC). These 2012
criteria were based on the latest research and science at the time including epidemiology studies
conducted in the 2000s. The criteria are designed to protect the public from exposure to harmful
levels of pathogens while participating in water-contact activities in recreational waters. An
important goal of the five-year review is to document the assessment of whether revisions to the
2012 RWQC are necessary. A key component of that assessment is a consideration of the state of
the science and advances made since the previous five-year review that support the
recommended RWQC and enhance its implementation. The EPA’s RWQC Review will be the
second five-year review. Systematic literature search and review will be conducted on five topics
relevant to the 2
nd
five-year RWQC Review:
1. Advances in molecular microbial source tracking (MST) for recreational water
applications (supports Section III.E of this report).
2. Advances in molecular methods (supports Section III.F of this report).
3. Advances in quantitative microbial risk assessment (QMRA) and epidemiological studies
(supports Sections III.A.1, III.A.2, III.A.3 of this report).
4. Characterization of children’s risk from exposure to recreational water contaminated by
fecal contamination (supports Sections III.A.4 of this report).
5. Cyanotoxins: microcystins, cylindrospermopsin, and anatoxin-a (supports Section III.C
of this report).
This document includes the literature search strategies for the above topics. Each of the topic
areas have associated focus areas and research questions. The literature search for Topics 1 and 2
were conducted in a single effort as they are closely related by focusing on methods. In addition,
articles that were relevant for more than one topic area, were cross-tagged between topics, except
for cyanotoxins which was conducted under a separate effort.
Systematic Approach
The basic steps of this systemic review include:
1) Planning: scoping, research questions, keywords, title/abstract (Ti/Abs) eligibility criteria
2) Literature search
3) Prioritization, clustering (optional depending on how many Ti/Abs obtained)
4) Ti/Abs screening (optional machine learning)
5) Literature retrieval
6) Study quality metrics evaluation

A-2
7) Data extraction
8) Data analysis and visualization
This document includes the literature search and the eligibility (inclusion/exclusion) criteria for
Ti/Abs screening and study quality metrics.
2. Topics 1 and 2: Methodological Progress
This search strategy specifically focuses on 1) advances in MST (or fecal source identification
and bacterial source tracking) for recreational water applications, and 2) advances on molecular
methods. The first topic includes advances in method standardization, standard control or
reference materials, water sample inhibition or matrix interference, genetic markers, field studies
to identify and/or remediate fecal sources. The second topic focuses on advances in the
development of quantitative polymerase chain reaction (qPCR), digital polymerase chain
reaction (PCR), reverse transcription PCR (RT-PCR), next generation sequencing, metagenomic
deoxyribonucleic acid (DNA) sequencing, and hypervariable 16S ribosomal ribonucleic acid
(RNA) gene sequencing used in recreational waters to measure indicators of fecal contamination.
Focus Areas:
• Advances in method standardization.
• Development of standard control or reference materials.
• Occurrence of amplification inhibition and/or matrix interference.
• Statistical analysis or other approaches to address non-detects or results below the level
of quantification.
• Field studies to identify and/or remediate fecal sources.
• Advances in the development of qPCR, digital PCR, RT-PCR, next generation
sequencing, metagenomic DNA sequencing, and hypervariable 16S ribosomal RNA gene
sequencing used in recreational waters to measure indicators of fecal contamination,
pathogen indicators or waterborne pathogens.
• Advances in non-molecular yet novel approaches to culture methods and combinations of
FIB.
• Data interpretation statistical/modeling approaches (fecal score, QMRA, censored data).
Research Question: What methodological (tools, markers, approaches) advances have been made
since 2016 in the area of recreational surface water-based monitoring (e.g., fecal indicator
organisms, pathogens, or MST) including method standardization (standards or reference
material) and controls (inhibition or quality control)?
Literature Search Strategy for Topics 1 and 2: Methodological Progress
The searches are limited to the English language only.
Databases for searches include PubMed (terms searched in All Fields) and Web of Science
(terms searched in Topic).

A-3
Dates: January 2016 to November 9, 2021 (described below)
Keywords were tested to develop the strategies.
Topic 1 and 2: Methodological Progress Keyword Test Summary
Search Date Date Limit Search Strategy Sets
Number of Title/Abstracts
PubMed WoS
Unique
Total
November 9, 2021
January 2016–search date
Organism AND Water AND
Detail AND Methodology
570
326
717
Topic 1 and 2: Database Search: PubMed
Date of Search: November 9, 2021 (range January 2016 through November 9, 2021)
Fields Searched: All Fields
Limits: English Only; January 1, 2016, November 9, 2021
Set Name Search Strategy for PubMed
Results (Number of
Titles)
Organism (“microbial source tracking” OR “MST” OR “microbial” OR
“microorganism*” OR “fecal source identification” OR “bacterial
source tracking” OR “indicator*” OR “fecal source tracking” OR
“HF183” OR “fecal pollution” OR “faecal pollution” OR “fecal
contamination” OR “faecal contamination” OR
“Feces/microbiology”[MeSH])
378,129
Water (“seawater*” OR “beach water*” OR “coastal water*” OR
“recreational water*” OR “environmental water*” OR “source water*”
OR “water quality” OR “water data” OR “water microbiology”)
36,185
Detail (“amplification inhibition” OR “matrix interference” OR “biomarker*”
OR “qPCR” OR “quantitative polymerase chain reaction” OR “RT-
qPCR” OR “real-time reverse transcription polymerase chain reaction”
OR “real-time reverse transcription PCR” OR “real time reverse
transcription polymerase chain reaction” OR “real time reverse
transcription PCR” OR “real-time PCR” OR “quantitative PCR” OR
“Real-Time Polymerase Chain Reaction” OR “dPCR” OR “digital
PCR” OR “digital polymerase chain reaction” OR “ddPCR” OR
“droplet digital PCR” OR “droplet digital polymerase chain reaction”
OR “metagenomic” OR “16S RNA” OR “genetic sequencing” OR
“censored data” OR “below limit of quantification” OR “statistical
analysis” OR “statistical analyses” OR “water quality analysis” OR
“microbial biosensor*”)
481,175
Methodology (“method” OR “methods”) 3,088,876
Total (Organism AND Water AND Detail AND Methodology) 570

A-4
Screenshot of Topic 1 and Topic 2 PubMed Search
Topic 1 and 2: Database Search: Web of Science
Date of Search: November 9, 2021 (range 2016 through 2021)
Fields Searched: Topic
Limits: English Only; 2016 to 2021
Set Name Search Strategy for Web of Science
Results (Number of
Titles)
Organism (“microbial source tracking” OR “MST” OR “microbial” OR
“microorganism*” OR “fecal source identification” OR “bacterial
source tracking” OR “indicator*” OR “fecal source tracking” OR
“HF183” OR “fecal pollution” OR “faecal pollution” OR “fecal
contamination” OR “faecal contamination”)
388,870
Water (“seawater*” OR “beach water*” OR “coastal water*” OR
“recreational water*” OR “environmental water*” OR “source water*”
OR “water quality” OR “water data” OR “water microbiology”)
76,157

A-5
Set Name Search Strategy for Web of Science
Results (Number of
Titles)
Detail
(“amplification inhibition” OR “matrix interference” OR “biomarker*”
OR “qPCR” OR “quantitative polymerase chain reaction” OR “RT-
qPCR” OR “real-time reverse transcription polymerase chain reaction”
OR “real-time reverse transcription PCR” OR “real time reverse
transcription polymerase chain reaction” OR “real time reverse
transcription PCR” OR “real-time PCR” OR “quantitative PCR” OR
“Real-Time Polymerase Chain Reaction” OR “dPCR” OR “digital
PCR” OR “digital polymerase chain reaction” OR “ddPCR” OR
“droplet digital PCR” OR “droplet digital polymerase chain reaction”
OR “metagenomic” OR “16S RNA” OR “genetic sequencing” OR
“censored data” OR “below limit of quantification” OR “statistical
analysis” OR “statistical analyses” OR “water quality analysis” OR
“microbial biosensor*”)
423,029
Methodology (“method” OR “methods”) 3,573,911
Total (Organism AND Water AND Detail AND Methodology) 326
Screenshot of Web of Science Topic 1 and Topic 2 Search

A-6
Topic 1 and 2: Gray Literature Search
Date of Search: November 9, 2021
NOTE: Google uses strict character limits. Strings containing more than 32 words are not
allowed.
Organization URL Keywords /Search string
Results
(Duplicates
Removed)
FDA FDA.gov
site:fda.gov AND (“microbial source” OR
“MST” OR “fecal source” OR “bacterial
source” OR “HF183” OR “fecal pollution”)
AND (“standard” OR “reference” OR
“inhibition” OR “quality” OR “PCR” OR
“indicator*”) AND (water OR “beach”) AND
(“PCR” OR “polymerase chain reaction” OR
metagenomic OR “censored data” OR
“genetic sequencing” OR “statistical
analysis”)
14
NPS NPS.gov
site:nps.gov AND (“microbial source” OR
“MST” OR “fecal source” OR “bacterial
source” OR “HF183” OR “fecal pollution”)
AND (“standard” OR “reference” OR
“inhibition” OR “quality” OR “PCR” OR
“indicator*”) AND (water OR “beach”) AND
(“PCR” OR “polymerase chain reaction” OR
metagenomic OR “censored data” OR
“genetic sequencing” OR “statistical
analysis”)
3
FWS FWS.gov
site:fws.gov AND (“microbial source” OR
“MST” OR “fecal source” OR “bacterial
source” OR “HF183” OR “fecal pollution”)
AND (“standard” OR “reference” OR
“inhibition” OR “quality” OR “PCR” OR
“indicator*”) AND (water OR “beach”) AND
(“PCR” OR “polymerase chain reaction” OR
metagenomic OR “censored data” OR
“genetic sequencing” OR “statistical
analysis”)
2
NAS Nap.edu
site:nap.edu AND (“microbial source” OR
“MST” OR “fecal source” OR “bacterial
source” OR “HF183” OR “fecal pollution”)
AND (“standard” OR “reference” OR
“inhibition” OR “quality” OR “PCR” OR
“indicator*”) AND (water OR “beach”) AND
(“PCR” OR “polymerase chain reaction” OR
metagenomic OR “censored data” OR
“genetic sequencing” OR “statistical
analysis”)
4
WHO Who.int
site:who.int AND (“microbial source” OR
“MST” OR “fecal source” OR “bacterial
source” OR “HF183” OR “fecal pollution”)
AND (“standard” OR “reference” OR
“inhibition” OR “quality” OR “PCR” OR
17

A-7
Organization URL Keywords /Search string
Results
(Duplicates
Removed)
“indicator*”) AND (water OR “beach”) AND
(“PCR” OR “polymerase chain reaction” OR
metagenomic OR “censored data” OR
“genetic sequencing” OR “statistical
analysis”)
TOTAL
40
Date Limited: 2016 to November 9, 2021 [Present]
Topic 1 and 2: Title/Abstract Screening Criteria and Tagging
Two separate individuals screened and applied the following tags to each title/abstract. In cases
where screener 1 and 2 disagreed a subject matter expert provided conflict resolution and
determined the final tagging result. Each title/abstract was tagged as follows:
Screening Tag Description of Tag
Top Level Tags – choose one [include, unclear, or exclude]:
Include Topic 1 Obtain full text, advance to full text screening (see topic description below).
Include Topic 2 Obtain full text, advance to full text screening (see topic description below).
Unclear
Requires full text to determine whether within scope. Unclear if applicable to
recreational waters, for example, drinking water, groundwater, wastewater
studies. OK to include “other possible tags.”
Exclude
Do not obtain full text. Not within scope. OK to include notes. OK to include
“other possible tags.”
If Include – choose all that apply [these four tags only apply if ‘include’]:
MST (Topic 1)
• Advances in method standardization related to MST.
• Development of standard control or reference materials for MST.
• Occurrence of amplification inhibition and/or matrix interference for
methods used for MST.
• Field studies to identify and/or remediate fecal sources.
• Risk assessment with MST genetic markers (epidemiological studies,
QMRA).
• In Scope:
MST in natural ambient surface water samples.
HF183 (human fecal marker), markers for livestock, wildlife,
other sources of fecal contamination.
Review Articles about MST data or methods.
• NOT in Scope:
MST for other water types: drinking water, ground water,
wastewater.

A-8
Screening Tag Description of Tag
MST that does not use to microbial markers (e.g., caffeine,
detergent brighteners, plant viruses, other indicators of water
quality such as dissolved oxygen, turbidity).
Molecular Methods (Topic 2)
• Advances in the development of qPCR, digital PCR, rt-PCR, next
generation sequencing, metagenomic DNA sequencing, and hypervariable
16S RNA gene sequencing used in recreational waters to measure
indicators of fecal contamination, pathogen indicators or waterborn
e
pa
thogens.
• Advances in statistics/modeling for data interpretation (fecal score, QMRA,
censored data, etc.).
• In Scope:
Methods applied to natural ambient surface water samples.
Methods that detect (presence/absence) or enumerate (quantify)
pathogens or indicators (including but not limited to fecal
coliform, enterococci, E. coli, coliphage, norovirus,
Cryptosporidium, Giardia).
Methods that use spiked samples if the method is for enumeratin
g
microbes in ambient waters.
Review articles for methods.
• NOT in Scope:
Methods for drinking water, ground water, wastewater.
Methods that do not pertain to microbes (e.g., caffeine, detergent
brighteners, plant viruses, other indicators of water quality such as
dissolved oxygen, turbidity).
Priority or Review (for Topic 1)
• Articles that provide a review of one of the major topics will be prioritized.
Other papers that seem particularly of interest and should be prioritized can
also have this tag. Use judgment with this tag. Not all review articles need
to have this tag—just the review articles that are particularly on point.
Priority or Review (for Topic 2)
• Articles that provide a review of one of the major topics will be prioritized.
Other papers that seem particularly of interest and should be prioritized can
also have this tag. Use judgment with this tag. Not all review articles need
to have this tag—just the review articles that are particularly on point.
Other Possible Tags – choose all that apply [Ti/Abs indicates the paper may contain information on]:
QMRA or Epi (Topic 3)
• Advances in QMRA and epidemiological studies in analyzing indicators of
fecal contamination (specifically: coliform bacteria, fecal streptococci,
anaerobic bacteria) in recreational waters.
• Do not include if only bacteriophages.
Children’s risk (Topic 4)
• Health effects that children experience following exposure to microbially
contaminated surface waters, including illness rates/health outcomes from
outbreak reports, epidemiological or other health studies.
Explanation – Free text notes
field
• *Optional additional keywords including but not limited to: antimicrobial
resistance (AMR), combined sewer overflows (CSO), sanitary sewer
overflow (SSO), somatic coliphage, male-specific coliphage (MSC), F-
specific coliphage (MSC).
• Additional notes about the paper.
A-9
Topic 1: Study Quality Evaluation
Articles that were ‘include’ or ‘unclear’ moved to full text screening. The full text was reviewed
for scope to confirm the article is within the scope. Articles that pass scope were screened for
study quality and articles that pass study quality were qualified for data extraction.
Scope
Is the study within the Topic 1 scope? Does it include information on microbial source tracking
in recreational waters?
• Yes – continue with study quality.
• No – stop evaluation.
Study Quality Metrics – If Any of the Metrics Are Unacceptable, the Study Was Not
Included for Data Extraction.
Sampling Methodology:
• Acceptable – Sampling is described or referenced via a citation. Information may include
equipment, sampling depth, time of day, process/reasons for selection of sampling sites.
Sampling location map is best, but not required. Site is described, including at a
minimum the water type (fresh or marine).
• Unacceptable – If any of the following is missing, rate as unacceptable: date of sampling,
water type (matrix), sample storage before assay. Not enough information is included to
evaluate the sampling or the sampling procedures have critical problems.
Analytical Methodology:
• Acceptable – Methodology is well described. Information may include
filtration/concentration and extraction methods, analytical equipment, reagents,
instrument calibration, level of detection (LOD), level of quantification (LOQ), controls,
standards, recovery, matrix adjustments, cycling protocol, primers.
• Unacceptable – Controls are not described and it is unclear whether controls were
conducted. Or not enough information is included to evaluate the method.
Spatial and Temporal Variability:
• Acceptable – sample size is more than one. Replicates and multiple timepoints may be
included.
• Unacceptable – Sample size is not reported or only one sample was taken or sample size
is unclear or indecipherable for the summary statistics.
Reporting of Results:
A-10
• Acceptable – Raw data are included (check supplemental materials) or summary results
are clearly described, such as GM, arithmetic mean, SD, SE, median, and other
percentiles. Frequency of detection is reported. Variability and uncertainty are
discussed/captured in summary statistics.
• Unacceptable – Samples described in the methods are not reported in the results or vice
versa. Unclear whether summary data include non-detects.
Quality Assurance:
• Acceptable – Quality assurance is discussed or can be implied given discussion of
controls and recoveries.
• Unacceptable – Not enough information is included to evaluate whether quality assurance
(QA) was an issue, or QA issues have been identified that interfere with the reliability of
the study, or the study is a non-peer-reviewed publication.
Topic 2: Study Quality Evaluation
Articles that were ‘include’ or ‘unclear’ moved to full text screening. The full text was reviewed
for scope to confirm the article is within the scope. Articles that pass scope were screened for
study quality and articles that pass study quality were qualified for data extraction.
Scope
Is the study within the Topic 2 scope? Does it include information on methods for measuring
indicators (e.g., E. coli, enterococci, coliphages) or pathogens in recreational waters?
• Yes – continue with study quality.
• No – stop evaluation.
Study Quality Metrics – If Any of the Metrics Are Unacceptable, the Study Was Not
Included for Data Extraction.
Sampling Methodology:
• Acceptable – Sampling is described or referenced via a citation. Information may include
equipment, sampling depth, time of day, process/reasons for selection of sampling sites.
Sampling location map is best, but not required. Site is described, including at a
minimum the water type (fresh or marine).
• Unacceptable – If any of the following is missing, rate the study as unacceptable: date of
sampling (including the range of sampling dates), water type, sample storage before
assay. Not enough information is included to evaluate the sampling, or the sampling
procedures have critical problems.
A-11
Analytical Methodology:
• Acceptable – Methodology is well described. Information provided may include
filtration/concentration and extraction methods, analytical equipment, reagents,
instrument calibration, LOD, LOQ, controls, standards, recovery, matrix adjustments,
cycling protocol, primers.
• Unacceptable – Controls are not described, it is unclear whether controls were conducted,
or not enough information is included to evaluate the methodology.
Spatial and Temporal Variability:
• Acceptable – Sample size is more than one. Replicates and multiple timepoints may be
included.
• Unacceptable – Sample size is not reported, or only one sample was taken, or sample size
is unclear or indecipherable for the summary statistics.
Reporting of Results:
• Acceptable – Raw data are included (check supplemental materials) or summary results
are clearly described, such as geometric mean (GM), arithmetic mean, standard deviation
(SD), standard error (SE), median, and other percentiles. Frequency of detection is
reported. Variability and uncertainty are discussed/captured in summary statistics.
• Unacceptable – Samples described in the methods are not reported in the results or vice
versa. Unclear whether summary data include non-detects (i.e., an analytical sample
where the concentration is deemed to be lower than could be detected using the method
employed by the laboratory).
Quality Assurance:
• Acceptable – QA is discussed or can be implied given discussion of controls and
recoveries.
• Unacceptable – Not enough information is included to evaluate whether QA was an issue,
or QA issues have been identified that interfere with the reliability of the study, or the
study is a non-peer-reviewed publication.

A-12
Topic 1: PRISMA Diagram

A-13
Topic 2: PRISMA Diagram

A-14
3. Topic 3: Quantitative Microbial Risk Assessment (QMRA) and Epidemiological Studies
This topic focuses on advances estimating human health risks from exposure to fecal
contamination using QMRA and findings from epidemiological studies in measuring indicators
of fecal contamination except bacteriophages (e.g., coliform bacteria, fecal streptococci,
anaerobic bacteria, pathogens) in recreational waters. The search will also determine if
epidemiological studies and QMRA measured for exposure and health effects endpoints, as well
as for indicators of fecal contamination, MST, or pathogens. This systematic literature review
will be conducted from January 2016 to November 22, 2021.
Focus Areas:
• Advances in risk characterization
• Parameter selection for exposure and health outcomes assessments
• Approaches used to describe microbial distributions
• Approaches used to characterize the source(s) of pathogens or indicators e.g., MST
Research Question: What advances have been made in estimating human health risks from
exposure to fecal contamination in recreational waters using QMRA and epidemiological
studies?
Literature Search Strategy for Topic 3: Quantitative Microbial Risk Assessment (QMRA) and
Epidemiological Studies
The searches are limited the language to English only.
Databases for searches include: PubMed (terms searched in All Fields) and Web of Science
(terms searched in Topic)
Dates: January 2016 to November 22, 2021 [present]
Keywords were tested to develop the strategies.
Topic 3: Quantitative Microbial Risk Assessment (QMRA) and Epidemiological Studies
Keyword Test Summary
Search Date Date Limit Search Strategy Sets
Number of Title/Abstracts
PubMed WoS
Unique
Total
November 22, 2021
January 2016–search date
Organism AND Source AND
Water AND Detail
777
976
1293
A-15
Topic 3 Database Search: PubMed
Date of Search: November 22, 2021 (range January 2016 through November 22, 2021)
Fields Searched: All Fields
Limits: English Only; January 1, 2016, to November 22, 2021
Set Name Search Strategy for PubMed
Results (Number of
Titles)
Organism ((“quantitative microbial risk assessment” OR “QMRA” OR
“microbial” OR “antimicrobial resistant” OR “epidemiol*” OR
“prospective cohort*” OR “randomized control trial” OR “randomized
controlled trial” OR “longitudinal” OR “case control” OR “case-
control” OR “surveillance” OR “outbreak” OR “Epidemiology”[Mesh]
OR “Molecular Epidemiology”[Mesh]) NOT (“toxicology” OR
“animal study” OR “animal studies”))
1,296,600
Source (“fecal contamination” OR “feces” OR “fecal source” OR
“contaminat*” OR “contaminant*” OR “faecal” OR “untreated” OR
“bacteria*”)
492,062
Water (“recreational water*” OR “surface water” OR “beach” OR “coastal
water*”)
20,643
Detail (“Exposure” OR “ingestion” OR “dermal” OR” inhalation” OR “Dose
response” OR” reference pathogen” OR “pathogen” OR “Infection”
OR “illness” OR “morbidity” OR “gastrointestinal” OR “respiratory”
OR “non-detects” OR “data replacement” OR “limit of detection” OR
“level of quantitation” OR “pathogen*” OR “indicator*” OR “health
effect*” OR “risk*”)
Quoted phrase not found: “level of quantitation”
2,435,377
Total (Organism AND Source AND Water AND Detail) 777

A-16
Screenshot of Topic 3 PubMed Search

A-17
Topic 3 Database Search: Web of Science
Date of Search: November 22, 2021 (range 2016 through 2021)
Fields Searched: Topic
Limits: English Only; 2016 to 2021
Search run via Google Scraper Tool
Set Name Search Strategy for Web of Science
Results (Number of
Titles)
Organism ((“quantitative microbial risk assessment” OR “QMRA” OR
“microbial” OR “antimicrobial resistant” OR “epidemiol*” OR
“prospective cohort*” OR “randomized control trial” OR “randomized
controlled trial” OR “longitudinal” OR “case control” OR “case-
control” OR “surveillance” OR “outbreak” OR “Molecular
Epidemiology”) NOT (“toxicology” OR “animal study” OR “animal
studies”))
747,925
Source
(“fecal contamination” OR “feces” OR “fecal source” OR
“contaminat*” OR “contaminant*” OR “faecal” OR “untreated” OR
“bacteria*”)
538,995
Water (“recreational water*” OR “surface water” OR “beach” OR “coastal
water*”)
38,874

A-18
Set Name Search Strategy for Web of Science
Results (Number of
Titles)
Detail
(“Exposure” OR “ingestion” OR “dermal” OR” inhalation” OR “Dose
response” OR” reference pathogen” OR “pathogen” OR “Infection”
OR “illness” OR “morbidity” OR “gastrointestinal” OR “respiratory”
OR “non-detects” OR “data replacement” OR “limit of detection” OR
“level of quantitation” OR “pathogen*” OR “indicator*” OR “health
effect*” OR “risk*”)
2,553,267
Total
(Organism AND Source AND Water AND Detail)
976
Screenshot of Web of Science Topic 3 Search

A-19
Topic 3 Gray Literature Search
Date of Search: November 19, 2021
Limits: 2016 to 2021; PDFs only
NOTE: Google uses strict character limits. Strings containing more than 32 words are not
allowed.
Organization URL Keywords /Search string
Results
(Duplicates
Removed)
FDA FDA.gov
site:fda.gov AND (“risk assessment” OR
“QMRA” OR “epidemiol*” OR “cohort”
“longitudinal” OR “outbreak”) AND (Feces
OR fecal) AND (“recreational water”) AND
(“Exposure” OR “ingestion” OR “dermal” OR
”inhalation” OR “Dose response” OR
“Infection” OR “illness” OR “morbidity” OR
“gastrointestinal” OR “respiratory” OR
“detection” OR “pathogen*” OR “indicator*”
OR “health effect”) NOT (“toxicology”)
52
NPS NPS.gov
site:nps.gov AND (“risk assessment” OR
“QMRA” OR “epidemiol*” OR “cohort”
“longitudinal” OR “outbreak”) AND (Feces
OR fecal) AND (“recreational water”) AND
(“Exposure” OR “ingestion” OR “dermal” OR
”inhalation” OR “Dose response” OR
“Infection” OR “illness” OR “morbidity” OR
“gastrointestinal” OR “respiratory” OR
“detection” OR “pathogen*” OR “indicator*”
OR “health effect”) NOT (“toxicology”)
2
FWS Fws.gov
site:fws.gov AND (“risk assessment” OR
“QMRA” OR “epidemiol*” OR “cohort”
“longitudinal” OR “outbreak”) AND (Feces
OR fecal) AND (“recreational water”) AND
(“Exposure” OR “ingestion” OR “dermal” OR
”inhalation” OR “Dose response” OR
“Infection” OR “illness” OR “morbidity” OR
“gastrointestinal” OR “respiratory” OR
“detection” OR “pathogen*” OR “indicator*”
OR “health effect”) NOT (“toxicology”)
1
NAS Nap.edu
Site:nap.edu AND (“risk assessment” OR
“QMRA” OR “epidemiol*” OR “cohort”
“longitudinal” OR “outbreak”) AND (Feces
OR fecal) AND (“recreational water”) AND
(“Exposure” OR “ingestion” OR “dermal” OR
”inhalation” OR “Dose response” OR
“Infection” OR “illness” OR “morbidity” OR
“gastrointestinal” OR “respiratory” OR
“detection” OR “pathogen*” OR “indicator*”
OR “health effect”) NOT (“toxicology”)
0
WHO Who.int
Site:who.int AND (“risk assessment” OR
“QMRA” OR “epidemiol*” OR “cohort”
“longitudinal” OR “outbreak”) AND (Feces
OR fecal) AND (“recreational water”) AND
6

A-20
Organization URL Keywords /Search string
Results
(Duplicates
Removed)
(“Exposure” OR “ingestion” OR “dermal” OR
”inhalation” OR “Dose response” OR
“Infection” OR “illness” OR “morbidity” OR
“gastrointestinal” OR “respiratory” OR
“detection” OR “pathogen*” OR “indicator*”
OR “health effect”) NOT (“toxicology”)
Total All domains Same search in each domain 61
Topic 3 Prioritization of Ti/Abs for Screening
Using prioritization tools built into litstream were used to evaluate the Ti/Abs that were returned
from the above data searches.
Supervised clustering litstream tools were used to predict whether the Ti/Abs are of interest
based on six different algorithms. Seed papers feed into the prediction algorithm.
Seed papers for Topic 3 included:
1. Abdelzaher, A.M., Wright, M.E., Ortega, C., Rasem Hasan, A., Shibata, T., Solo-
Gabrieie, H.-M., Kish, J., Withum, K., He, G., Elmir, S.M., Bonilla, J.A., Bonilla, T.D.,
Palmer, C.J., Scott, T.M., Lukasik, J., Harwood, V.J., McQuaig, S., Sinigalliano, C.D.,
Gidley, M.L., Wanless, D., Plano, L.R., Garza, A.C., Zhu, X., Stewart, J.R., Dickerson,
J.W., Yampara-Iquise, H., Carson, C., Fleisher, J.M., Fleming, L.E. 2011. Daily measures
of microbes and human health at a non-point source marine beach. Journal of Water and
Health, 9(3): 443-457.
2. Ashbolt, N.J., Bruno, M. 2003. Application and refinement of the WHO risk framework
for recreational waters in Sydney Australia. Journal of Water and Health, 1(3): 125-131.
3. Ashbolt, N.J., Schoen, M.E., Soller, J.A., Roser, D.J. 2010. Predicting pathogen risks to
aid beach management: The real value of quantitative microbial risk assessment
(QMRA). Water Research, 44(16): 4692-4703.
4. Boehm, A.B., Soller, J.A., Shanks, O.C. 2015. Human-associated fecal quantitative
polymerase chain reaction measurements and simulated risk of gastrointestinal illness in
recreational waters contaminated with raw sewage. Environmental Science &
Technology Letters, 2(10): 270-275.
5. Boehm, A.B., Graham, K.E., Jennings, W.C. 2018. Can We Swim Yet? Systematic
Review, Meta-Analysis, and Risk Assessment of Aging Sewage in Surface Waters.
Environ Sci Technol. 52(17):9634-9645.
6. Colford, J., Wade, T., Sandhu, S., Wright, C., Lee, S., Shaw, S., Fox, K., Burns, S.,
Benker, A., Brookhart, M., van der Laan, M., Levy, D. 2005. A randomized control trial
of in-home drinking water intervention to reduce gastrointestinal illness. American
Journal of Epidemiology 161(5): 472-82.
A-21
7. Colford, J.M., Jr., Wade, T.J., Schiff, K.C., Wright, C.C., Griffith, J.F., Sandhu, S.K.,
Burns, S., Sobsey, M., Lovelace, G., Weisberg, S.B., 2007. Water quality indicators and
the risk of illness at beaches with nonpoint sources of fecal contamination. Epidemiology
18(1): 27-35.
8. Colford, J., Schiff, K.C., Griffith, J.F., Yau, V., Arnold, B.F., Wright, C.C., Gruber, J.S.,
Wade, T.J., Burns, S., Hayes, J., McGee, C., Gold, M., Cao, Y., Noble, R.T., Haugland,
R., Weisberg, S.B. 2012. Using rapid indicators for Enterococcus to assess the risk of
illness after exposure to urban runoff contaminated marine water. Water Research 46:
2176-2186.
9. Fleisher, J.M., Kay, D., Salmon, R.L., Jones, F., Wyer, M.D., Godfree, A.F. 1996.
Marine waters contaminated with domestic sewage: Nonenteric illnesses associated with
bather exposure in the United Kingdom. American Journal of Public Health 86(9): 1228-
1234
10. Fleisher, J.M., Fleming, L.E., Solo-Gabriele, H.M., Kish, J.K., Sinigalliano, C.D., Plano,
L., Elmir, S.M., Wang, J.D., Withum, K., Shibata, T., Gidley, M.L., Abdelzaher, A., He,
G., Ortega, C., Zhu, X., Wright, M., Hollenbeck, J., Backer, L.C. 2010. The BEACHES
Study: Health effects and exposures from non-point source microbial contaminants in
subtropical recreational marine waters. International Journal of Epidemiology 39(5):
1291-1298.
11. Kay, D., Fleisher, J.M., Salmon, R.L., Jones, F., Wyer, M.D., Godfree, A.F., Zelenauch-
Jacquotte, Z., Shore, R. 1994. Predicting likelihood of gastroenteritis from sea bathing:
Results from randomised exposure. Lancet 344(8927): 905-909.
12. Lamparelli, C.C., Pogreba-Brown, K., Verhougstraete, M., Zanoli Sato, M.I.Z., de Castro
Bruni, A., Wade, T.J., Eisenberg, J.N.S. 2015. Are fecal indicator bacteria appropriate
measures of recreational water risks in the tropics: A cohort study of beach goers in
Brazil? Water Research 87:59-68.
13. Marion, J.W., Lee, C., Lee, C.S., Wang,Q., Lemeshow, S., Buckley, T.J., Saif, L.J., Lee,
J. 2014. Integrating Bacterial and Viral Water Quality Assessment to Predict Swimming-
Associated Illness at a Freshwater Beach: A Cohort Study. PloS ONE 9(11): e112029.
Doi:10.1371/journal.pone.0112029
14. McBride, G.B., Stott, R., Miller, W., Bambic, D., Wuertz, S. 2013. Discharge-based
QMRA for estimation of public health risks from exposure to stormwater-borne
pathogens in recreational waters in the United States. Water Research, 47:5282-5297.
15. Rijal, G., Tolson, J., Petropoulou, C., Granato, T., Glymph, A., Gerba, C., DeFlaun, M.,
O’Connor, C., Kollias, L., Lanyon, R. 2011. Microbial risk assessment for recreational
use of the Chicago Area Waterway System. Journal of Water and Health, 9(1): 169-186.
16. Sales-Ortells, H., Medema, G. 2014. Screening-level microbial risk assessment of urban
water locations: A tool for prioritization. Environmental Science & Technology, 48(16):
9780-9789.
17. Schets, F.M., Schijven, J.F., de Roda Husman, A.M. 2011. Exposure assessment for
swimmers in bathing waters and swimming pools. Water Research. 45:2392-2400.
18. Schijven, J., de Roda Husman, A.M. 2006. A survey of diving behavior and accidental
ingestion among Dutch occupational and sport divers to assess the risk of infection with
A-22
waterborne pathogenic microorganisms. Environmental Health Perspectives, 114(5): 712-
717.
19. Schoen, M.E., Ashbolt, N.J. 2010. Assessing pathogen risk to swimmers at non-sewage
impacted recreational beaches. Environmental Science & Technology, 44: 2286-2291.
20. Schoen, M.E., Soller, J.A., Ashbolt, N.J. 2011. Evaluating the importance of faecal
sources in human-impacted waters. Water Research, 45: 2670-2680.
21. Sinigalliano, C.D., Fleisher, J.M., Gidley, M.L., Solo-Gabriele, H.M., Shibata, T., Plano,
L.R.W., Elmir, S.M., Wanless, D., Bartkowiak, J., Boiteau, R., Withum, K., Abdelzaher,
A.M., He, G., Ortega, C., Zhu, X., Wright, M.E., Kish, J., Hollenbeck, J., Scott, T.,
Backer, L.C., Fleming, L.E. 2010. Traditional and molecular analyses for fecal indicator
bacteria in non-point source subtropical recreational marine waters. Water Research 44:
3763-3772.
22. Soller, J.A., Olivieri, A., Crook, J., Parkin, R., Spear, R., Tchobanoglous, G., Eisenberg,
J.N.S. 2003. Risk-based approach to evaluate the public health benefit of additional
wastewater treatment. Environmental Science & Technology, 37(9): 1882-1891.
23. Soller, J.A., Eisenberg, J.N.S., DeGeorge, J.F., Cooper, R.C., Tchobanoglous, G.,
Olivieri, A.W. 2006. A public health evaluation of recreational water impairment. Journal
of Water and Health, 4(1): 1-19.
24. Soller, J.A., Bartrand, T., Ashbolt, N.J., Ravenscroft, J., Wade, T.J. 2010a. Estimating the
primary etiologic agents in recreational freshwaters impacted by human sources of faecal
contamination. Water Research, 44(16): 4736-4747.
25. Soller, J.A., Schoen, M.E., Bartrand, T., Ravenscroft, J., Wade, T.J. 2010b. Estimated
human health risks from exposure to recreational waters impacted by human and non-
human sources of faecal contamination. Water Research, 44(16): 4674-4691.
26. Soller, J.A., Schoen, M.E., Varghese, A., Ichida, A.M., Boehm, A.B., Eftim, S., Ashbolt,
N.J., Ravenscroft, J.E. 2014. Human health risk implications of multiple sources of faecal
indicator bacteria in a recreational waterbody. Water Research, 66:254–264.
27. Soller, J., Bartrand, T., Ravenscroft, J., Molina, M., Whelan, G., Schoen, M., Ashbolt, N.
2015. Estimated Human Health Risks from Recreational Exposures to Stormwater
Runoff Containing Animal Faecal Material, Environmental Modelling & Software,
72:21-32.
28. Soller, J.A., Eftim, S., Wade, T.J., Ichida, A.M., Clancy, J.L., Johnson, T.B., Schwab, K.,
Ramirez-Toro, G., Nappier, S., Ravenscroft, J.E. 2016. Use of quantitative microbial risk
assessment to improve interpretation of a recreational water epidemiological study.
Microbial Risk Analysis.1:2-11.
29. Soller, J.A., Schoen, M., Steele, J.A., Griffith, J.F., Schiff, K.C. 2017. Incidence of
gastrointestinal illness following wet weather recreational exposures: Harmonization of
quantitative microbial risk assessment with an epidemiologic investigation of surfers.
Water Research 121:280-289.
30. Sunger, N., Hamilton, K.A., Morgan, P.M., Haas, C.N. 2018. Comparison of pathogen-
derived ‘total risk’ with indicator-based correlations for recreational (swimming)
exposure. Environmental Science and Pollution Research International. Doi:
10.1007/s11356-018-1881-x. [Epub ahead of print]
A-23
31. Tseng, L.Y., Jiang, S.C. 2012. Comparison of recreational health risks associated with
surfing and swimming in dry weather and post-storm conditions at Southern California
beaches using quantitative microbial risk assessment (QMRA). Marine Pollution
Bulletin, 64: 912-918.
32. Viau, E.J., Lee, D., Boehm, A.B. 2011. Swimmer risk of gastrointestinal illness from
exposure to tropical coastal waters impacted by terrestrial dry-weather runoff.
Environmental Science & Technology, 45: 7158-7165.
33. Wade, T.J., Calderon, R.L., Sams, E., Beach, M., Brenner, K.P., Williams, A.H., Dufour,
A.P. 2006. Rapidly measured indicators of recreational water quality are predictive of
swimming-associated gastrointestinal illness. Environmental Health Perspectives 114(1):
24-28.
34. Wade, T.J., Calderon, R.L., Brenner, K.P., Sams, E., Beach, M., Haugland, R., Wymer,
L., Dufour, A.P. 2008. High sensitivity of children to swimming-associated
gastrointestinal illness – results using a rapid assay of recreational water quality.
Epidemiology 19(3): 375-383.
35. Wade, T.J., Sams, E., Brenner, K.P., Haugland, R., Chern, E., Beach, M., Wymer, L.,
Rankin, C.C., Love, D., Li, Q., Noble, R., Dufour, A.P. 2010. Rapidly measured
indicators or recreational water quality and swimming-associated illness at marine
beaches: A prospective cohort study. Environmental Health 9: 66.
36. Wiedenmann, A., Krüger, P., Dietz, K., López-Pila, J.M., Szewzyk, R., Botzenhart, K.
2006. A randomized controlled trial assessing infectious disease risks from bathing in
fresh recreational waters in relation to the concentration of E. coli, intestinal enterococci,
Clostridium perfringens, and somatic coliphages. Environmental Health Perspectives
114(2): 228-236.
37. Yau, V., Schiff, K., Arnold, B., Griffith, J., Gruber, J., Wright, C., Wade, T., Burns, S.,
Hayes, J., McGee, C., Gold, M., Cao, Y., Boehm, A., Weisber, S., Colford, J. 2014.
Effect of submarine groundwater discharge on bacterial indicators and swimmer health at
Avalon Beach, California, USA. Water Research 59: 23-36.
The ‘ensemble score’ is the number of algorithms that predicted the Ti/Abs would be relevant.
The ‘count of ensemble score’ is the number of Ti/Abs that got each ensemble score. The ‘# of
seeds’ is the number of the seed articles that got each ensemble score. Ti/Abs that received a
score of 1 to 6 were included in the screening. Ti/Abs that received a score of 0 were not
screened.

A-24
Topic 3 Title/Abstract Screening Criteria and Tagging
Two separate individuals screened and applied the following tags to each title/abstract. In cases
where screener 1 and 2 disagreed a subject matter expert provided conflict resolution and
determined the final tagging result. Each title/abstract was tagged as follows:
Screening Tag Description of Tag
Top Level Tags – choose one [include, unclear, or exclude]:
Include (Topic 3 QMRA/epi)
Obtain full text, advance to full text screening
• Advances in QMRA and epidemiological studies in analyzing indicators of
fecal contamination (specifically: coliform bacteria, fecal streptococci,
anaerobic bacteria) in recreational waters.
• Do not include if only bacteriophages.
• In Scope:
QMRA (or MRA) modeling infectious disease risk in ambient surface
water samples. Or QMRAs that link risk to fecal indicators.
Epidemiological studies conducted on recreational exposures t
o
s
urface waters. Can be primary (e.g., swimming) or secondary (e.g.,
boating) contact exposure.
Papers that advance specific parameters for QMRA might also be of
interest. Use your judgment. The authors should talk about how the
paper fits into QMRA.
Review Articles about QMRA or epi in rec waters are also OK.
• NOT in Scope:
Studies for other water types: drinking water, ground water,
wastewater.
Studies prior to 2016 (2016 and earlier) are not in scope.
Unclear
Requires full text to determine whether within scope. Unclear if applicable to
recreational waters, for example, drinking water, groundwater, wastewater
studies. OK to include “other possible tags.”
Exclude
Do not obtain full text. Not within scope. OK to include notes. OK to include
“other possible tags.”
If “Include” this tag might also apply [this tag only applies if ‘include’]:
Priority or Review (for Topic 3)
• Articles that provide a review of one of the major topics will be prioritized.
Other papers that seem particularly of interest and should be prioritized can
also have this tag. Use judgment with this tag. Not all review articles need
to have this tag – just the review articles that are particularly on point

A-25
Screening Tag Description of Tag
Other Possible Tags – choose all that apply [Ti/Abs indicates the paper may contain information on]:
MST (Topic 1)
• Advances in method standardization related to MST.
• Development of standard control or reference materials for MST.
• Occurrence of amplification inhibition and/or matrix interference for
methods used for MST.
• Field studies to identify and/or remediate fecal sources.
• Risk assessment with MST genetic markers (epidemiological studies,
QMRA).
Molecular Methods (Topic 2)
• Advances in the development of qPCR, digital PCR, rt-PCR, next
generation sequencing, metagenomic DNA sequencing, and hypervariable
16S RNA gene sequencing used in recreational waters to measure
indicators of fecal contamination, pathogen indicators or waterborne
pathogens.
• Advances in statistics/modeling for data interpretation (fecal score, QMRA,
censored data, etc.).
Children’s Risk (Topic 4)
• Health effects that children experience following exposure to microbially
contaminated surface waters, including illness rates/health outcomes from
outbreak reports, epidemiological or other health studies.
Explanation – Free Text Notes
Field
• *Optional additional keywords including but not limited to: antimicrobial
resistance (AMR), combined sewer overflows (CSO), sanitary sewer
overflow (SSO), somatic coliphage, male-specific coliphage (MSC), F-
specific coliphage (MSC).
• Additional notes about the paper.
Topic 3 Study Quality Evaluation
Articles that were ‘include’ or ‘unclear’ moved to full text screening. The full text was reviewed
for scope to confirm the article is within the scope. Articles that pass scope were screened for
study quality and articles that pass study quality were qualified for data extraction.
Scope
Is the study within the Topic 3 scope? Does it include information on QMRA characterizing risk
in fecally contaminated recreational waters or recreational water epidemiological studies
including indicators of fecal contamination (e.g., coliform bacteria, fecal streptococci, anaerobic
bacteria)?
• Yes – continue with study quality.
• No – stop evaluation. (Choose ‘no’ if the study is only about bacteriophages/coliphages,
does not include a recreational water exposure route [e.g., incidental ingestion, inhalation,
dermal].)
Study Quality Metrics – If Any of the Metrics Are Unacceptable, the Study Was Not
Included for Data Extraction.
QMRA Documentation:
A-26
• Acceptable – The narrative defines the concern driving the risk assessment, the purpose,
and objectives of the risk assessment, the scope of analysis, some sort of conceptual
model, and analytical approaches are described. Media or matrix is clear. Health endpoint
is clear.
• Unacceptable – Not enough information is included to evaluate the QMRA methods or
critical problems are identified. Matrix/media is not identified. Health endpoint is not
clear.
• NA – Not a QMRA.
QMRA Parameters:
• Acceptable – Parameters are well described. Quantitative parameters include some
justification/logic/basis for selection.
• Unacceptable – Numeric parameters are not provided, not adequately referenced or are
unclear. Or not enough information is included to evaluate the parameters.
• NA – Not a QMRA.
Epi Documentation:
• Acceptable – Site and conditions at the site are described. Population is described. Study
design is described. Odd ratios or other risk metrics are included and clearly presented.
Confounding variables, if included for some analyses, are articulated and presented.
• Unacceptable – Unclear or indecipherable methods. Type of epidemiological study
design is not included or cannot be evaluated with the information provided.
• NA – Not an epidemiological study.
Reporting of Results:
• Acceptable – Raw data are included (check supplemental materials) or summary results
are clearly described. Variability and uncertainty are discussed or captured in summary
statistics.
• Unacceptable – Reporting of results is unclear, does not match methods (results with no
method description or methods with no results reported), or is insufficient to evaluate the
study.
Quality Assurance:
• Acceptable – Quality assurance is discussed or can be implied in the discussion. Peer-
reviewed or reputable government or institutional gray literature are acceptable.
• Unacceptable – Not enough information is included to evaluate whether QA was an issue,
or QA issues have been identified that interfere with the reliability of the study, or the
study is a non-peer-reviewed publication.
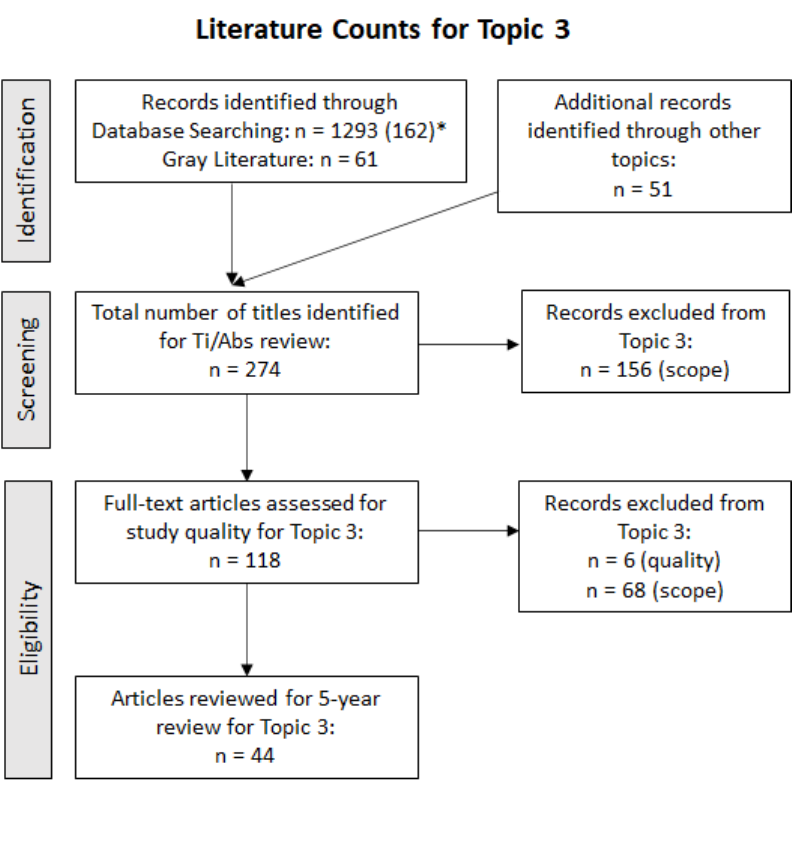
A-27
Topic 3: PRISMA Diagram
*
Automated prioritization tools were used to bin Ti/Abs. The most relevant Ti/Abs group was screened.

A-28
4. Topic 4: Characterization of Children’s Risk from Exposure to Recreational Water
Contaminated by Fecal Contamination
This topic specifically focuses on the health effects that children experience following exposure
to fecal-contaminated surface waters, including infection or illness rates/health outcomes from
outbreak reports, epidemiological or other health studies, exposure behavior studies conducted in
swimming pools that may have been performed for children or a comparison between adults and
children. The systematic literature review will be conducted from January 2018 to November 8,
2021.
Focus Areas:
• Advances in recreational exposure description for children
• Characterization of children’s illness susceptibility
• Observed illness rates in children upon exposure
Research Question: What advances have been made in characterizing the differential
susceptibility between children and adults and how exposure behaviors (contact, inhalation, and
ingestion patterns) to waterborne fecal pathogens for children differ from adults?
Literature Search Strategy for Topic 4: Characterization of Children’s Risk from Exposure to
Recreational Water Contaminated by Fecal Contamination
The searches are limited the language to English only.
Databases for searches include: PubMed (terms searched in All Fields) and Web of Science
(terms searched in Topic)
Dates: January 2018 to November 8, 2021 [present]
Keywords were tested to develop the strategies.
Topic 4: Characterization of Children’s Risk from Exposure to Recreational Water
Contaminated by Fecal Contamination Keyword Test Summary
Search Date Date Limit Search Strategy Sets
Number of Title/Abstracts
PubMed
WoS
Unique Total
November 8,
2021
January 2018–search
date
Organism AND Source
AND Water AND Detail
AND Population
399
188
484
Topic 4: Database Search: PubMed
Date of Search: November 8, 2021 (range January 2018 through November 8, 2021)
Fields Searched: All Fields
Limits: English Only; January 1, 2018, to November 8, 2021

A-29
Set Name Search Strategy for PubMed
Results (Number of
Titles)
Organism
((“quantitative microbial risk assessment” OR “QMRA” OR
“epidemiol*” OR “prospective cohort*” OR “randomized control trial”
OR “randomized controlled trial” OR “survey*” OR “longitudinal”
OR “case control” OR “case-control” OR “surveillance” OR
“outbreak” OR “Epidemiology”[Mesh]) NOT (“toxicology” OR
“animal study” OR “animal studies”))
975,149
Source (“fecal contamination” OR “feces” OR “fecal source” OR
“contaminated” OR “contaminant*”)
57,458
Water (“recreational water*” OR “surface water” OR “ambient water” OR
“water” OR “beach*”)
253,558
Detail (“ingestion” OR “inhalation” OR “dermal” OR “ear” OR “eye” OR
“behavior” OR “exposure*” OR “recreational exposure*” OR
“duration” OR “pathogen*” OR “dose” OR “infection” OR “illness”
OR “gastrointestinal illness” OR “respiratory illness” OR “waterborne
gastrointestinal illness*” OR “waterborne illness” OR “health burden”
OR “outbreak” OR “surveillance” OR “severity” OR “susceptibility”
OR “risk” OR “lifestage” OR “life stage” OR “negative health
outcome*” OR “adverse health outcome*” OR “health outcome*”)
1,929,700
Population (“child” OR “Child”[Mesh] OR “children*”) 494,592
Total
(Organism AND Source AND Water AND Detail AND Population)
399
A-30
Screenshot of Topic 4 PubMed Search

A-31
Topic 4 Database Search: Web of Science
Date of Search: November 8, 2021 (range 2018 through 2021)
Fields Searched: Topic
Limits: English Only; 2018 to 2021
Set Name Search Strategy for Web of Science
Results (Number of
Titles)
Organism ((“quantitative microbial risk assessment” OR “QMRA” OR
“epidemiol*” OR “prospective cohort*” OR “randomized control trial”
OR “randomized controlled trial” OR “survey*” OR “longitudinal”
OR “case control” OR “case-control” OR “surveillance” OR
“outbreak”) NOT (“toxicology” OR “animal study” OR “animal
studies”))
692,505
Source (“fecal contamination” OR “feces” OR “fecal source” OR
“contaminated” OR “contaminant*”)
77,511
Water (“recreational water*” OR “surface water” OR “ambient water” OR
“water” OR “beach*”)
674,960
Detail (“ingestion” OR “inhalation” OR “dermal” OR “ear” OR “eye” OR
“behavior” OR “exposure*” OR “exposure and frequency” OR
“recreational exposure*” OR “duration” OR “pathogen*” OR “dose”
OR “infection” OR “illness” OR “gastrointestinal illness” OR
“respiratory illness” OR “waterborne gastrointestinal illness*” OR
“waterborne illness” OR “health burden” OR “outbreak” OR
“surveillance” OR “severity” OR “susceptibility” OR “risk” OR
“lifestage” OR “life stage” OR “negative health outcome*” OR
“adverse health outcome*” OR “health outcome*”)
2,483,216
Population (“child” OR “children*”) 337,190
Total (Organism AND Source AND Water AND Detail AND Population) 188

A-32
Screenshot of Web of Science Topic 4 Search

A-33
Topic 4: Gray Literature Search
Date of Search: November 18, 2021
Limits: English Only; 2018 to 2021
Search run via Google Scraper Tool
NOTE: Google uses strict character limits. Strings containing more than 32 words are not
allowed.
Organization URL Keywords /Search string Results
FDA FDA.gov
site:fda.gov AND (“risk assessment” OR
“QMRA” OR “epidemiol*” OR “cohort” OR
“randomized” OR “longitudinal” OR
“control” OR “survey”) AND (Feces OR
“contaminant”) AND (“Water” OR “beach”)
AND (child*) AND (“ingestion” OR
“inhalation” OR “dermal” OR “ear” OR “eye”
OR “behavior” OR “exposure*” OR
“pathogen*” OR “infection” OR “illness” OR
“outbreak” OR “surveillance” OR
“susceptibility” OR “risk” OR “health
outcome”)
7
NPS Nps.gov
site:nps.gov AND (“risk assessment” OR
“QMRA” OR “epidemiol*” OR “cohort” OR
“randomized” OR “longitudinal” OR
“control” OR “survey”) AND (Feces OR
“contaminant”) AND (“Water” OR “beach”)
AND (child*) AND (“ingestion” OR
“inhalation” OR “dermal” OR “ear” OR “eye”
OR “behavior” OR “exposure*” OR
“pathogen*” OR “infection” OR “illness” OR
“outbreak” OR “surveillance” OR
“susceptibility” OR “risk” OR “health
outcome”)
4
FWS Fws.gov
site:fws.gov AND (“risk assessment” OR
“QMRA” OR “epidemiol*” OR “cohort” OR
“randomized” OR “longitudinal” OR
“control” OR “survey”) AND (Feces OR
“contaminant”) AND (“Water” OR “beach”)
AND (child*) AND (“ingestion” OR
“inhalation” OR “dermal” OR “ear” OR “eye”
OR “behavior” OR “exposure*” OR
“pathogen*” OR “infection” OR “illness” OR
“outbreak” OR “surveillance” OR
“susceptibility” OR “risk” OR “health
outcome”)
9
NAS Nap.edu
site:nap.edu AND (“risk assessment” OR
“QMRA” OR “epidemiol*” OR “cohort” OR
“randomized” OR “longitudinal” OR
“control” OR “survey”) AND (Feces OR
“contaminant”) AND (“Water” OR “beach”)
0

A-34
Organization URL Keywords /Search string Results
AND (child*) AND (“ingestion” OR
“inhalation” OR “dermal” OR “ear” OR “eye”
OR “behavior” OR “exposure*” OR
“pathogen*” OR “infection” OR “illness” OR
“outbreak” OR “surveillance” OR
“susceptibility” OR “risk” OR “health
outcome”)
WHO Who.int
site:who.int AND (“risk assessment” OR
“QMRA” OR “epidemiol*” OR “cohort” OR
“randomized” OR “longitudinal” OR
“control” OR “survey”) AND (Feces OR
“contaminant”) AND (“Water” OR “beach”)
AND (child*) AND (“ingestion” OR
“inhalation” OR “dermal” OR “ear” OR “eye”
OR “behavior” OR “exposure*” OR
“pathogen*” OR “infection” OR “illness” OR
“outbreak” OR “surveillance” OR
“susceptibility” OR “risk” OR “health
outcome”)
10
Total All domains Same search in each domain
30
Topic 4: Title/Abstract Screening Criteria and Tagging
Two separate individuals screened and applied the following tags to each title/abstract. In cases
where screener 1 and 2 disagreed a subject matter expert provided conflict resolution and
determined the final tagging result. Each title/abstract was tagged as follows:
Screening Tag Description of Tag
Top Level Tags – choose one [include, unclear, or exclude]:
Include (Topic 4 Children’s
Risk)
Obtain full text, advance to full text screening.
• Health effects that children experience following exposure to microbially
contaminated surface waters, including illness rates/health outcomes from
outbreak reports, epidemiological or other health studies.
• In Scope:
Papers on children’s risks from exposure to microbes in surface waters
Review Articles about children’s risk in rec waters are also OK
Case studies, surveys
• NOT in Scope:
Studies for other water types: drinking water, ground water,
wastewater.
Non-microbial risks (drowning, animal related injuries).
Drinking surface water.
Schistosomes/Schistosomiasis (and other waterborne diseases that are
not typically found in U.S.).
Unclear
Requires full text to determine whether within scope. Unclear if applicable to
recreational waters, for example, drinking water, groundwater, wastewater
studies. OK to include “other possible tags.”

A-35
Screening Tag Description of Tag
Exclude
Do not obtain full text. Not within scope. OK to include notes. OK to include
“other possible tags.”
If “Include” this tag might also apply [this tag only applies if ‘include’]:
Priority or Review (for Topic 4)
Articles that provide a review of one of the major topics will be prioritized.
Other papers that seem particularly of interest and should be prioritized can also
have this tag. Use judgment with this tag. Not all review articles need to have
this tag – just the review articles that are particularly on point.
Other Possible Tags – choose all that apply [Ti/Abs indicates the paper may contain information on]:
MST (Topic 1)
• Advances in method standardization related to MST.
• Development of standard control or reference materials for MST.
• Occurrence of amplification inhibition and/or matrix interference for
methods used for MST.
• Field studies to identify and/or remediate fecal sources.
• Risk assessment with MST genetic markers (epidemiological studies,
QMRA).
Molecular Methods (Topic 2)
• Advances in the development of qPCR, digital PCR, rt-PCR, next
generation sequencing, metagenomic DNA sequencing, and hypervariable
16S RNA gene sequencing used in recreational waters to measure
indicators of fecal contamination, pathogen indicators or waterborne
pathogens.
• Advances in statistics/modeling for data interpretation (fecal score, QMRA,
censored data, etc.).
QMRA or Epi (Topic 3)
• Advances in QMRA and epidemiological studies in analyzing indicators of
fecal contamination (specifically: coliform bacteria, fecal streptococci,
anaerobic bacteria) in recreational waters.
• Do not include if only bacteriophages.
Explanation – Free text notes
field
• *Optional additional keywords including but not limited to: antimicrobial
resistance (AMR), combined sewer overflows (CSO), sanitary sewer
overflow (SSO), somatic coliphage, male-specific coliphage (MSC), F-
specific coliphage (MSC).
• Additional notes about the paper.
Topic 4: Study Quality Evaluation
Articles that were ‘include’ or ‘unclear’ moved to full text screening. The full text was reviewed
for scope to confirm the article is within the scope. Articles that pass scope were screened for
study quality and articles that pass study quality were qualified for data extraction.
Scope
Is the study within the Topic 5 scope? Does it include information on health effects that children
experience following exposure to microbially contaminated surface waters, including illness
rates/health outcomes from outbreak reports, epidemiological or other health studies, published
risk assessments (e.g., quantitative microbial risk assessments) that may have been performed for
children or a comparison between adult and children’s risk? Note that studies that have been
conducted in ambient waters using ingestion estimates are relevant to this topic.
A-36
• Yes – continue with study quality.
• No – stop evaluation.
Study Quality Metrics – If Any of the Metrics Are Unacceptable, the Study Was Not
Included for Data Extraction.
Study Documentation:
• Acceptable – The narrative defines the concern driving the study, the purpose, and
objectives of the study, and the scope of analysis. Some sort of conceptual model is
discussed and analytical approaches are described.
• Unacceptable – Not enough information is included to evaluate the study or critical
problems are identified.
Study Parameters:
• Acceptable – Parameters are well described. Quantitative parameters include some
justification/logic/basis for selection.
• Unacceptable – Numeric parameters are not provided or are unclear, or not enough
information is included to evaluate the parameters.
• NA – not a QMRA.
Epidemiological Documentation:
• Acceptable – Site, conditions at the site, study population, and study design are
described. Odd ratios or other risk metrics are included and clearly presented.
Confounding variables, if included for some analyses, are articulated and presented.
• Unacceptable – Unclear or indecipherable methods. Type of epidemiological study
design is not included or cannot be evaluated with the information provided.
• NA – not an epi study.
Reporting of Results:
• Acceptable – Raw data are included (check supplemental materials) or summary results
are clearly described. Variability and uncertainty are discussed or captured in summary
statistics.
• Unacceptable – Reporting of results is unclear, does not match methods (results with no
method description or methods with no results reported), or is insufficient to evaluate the
study.
Quality Assurance:
• Acceptable – Quality assurance (QA) is discussed or can be implied in the discussion.
Peer-reviewed or reputable government or institutional gray literature are acceptable.
A-37
• Unacceptable – Not enough information is included to evaluate whether QA was an issue,
or QA issues have been identified that interfere with the reliability of the study, or the
study is a non-peer-reviewed publication.

A-38
Topic 4: PRISMA Diagram

A-39
5. Topic 5: Cyanotoxins
This topic is focused on characterizing the human health effects of cyanotoxins from all routes of
exposure. Specifically, the cyanotoxins of interest for this topic are anatoxins, BMAA,
cylindrospermopsin, microcystins, nodularins, and saxitoxins. The literature searches for
anatoxins, microcystins, and cylindrospermopsin built upon work previously conducted by the
Office of Water and was conducted from 2014 to April 2021. The literature searches for all other
cyanotoxins were conducted with no start date limit to April 2021. Topic 5 literature search was
conducted separately from the literatures searches on Topics 1 through 4.
Focus Areas: Human Health, Mechanistic, Toxicokinetic, and Pharmacokinetic
(PK)/Physiologically based Pharmacokinetic (PBPK) Models.
Research Question: What epidemiological and toxicological literature is available for anatoxins,
BMAA, cylindrospermopsin, microcystins, nodularins, and saxitoxins, that could be informative
for derivation of a human health toxicity value? In addition, what supplemental material is
available that could help support a human health risk assessment for these cyanotoxins?
Literature Search Strategy for Topic 5: Cyanotoxins
Please note that the original literature search (April 2021) did not separate the searches by
cyanotoxin; nor did it date limit searches for microcystin and cylindrospermopsin. Therefore, a
second literature search was conducted in April 2022 to replicate the April 2021 literature search
but separate the results by cyanotoxin and date limit the microcystin and cylindrospermopsin
results. Additionally, a third literature search was conducted to specifically capture anatoxin
studies from 2014, as those were outside the date limit set in the initial April 2021 search.
Results from all searches are shown below.
Databases for searches include: PubMed (terms searched in All Fields) and Web of Science
(terms searched in Topic) and Scopus (terms searched in title, abstract, and keyword fields).
After 2021, use of Scopus is no longer supported by HERO.
Dates: Listed for each search
Topic 5 Database Search: PubMed (April 2021)
Date of Search: April 13, 2021
• Anatoxins: January 2015 to present
• BMAA: no limit
• Cylindrospermopsin: no limit
• Microcystins: no limit
• Nodularins: no limit
• Saxitoxins: no limit
Fields Searched: All Fields

A-40
Search Strategy for PubMed (April 2021)
Results (Number of
Titles)
((“microcystins”[tw] OR “microcystin”[tw]) OR (“microcystin-LA”[tw] OR
“96180-79-9”[rn]) OR (“microcystin-LF” OR “154037-70-4”[rn]) OR
(“microcystin-LR”[tw] OR “101043-37-2”[rn]) OR (“microcystin-LY” [tw] OR
“123304-10-9” [rn]) OR (“microcystin-RR”[tw] OR “111755-37-4”[rn]) OR
(“microcystin-YR”[tw] OR “101064-48-6”[rn]) OR “microcystin-LW”[tw] OR
“[Asp3]MCRR”[tw] OR “Asp3,Dhb7]MCRR”[tw] OR “MCWR”[tw]) OR
((“nodularins”[tw] OR “nodularin”[tw]) OR (“nodularin-R”[tw] OR “118399-22-
7”[rn]) OR “Nodularin-Har”[tw] OR “Nodularin-V (motuporin)”[tw] OR “[D-
Asp1]NOD”[tw] OR “[DMAdda3]NOD”[tw] OR “[dhb5]NOD”[tw] OR
“[Glu4(Ome)]NOD”[tw] OR “[MeAdda3]NOD”[tw] OR “[(6Z)-
Adda3]NOD”[tw]) OR (((“anatoxins”[tw] OR “anatoxin”[tw]) OR (“anatoxin-
a”[tw] OR “64285-06-9”[rn]) OR “homoanatoxin-a”[tw] OR “dihydroanatoxin-
a”[tw] OR “2,3-epoxy-anatoxin-a”[tw] OR “4-hydroxy-anatoxin-a”[tw] OR “4-
oxo-anatoxin-a”[tw] OR “dihydrohomoanatoxin-a”[tw] OR “Anatoxin-a(s)”[tw]
OR “(guanitoxin (GNT))”[tw]) AND (2015/01/01 : 2021/04/01[dp])) OR
(“Cylindrospermopsin”[tw] OR (“7-epicylindro-spermopsin”[tw] OR “143545-
90-8”[rn]) OR “7-deoxycylindrospermopsin”[tw] OR “7-deoxy-desulfo-
cylindrospermopsin”[tw] OR “7-deoxy-desulfo-12-
acetylcylindrospermopsin”[tw]) OR (“BMAA”[tw] OR “beta-Methylamino-L-
alanine)”[tw]) OR ((“saxitoxins”[tw] OR “saxitoxins”[tw]) OR (“stx-
dihydrochloride”[tw] OR “35554-08-6”[rn]) OR “Gonyaulax-toxin”[tw] OR
“Saxitoxin”[mh]) OR (“Carbamate”[tw] OR “NeoSTX”[tw] OR “GTX1”[tw] OR
“GTX2”[tw] OR “GTX3”[tw] OR “GTX4”[tw] OR “GTX5 (B1)”[tw] OR “GTX6
(B2)”[tw]) OR (“Decarbamoyl”[tw] OR “dcSTX”[tw] OR “dcNEOSTX”[tw] OR
“dcGTX1”[tw] OR “dcGTX2”[tw] OR “dcGTX3”[tw] OR “dcGTX4
”[tw]) OR
(
“Deoxydecarbamoyl”[tw] OR “doSTX”[tw] OR “doGTX2”[tw] OR
“doGTX3”[tw])
15,263
Topic 5 Database Search: PubMed (April 2022)
Date of Search: April 14, 2022
• Anatoxins: January 2015 to April 2021
• BMAA: no limit to April 2021
• Cylindrospermopsin: January 2014 to April 2021
• Microcystins: January 2014 to April 2021
• Nodularins: no limit to April 2021
• Saxitoxins: no limit to April 2021
Fields Searched: All Fields
Cyanotoxin Search Strategy for PubMed
Results
(Number of
Titles)
Anatoxins
((“anatoxins”[tw] OR “anatoxin”[tw]) OR (“anatoxin-a”[tw] OR
“64285-06-9”[rn]) OR “homoanatoxin-a”[tw] OR “dihydroanatoxin-
a”[tw] OR “2,3-epoxy-anatoxin-a”[tw] OR “4-hydroxy-anatoxin-
a”[tw] OR “4-oxo-anatoxin-a”[tw] OR “dihydrohomoanatoxin-a”[tw]
OR “Anatoxin-a(s)”[tw] OR “(guanitoxin (GNT))”) AND (2015/01/01
: 2021/04/13[dp])
117

A-41
Cyanotoxin Search Strategy for PubMed
Results
(Number of
Titles)
BMAA
(“BMAA”[tw] OR “beta-Methylamino-L-alanine)”[tw]) AND
(1800/01/01:2021/04/13[dp])
459
Cylindrospermopsin
(Date Limited)
(“Cylindrospermopsin”[tw] OR (“7-epicylindro-spermopsin”[tw] OR
“143545-90-8”[rn]) OR “7-deoxycylindrospermopsin”[tw] OR “7-
deoxy-desulfo-cylindrospermopsin”[tw] OR “7-deoxy-desulfo-12-
acetylcylindrospermopsin”[tw]) AND (2014/01/01:2021/04/13[dp])
278
Microcystins
(Date Limited)
(“microcystins”[tw] OR “microcystin”[tw]) OR (“microcystin-
LA”[tw] OR “96180-79-9”[rn]) OR (“microcystin-LF” OR “154037-
70-4”[rn]) OR (“microcystin-LR”[tw] OR “101043-37-2”[rn]) OR
(“microcystin-LY” [tw] OR “123304-10-9” [rn]) OR (“microcystin-
RR”[tw] OR “111755-37-4”[rn]) OR (“microcystin-YR”[tw] OR
“101064-48-6”[rn]) OR “microcystin-LW”[tw] OR
“[Asp3]MCRR”[tw] OR “Asp3,Dhb7]MCRR”[tw] OR “MCWR”[tw])
AND (2014/01/01:2021/04/13[dp])
2,076
Nodularins
((“nodularin-R”[tw] OR “118399-22-7”[rn]) OR “Nodularin-Har”[tw]
OR “Nodularin-V (motuporin)”[tw] OR “[D-Asp1]NOD”[tw] OR
“[DMAdda3]NOD”[tw] OR “[dhb5]NOD”[tw] OR
“[Glu4(Ome)]NOD”[tw] OR “[MeAdda3]NOD”[tw] OR “[(6Z)-
Adda3]NOD”[tw]) AND (1800/01/01:2021/04/13[dp])
259
Saxitoxins ((“saxitoxins”[tw] OR “saxitoxins”[tw]) OR (“stx-
dihydrochloride”[tw] OR “35554-08-6”[rn]) OR “Gonyaulax-
toxin”[tw] OR “Saxitoxin”[mh]) OR (“Carbamate”[tw] OR
“NeoSTX”[tw] OR “GTX1”[tw] OR “GTX2”[tw] OR “GTX3”[tw]
OR “GTX4”[tw] OR “GTX5 (B1)”[tw] OR “GTX6 (B2)”[tw]) OR
(“Decarbamoyl”[tw] OR “dcSTX”[tw] OR “dcNEOSTX”[tw] OR
“dcGTX1”[tw] OR “dcGTX2”[tw] OR “dcGTX3”[tw] OR
“dcGTX4”[tw]) OR (“Deoxydecarbamoyl”[tw] OR “doSTX”[tw] OR
“doGTX2”[tw] OR “doGTX3”[tw]) AND
(1800/01/01:2021/04/13[dp])
9,887
Total Unique
References
12,692
Topic 5 Database Search: Web of Science (April 2021)
Date of Search: April 13, 2021
• Anatoxins: January 2015 to present
• BMAA: no limit
• Cylindrospermopsin: no limit
• Microcystins: no limit
• Nodularins: no limit
• Saxitoxins: no limit

A-42
Fields Searched: Topic
Search Strategy for Web of Science Results (Number of Titles)
((TS=”microcystins”OR TS=”microcystin”) OR (TS=”microcystin-LA” OR
TS=”96180-79-9”) OR (TS=”microcystin-LF” OR TS=”154037-70-4”) OR
(TS=”microcystin-LR” OR TS=”101043-37-2”) OR (TS=”microcystin-LY” OR
TS=”123304-10-9”) OR (TS=”microcystin-RR” OR TS=”111755-37-4”) OR
(TS=”microcystin-YR” OR TS=”101064-48-6”) OR TS=”microcystin-LW” OR
TS=”[Asp3]MCRR” OR TS=”Asp3,Dhb7]MCRR” OR TS=”MCWR”) OR
((TS=”nodularins” OR TS=”nodularin”) OR (TS=”nodularin-R” OR TS=”118399-
22-7”) OR TS=”Nodularin-Har” OR TS=”Nodularin-V (motuporin)” OR TS=”[D-
Asp1]NOD” OR TS=”[DMAdda3]NOD” OR TS=”[dhb5]NOD” O
R
TS
=”[Glu4(Ome)]NOD” OR TS=”[MeAdda3]NOD” OR TS=”[(6Z)-
Adda3]NOD”) OR (((TS=”anatoxins” OR TS=”anatoxin”) OR (TS=”anatoxin-a”
OR TS=”64285-06-9”) OR TS=”homoanatoxin-a” OR TS=”dihydroanatoxin-a”
OR TS=”2,3-epoxy-anatoxin-a” OR TS=”4-hydroxy-anatoxin-a” OR TS=”4-oxo-
anatoxin-a” OR TS=”dihydrohomoanatoxin-a” OR TS=”Anatoxin-a(s)” OR
TS=”(guanitoxin (GNT))”) AND (PY= 2015-2021)) OR
(TS=”Cylindrospermopsin” OR (TS=”7-epicylindro-spermopsin” O
R
TS
=”143545-90-8”) OR TS=”7-deoxycylindrospermopsin” OR TS=”7-deoxy-
desulfo-cylindrospermopsin” OR TS=”7-deoxy-desulfo-12-
acetylcylindrospermopsin”) OR (TS=”BMAA” OR TS=”(beta-Methylamino-L-
alanine)”) OR ((TS=”saxitoxin” OR TS=”saxitoxins”) OR (TS=”stx-
dihydrochloride” OR TS=”35554-08-6”) OR TS=”Gonyaulax-toxin”) OR
(TS=”Carbamate” OR TS=”
NeoSTX” OR TS=”G
TX1” OR TS=”GTX2” OR
TS=”GTX3” OR TS=”GTX4” OR TS=”GTX5 (B1)” OR TS=”GTX6 (B2)”) OR
(TS=”N-sulfocarbonyl”) OR (TS=”Decarbamoyl” OR TS=”dcSTX” OR
TS
=”dcNEOSTX” OR TS=”dcGTX1” OR TS=”dcGTX2” OR TS=”dcGTX3” O
R
TS
=”dcGTX4”) OR (TS=”Deoxydecarbamoyl” OR TS=”doSTX” O
R
TS
=”doGTX2” OR TS=”doGTX3”)
26,098
Topic 5 Database Search: Web of Science (April 2022)
Date of Search: April 14, 2022
• Anatoxins: January 2015 to April 2021
• BMAA: no limit to April 2021
• Cylindrospermopsin: January 2014 to April 2021
• Microcystins: January 2014 to April 2021
• Nodularins: no limit to April 2021
• Saxitoxins: no limit to April 2021
Fields Searched: Topic

A-43
Cyanotoxin Search Strategy for Web of Science
Results
(Number of
Titles)
Anatoxins ((TS=”anatoxins” OR TS=”anatoxin”) OR (TS=”anatoxin-a” OR
TS=”64285-06-9”) OR TS=”homoanatoxin-a” OR
TS=”dihydroanatoxin-a” OR TS=”2,3-epoxy-anatoxin-a” OR TS=”4-
hydroxy-anatoxin-a” OR TS=”4-oxo-anatoxin-a” OR
TS=”dihydrohomoanatoxin-a” OR TS=”Anatoxin-a(s)” OR
TS=”(guanitoxin (GNT))”) AND (DOP=2015-01-01/2021-04-13)
327
BMAA
(TS=”BMAA” OR TS=”(beta-Methylamino-L-alanine)”) AND
(DOP=1800-01-01/2021-04-13)
562
Cylindrospermopsin
(TS=”Cylindrospermopsin” OR (TS=”7-epicylindro-spermopsin” OR
TS=”143545-90-8”) OR TS=”7-deoxycylindrospermopsin” OR
TS=”7-deoxy-desulfo-cylindrospermopsin” OR TS=”7-deoxy-desulfo-
12-acetylcylindrospermopsin”) AND (DOP=2014-01-01/2021-04-13)
465
Microcystins
((TS=”microcystins”OR TS=”microcystin”) OR (TS=”microcystin-
LA” OR TS=”96180-79-9”) OR (TS=”microcystin-LF” OR
TS=”154037-70-4”) OR (TS=”microcystin-LR” OR TS=”101043-37-
2”) OR (TS=”microcystin-LY” OR TS=”123304-10-9”) OR
(TS=”microcystin-RR” OR TS=”111755-37-4”) OR
(TS=”microcystin-YR” OR TS=”101064-48-6”) OR TS=”microcystin-
LW” OR TS=”[Asp3]MCRR” OR TS=”Asp3,Dhb7]MCRR” OR
TS=”MCWR”) AND (DOP=2014-01-01/2021-04-13)
3,401
Nodularins ((TS=”nodularins” OR TS=”nodularin”) OR (TS=”nodularin-R” OR
TS=”118399-22-7”) OR TS=”Nodularin-Har” OR TS=”Nodularin-V
(motuporin)” OR TS=”[D-Asp1]NOD” OR TS=”[DMAdda3]NOD”
OR TS=”[dhb5]NOD” OR TS=”[Glu4(Ome)]NOD” OR
TS=”[MeAdda3]NOD” OR TS=”[(6Z)-Adda3]NOD”) AND
(DOP=1800-01-01/2021-04-13)
779
Saxitoxins (((TS=”saxitoxin” OR TS=”saxitoxins”) OR (TS=”stx-
dihydrochloride” OR TS=”35554-08-6”) OR TS=”Gonyaulax-toxin”)
OR (TS=”Carbamate” OR TS=”NeoSTX” OR TS=”GTX1” OR
TS=”GTX2” OR TS=”GTX3” OR TS=”GTX4” OR TS=”GTX5 (B1)”
OR TS=”GTX6 (B2)”) OR (TS=”N-sulfocarbonyl”) OR
(TS=”Decarbamoyl” OR TS=”dcSTX” OR TS=”dcNEOSTX” OR
TS=”dcGTX1” OR TS=”dcGTX2” OR TS=”dcGTX3” OR
TS=”dcGTX4”) OR (TS=”Deoxydecarbamoyl” OR TS=”doSTX” OR
TS=”doGTX2” OR TS=”doGTX3”)) AND (DOP=1800-01-01/2021-
04-13)
18,221
Total Unique
References
22,756
Topic 5 Database Search: Scopus (April 2021)
Date of Search: April 14, 2021
• Anatoxins: January 2015 to present
• BMAA: no limit

A-44
• Cylindrospermopsin: no limit
• Microcystins: no limit
• Nodularins: no limit
• Saxitoxins: no limit
Fields Searched: Title, Abstract, and Keywords
Search Strategy for Scopus Results (Number of Titles)
( ( TITLE-ABS-KEY ( {microcystins} ) OR TITLE-ABS-KEY ( {microcystin} )
) OR ( TITLE-ABS-KEY ( {microcystin-LA} ) OR TITLE-ABS-KEY (
{96180-79-9} ) ) OR ( TITLE-ABS-KEY ( {microcystin-LF} ) OR TITLE-
ABS-KEY ( {154037-70-4} ) ) OR ( TITLE-ABS-KEY ( {microcystin-LR} )
OR TITLE-ABS-KEY ( {101043-37-2} ) ) OR ( TITLE-ABS-KEY (
{microcystin-LY} ) OR TITLE-ABS-KEY ( {123304-10-9} ) ) OR ( TITLE-
ABS-KEY ( {microcystin-RR} ) OR TITLE-ABS-KEY ( {111755-37-4} ) ) OR
( TITLE-ABS-KEY ( {microcystin-YR} ) OR TITLE-ABS-KEY ( {101064-48-
6
} )
) OR TITLE-ABS-KEY ( {microcystin-LW} ) OR TITLE-ABS-KEY (
{[Asp3]MCRR} ) OR TITLE-ABS-KEY ( {Asp3,Dhb7]MCRR} ) OR TITLE-
ABS-KEY ( {MCWR} ) ) OR ( ( TITLE-ABS-KEY ( {nodularins} ) OR
TITLE-ABS-KEY ( {nodularin} ) ) OR ( TITLE-ABS-KEY ( {nodularin-R} )
OR TITLE-ABS-KEY ( {118399-22-7} ) ) OR TITLE-ABS-KEY ( {Nodularin-
Har} ) OR TITLE-ABS-KEY ( {Nodularin-V (motuporin)} ) OR TITLE-ABS-
KEY ( {[D-Asp1]NOD} ) OR TITLE-ABS-KEY ( {[DMAdda3]NOD} ) OR
TITLE-ABS-KEY ( {[dhb5]NOD} ) OR TITLE-ABS-KEY (
{[Glu4(OMe)]NOD} ) OR TITLE-ABS-KEY ( {[MeAdda3]NOD} ) OR
TITLE-ABS-KEY ( {[(6Z)-Adda3]NOD} ) ) OR ( ( ( TITLE-ABS-KE
Y
(
{anatoxins} ) OR TITLE-ABS-KEY ( {anatoxin} ) ) OR ( TITLE-ABS-KE
Y
(
{anatoxin-a} ) OR TITLE-ABS-KEY ( {64285-06-9} ) ) OR TITLE-ABS-KE
Y
(
{homoanatoxin-a} ) OR TITLE-ABS-KEY ( {dihydroanatoxin-a} ) OR
TITLE-ABS-KEY ( {2,3-epoxy-anatoxin-a} ) OR TITLE-ABS-KEY ( {4-
hydroxy-anatoxin
-a} )
OR TITLE-ABS-KEY ( {4-oxo-anatoxin-a} ) OR
TITLE-ABS-KEY ( {dihydrohomoanatoxin-a} ) OR TITLE-ABS-KEY (
{Anatoxin-a(s)} ) OR TITLE-ABS-KEY ( {(guanitoxin (GNT))} ) ) AND (
PUBYEAR > 2014 ) ) OR ( TITLE-ABS-KEY ( {Cylindrospermopsin} ) OR (
TITLE-ABS-KEY ( {7-epicylindro-spermopsin} ) OR TITLE-ABS-KEY (
{143545-90-8} ) ) OR TITLE-ABS-KEY ( {7-deoxycylindrospermopsin} ) OR
TITLE-ABS-KEY ( {7-deoxy-desulfo-cylindrospermopsin} ) OR TITLE-ABS-
KEY ( {7-deoxy-desulfo-12-acetylcylindrospermopsin} ) ) OR ( TITLE-ABS-
KEY ( {BMAA} ) OR TITLE-ABS-KEY ( {beta-Methylamino-L-alanine)} ) )
OR ( ( TITLE-ABS-KEY ( {saxitoxins} ) OR TITLE-ABS-KEY ( {saxitoxins} )
) OR ( TITLE-ABS-KEY ( {stx-dihydrochloride} ) OR TITLE-ABS-KEY (
{35554-08-6} ) ) OR TITLE-ABS-KEY ( {Gonyaulax-toxin} ) ) OR ( TITLE-
ABS-KEY ( {Carbamate} ) OR TITLE-ABS-KEY ( {NeoSTX} ) OR TITLE-
ABS-KEY ( {GTX1} ) OR TITLE-ABS-KEY ( {GTX2} ) OR TITLE-ABS-
KEY ( {GTX3} ) OR TITLE-ABS-KEY ( {GTX4} ) OR TITLE-ABS-KEY (
{GTX5 (B1)} ) OR TITLE-ABS-KEY ( {GTX6 (B2)} ) ) OR ( TITLE-ABS-
KEY ( {N-sulfocarbonyl} ) ) OR ( TITLE-ABS-KEY ( {Decarbamoyl} ) OR
TITLE-ABS-KEY ( {dcSTX} ) OR TITLE-ABS-KEY ( {dcNEOSTX} ) OR
TITLE-ABS-KEY ( {dcGTX1} ) OR TITLE-ABS-KEY ( {dcGTX2} ) OR
TITLE-ABS-KEY ( {dcGTX3} ) OR TITLE-ABS-KEY ( {dcGTX4} ) ) OR (
TITLE-ABS-KEY ( {Deoxydecarbamoyl} ) OR TITLE-ABS-KEY ( {doSTX} )
OR TITLE-ABS-KEY ( {doGTX2} ) OR
TITLE-ABS-K
EY ( {doGTX3} ) )
29,314

A-45
Topic 5 Database Search: Anatoxins 2014 (December 2021)
Date of Search: December 28, 2021
• Anatoxins: 2014
Fields Searched
• PubMed: All Fields
• Web of Science: Topic
• Scopus: Title, Abstract, and Keywords
Database Search Strategy for Database
Results
(Number of
Titles)
PubMed
(((“anatoxins”[tw] OR “anatoxin”[tw]) OR (“anatoxin-a”[tw] OR
“64285-06-9”[rn]) OR “homoanatoxin-a”[tw] OR “dihydroanatoxin-
a”[tw] OR “2,3-epoxy-anatoxin-a”[tw] OR “4-hydroxy-anatoxin-
a”[tw] OR “4-oxo-anatoxin-a”[tw] OR “dihydrohomoanatoxin-a”[tw]
OR “Anatoxin-a(s)”[tw] OR “(guanitoxin (GNT))”[tw]) AND (2014
[dp]))
28
Web of Science (((TS=”anatoxins” OR TS=”anatoxin”) OR (TS=”anatoxin-a” OR
TS=”64285-06-9”) OR TS=”homoanatoxin-a” OR
TS=”dihydroanatoxin-a” OR TS=”2,3-epoxy-anatoxin-a” OR TS=”4-
hydroxy-anatoxin-a” OR TS=”4-oxo-anatoxin-a” OR
TS=”dihydrohomoanatoxin-a” OR TS=”Anatoxin-a(s)” OR
TS=”(guanitoxin (GNT))”) AND (PY= 2014))
47
Scopus
( ( ( TITLE-ABS-KEY ( {anatoxins} ) OR TITLE-ABS-KEY (
{anatoxin} ) ) OR ( TITLE-ABS-KEY ( {anatoxin-a} ) OR TITLE-
ABS-KEY ( {64285-06-9} ) ) OR TITLE-ABS-KEY (
{homoanatoxin-a} ) OR TITLE-ABS-KEY ( {dihydroanatoxin-a} )
OR TITLE-ABS-KEY ( {2,3-epoxy-anatoxin-a} ) OR TITLE-ABS-
KEY ( {4-hydroxy-anatoxin-a} ) OR TITLE-ABS-KEY ( {4-oxo-
anatoxin-a} ) OR TITLE-ABS-KEY ( {dihydrohomoanatoxin-a} )
OR TITLE-ABS-KEY ( {Anatoxin-a(s)} ) OR TITLE-ABS-KEY (
{(guanitoxin (GNT))} ) ) AND ( PUBYEAR = 2014 ) )
48
Total Unique Studies 62
Topic 5 Database Search: Gray Literature
Date of Search: See “Date Searched” column
• No limit for any cyanotoxin
Source Search Strategy for Source
Results
(Number
of Titles)
Date Searched
EPA
Chemicals
Dashboard
ToxVal
database
Searched for each cyanotoxin by provided CAS RN or
name(s)
10 5/12/2021

A-46
Source Search Strategy for Source
Results
(Number
of Titles)
Date Searched
ECHA
registration
dossiers
Searched for each cyanotoxin by provided CAS RN or
name(s)
12 5/13/2021
EPA
ChemView
database
Searched for each cyanotoxin by provided CAS RN or
name(s)
0 5/13/2021
NTP CEBS
database
Searched for each cyanotoxin by provided CAS RN or
name(s)
4 5/13/2021
OECD SIDS
HPV
Searched for each cyanotoxin by provided CAS RN or
name(s)
0 5/13/2021
ECOTOX
database
*Search does not work - -
ATSDR Looked for cynatoxins of interest in listed profiles 0 5/19/2021
NAS Searched site for each cyanotoxin of interest 0 5/19/2021
NCI Searched for each cyanotoxin by provided name(s) 0 6/02/2021
FDA Searches for each cyanotoxin by provided name(s) 3 6/02/02021
NIEHS
Microcystin OR Nodularin OR anatoxin OR
Cylindrospermopsin OR BMAA OR Saxitoxin OR
Carbamate OR N-sulfocarbonyl OR Decarbamoyl OR
Deoxydecarbamoyl
L
imited search results to National Institute of
Environmental Health Sciences
5 5/25/2021
WHO/IARC
Microcystin OR Nodularin OR anatoxin OR
Cylindrospermopsin OR BMAA OR Saxitoxin OR
Carbamate OR N-sulfocarbonyl OR Decarbamoyl OR
Deoxydecarbamoyl
0 5/25/2021
Health
Canada
Searched for each cyanotoxin by provided name(s)
9 5/25/20215
CalEPA
Microcystin OR Nodularin OR anatoxin OR
Cylindrospermopsin OR BMAA OR Saxitoxin OR
11 5/26/2021

A-47
Source Search Strategy for Source
Results
(Number
of Titles)
Date Searched
Carbamate OR N-sulfocarbonyl OR Decarbamoyl OR
Deoxydecarbamoyl
Australian
Gov.
Department
of
Agriculture,
Water, and
the
Environment
Microcystin OR Nodularin OR anatoxin OR
Cylindrospermopsin OR BMAA OR Saxitoxin OR
Carbamate OR N-sulfocarbonyl OR Decarbamoyl OR
Deoxydecarbamoyl
1 5/27/2021
Water
Research
Australia
Microcystin OR Nodularin OR anatoxin OR
Cylindrospermopsin OR BMAA OR Saxitoxin OR
Carbamate OR N-sulfocarbonyl OR Decarbamoyl OR
Deoxydecarbamoyl
25 5/27/2021
European
Food Safety
Authority
Microcystin OR Nodularin OR anatoxin OR
Cylindrospermopsin OR BMAA OR Saxitoxin OR
Carbamate OR N-sulfocarbonyl OR Decarbamoyl OR
Deoxydecarbamoyl
1 6/02/2021
USGS
Searched for each cyanotoxin by provided name(s)
80 6/03/2021
CDC MMWR
Searched for each cyanotoxin by provided name(s)
F
YI – Can use the search bar at this link to search only
within MMWR:
https://www.cdc.gov/mmwr/mmwr_trends.html
12 06/14/2021
Total Unique
References
171
Topic 5 Title/Abstract Screening Criteria and Tagging
Two separate individuals screened the available literature based on the populations, exposures,
comparators, and outcomes (PECO) criteria and supplemental material categories in the tables
that follow. Studies were “included” if they met PECO criteria or fell into one of the
supplemental material categories. In cases where screener 1 and 2 disagreed, a third screener was
consulted to provide conflict resolution.

A-48
Populations, Exposures, Comparators, and Outcomes (PECO) Criteria
PECO
Element
Description
Populations
Human: Any population and lifestage (occupational or general population, including children and
other sensitive populations).
Animal: Non-human mammalian animal species (whole organism) of any lifestage (including
preconception, in utero, lactation, peripubertal, and adult stages). Examples include: rat, mouse,
rabbit, guinea pig, hamster, monkey, dog, mink.
-Studies of transgenic animals will be tracked as mechanistic studies under “potentially relevant
supplemental material.”
-Studies on alternative animal models and aquatic animal species will be tracked as
nonmammalian models system under “potentially supplemental material.”
-In vitro studies (including human or animal cells, tissues, or organs (not whole animals);
bacteria, nonmammalian eukaryotes) will be tracked as mechanistic information under
“potentially supplemental material.”
Exposures
Relevant forms:
Harmful algal blooms or their associated toxins (all forms of: anatoxins, BMAA,
cylindrospermopsin, microcystins, nodularins, and saxitoxins)
Metabolites used to estimate exposures
Human and Animal: Any exposure to oral, inhalation, dermal, intraperitoneal, or intravenous
injection, intravenous by dialysis routes of >1-day duration, or any duration assessing exposure
during reproduction or development.
Studies will also be included if biomarkers of exposure are evaluated (e.g., measured chemical or
metabolite levels in tissues or bodily fluids, anatoxin-a levels in human blood) but the exposure
route is unclear or likely from multiple routes.
Exposure measured in water (e.g., fresh, salt, surface) soil, fish or shellfish, dietary supplements,
edible plants will be tracked as “potentially supplemental material.”
Comparators
Human: A comparison or referent population exposed to lower levels (or no exposure/exposure
below detection limits), or exposure for shorter periods of time, or cases versus controls, or a
repeated-measures design. Case reports or case series of >3 people will be considered to meet
PECO criteria, while case reports describing findings in 1–3 people will be tracked as “potentially
relevant supplemental material.”
Animal: A concurrent control group exposed to vehicle-only treatment and/or untreated control
(control could be a baseline measurement e.g., acute toxicity studies of mortality, or a repeated-
measures design).
Outcomes
All health outcomes (cancer and noncancer). In general, endpoints related to clinical diagnostic
criteria, disease outcomes, histopathological examination, or other apical/phenotypic outcomes
are considered to meet PECO criteria and prioritized for evidence synthesis over outcomes such
as biochemical measures.

A-49
Categories of Potentially Relevant Supplemental Material
Category Evidence
Mechanistic
information
Studies reporting measurements related to a health outcome that inform the biological or
chemical events associated with phenotypic effects, in both mammalian and
nonmammalian model systems, including in vitro, in vivo (by any route of exposure,
includes transgenic models), ex vivo, and in silico studies. Genotoxicity tests are
considered “mechanistic.” Studies where the chemical is used as a laboratory reagent
generally do not need to be tagged (e.g., as a chemical probe used to measure antibody
response).
Toxicokinetic
(ADME)
Toxicokinetic (absorption, distribution, metabolism, and excretion; ADME) studies are
primarily controlled experiments, where defined exposures usually occur by intravenous,
oral, inhalation, or dermal routes, and the concentration of particles, a chemical, or its
metabolites in blood or serum, other body tissues, or excreta are then measured. These
data are used to estimate the amount absorbed (A), distributed (D), metabolized (M),
and/or excreted (E) through urine, breath, feces.
• The most informative studies involve measurements over time such that the
initial increase and subsequent concentration decline is observed, preferably at
multiple exposure levels. However, data collected from multiple tissues or
excreta at a single time-point also inform distribution.
• ADME data can also be collected from human subjects who have had
environmental or workplace exposures that are not quantified or fully defined.
However, to be useful such data must involve either repeated measurements over
a time-period when exposure is known (e.g., is zero because previous exposure
ended) *or* time- and subject-matched tissue or excreta concentrations (e.g.,
plasma and urine, or maternal and cord blood).
• ADME data, especially metabolism and tissue partition coefficient information,
can be generated using in vitro model systems. Although in vitro data may not be
as definitive as in vivo data, these studies should also be tracked as ADME. For
large evidence bases it may be appropriate to separately track the in vitro ADME
studies.
*Studies describing environmental fate and transport or metabolism in bacteria are not
tagged as ADME.
Classical
Pharmacokinetic
(PK) Model
Studies, or
Physiologically
based
Pharmacokinetic
(PBPK) Model
studies
Classical Pharmacokinetic (PK) or Dosimetry Model Studies: Classical PK or
dosimetry modeling usually divides the body into just one or two compartments, which are
not specified by physiology, where movement of a chemical into, between, and out of the
compartments is quantified empirically by fitting model parameters to ADME (absorption,
distribution, metabolism, and excretion) data. This category is for papers that provide
detailed descriptions of PK models, that are not a PBPK model.
• The data are typically the concentration time-course in blood or plasma after oral
and or intravenous exposure, but other exposure routes can be described.
• A classical PK model might be elaborated from the basic structure applied in
standard PK software, for example to include dermal or inhalation exposure, or
growth of body mass over time, but otherwise does not use specific tissue
volumes or blood flow rates as model parameters.
• Such models can be used for extrapolation like PBPK models, although such use
might be more limited.

A-50
Category Evidence
Physiologically based Pharmacokinetic (PBPK) or Mechanistic Dosimetry Model
Studies: PBPK models represent the body as various compartments (e.g., liver, lung,
slowly perfused tissue, richly perfused tissue) to quantify the movement of chemicals or
particles into and out of the body (compartments) by defined routes of exposure,
metabolism and elimination, and thereby estimate concentrations in blood or target tissues.
• Usually specific to humans or defined animal species; often a single model
structure is calibrated for multiple species.
• Some mechanistic dosimetry models might not be compartmental PBPK models
but predict dose to the body or specific regions or tissues based on mechanistic
data, such as ventilation rate and airway geometry.
• A defining characteristic is that key parameters are determined from
a
s
ubstance’s physicochemical parameters (e.g., particle size and distribution,
octanol-water partition coefficient) and physiological parameters (e.g., ventilation
rate, tissue volumes); that is, data that are independent of in vivo ADME data that
are otherwise used to estimate model parameters.
• Chemical-specific information on metabolism (e.g., Vmax, Km) or other
molecular processes (e.g., protein binding) might be obtained by fitting the model
to in vivo ADME data or determined from in vitro experiments and extrapolated
to in vivo predictions.
• They allow extrapolation between species, routes of exposure, or exposure
durations and levels; that is, they do not just quantify ADME for specific
experiments to which they have been fitted.
Nonmammalian
model systems
Studies in nonmammalian model systems (e.g., fish, birds, C. elegans). Ecotoxicity
studies, such as those on daphnia, algae, or aquatic plants, will be excluded unless they are
relevant to human health outcomes.
Non-PECO routes
of exposure
Exposure measured in water (e.g., fresh, salt, surface) soil, fish or shellfish, dietary
supplements, edible plants.
Exposure
characteristics (no
health outcome
assessment)
Exposure characteristic studies include data that are unrelated to health outcomes, but
which provide information on exposure sources or measurement properties of the
environmental agent (e.g., demonstrate a biomarker of exposure).
Mixture studies
Mixture studies are not considered to meet PECO criteria unless they use methods that
allow investigation of the exposure of interest by itself, rather than only evaluating effects
of the whole mixture. Methods used to assess investigation of the exposure by itself may
not be clear from the abstract, in particular for epidemiology studies. When unclear, the
study should be advanced to full text review to determine eligibility.
Case reports
Case reports of ≤3 subjects that describe health outcomes after exposure.
Records with no
original data
Records that do not contain original data, such as other agency assessments, informative
scientific literature reviews, editorials, or commentaries.
Conference
abstracts/abstract
only
Records that do not contain sufficient documentation to support study evaluation and data
extraction.

A-51
Topic 5: PRISMA Diagram
*Filters were applied to identify animal toxicology, epidemiology, and in vitro evidence.
B-1
Appendix B. Summaries of Studies Reviewed by EPA
This appendix includes summaries of studies that EPA found during the literature searches for
four topics, including:
1. Advances in molecular microbial source tracking (MST) for recreational water
applications.
2. Advances in molecular methods.
3. Advances in quantitative microbial risk assessment (QMRA) and epidemiological
studies.
4. Characterization of children’s risk from exposure to recreational water contaminated
by fecal contamination.
Only studies that passed the scope and study quality criteria (see Appendix A for the Literature
Search Strategies) are included in the table below. There are studies that are cited in the five-year
review that are not included in this table because those studies were for different topics or were
provided by subject matter experts independent of the literature search.

B-2
Table B-1. Brief Summary of Studies for Topics 1 through 4
Study Name
(*Denotes EPA
Authors)
Location
Study Type
(Search Topic)
Contamination
Sources
Water Quality
Metrics
Summaries
Abello et al.
(2021)
Laguna Lake,
Philippines
MST (culture
and molecular
methods)
Human, cattle,
swine
E. coli
A library-independent method of microbial source
tracking was used to isolate E. coli and detect heat-labile
toxin (LTIIA), heat-stable II (STII) genes, and human
biomarker mitochondrial DNA (mtDNA) NADH gene in
samples. Authors encouraged the use of E. coli LTIIa and
STII toxin genes for MST because they are host
associated and do not require large water sample volumes
for detection. Although fecal contamination at the study
site was attributed to agricultural sources (cow and pig),
the mtDNA was found to be a geographically stable
marker that was not influenced by diet (when compared to
fecal indicator bacteria).
Abia et al. (2016)
South Africa
QMRA
Human
E. coli, V. cholerae,
Salmonella spp. and
Shigella spp.
QMRA was conducted for the Apies River, Gauteng,
South Africa. Water samples were evaluated for E. coli
levels and the presence or absence of V. cholerae,
Salmonella spp. and Shigella spp. The study used the rate
of detection for calculating the probability of infection.
Based on information from the literature, the authors
assumed that 8% of E. coli counts were pathogenic and
estimated a probability of infection for pathogenic E. coli.
Ingestion rates used were 1 mL, 50 mL, and 100 mL to
represent different types of exposure, from incidental
ingestion to intentional ingestion.
Ahmad et al.
(2017)
NA – not
environmental
sampling
Method
development
NA – spiked
samples
Enterococcus
faecalis (ATCC,
19433); E. coli K12
strain C3000; Set of
six specific LAMP
primers (F3, B3,
FIP, BIP, LF, and
LB) for each target
gene
Using laboratory spiked samples, this study demonstrated
that Most Probable Number – Loop-Mediated Isothermal
Amplification (MPN-LAMP) can be used to quantify
bacteria in water using PCR without performing DNA
extraction. MPN-LAMP was demonstrated in both 25 μL
standard reaction volumes and in 1 μL microchip
reactions. Direct DNA amplification from bacterial cells
would eliminate sample processing steps and reduce the
complexity of gene analysis instruments. The authors
suggest that MPN-LAMP has the potential to replace the
conventional MPN method and would be valuable for use
with simple, sensitive, and rapid (25 minutes) integrated

B-3
Study Name
(*Denotes EPA
Authors)
Location
Study Type
(Search Topic)
Contamination
Sources
Water Quality
Metrics
Summaries
gene analysis systems with sample-in-answer-out
capability.
Ahmed et al.
(2016a)*
Brisbane River,
Brisbane
Australia;
Freshwater
creek in
Kissimmee
Florida
MST (culture,
qPCR)
Avian
E. coli, Fecal
coliforms,
Enterococcus spp.,
Helicobacter spp.
associated GFD
marker
The host specificity and prevalence of Helicobacter spp.
GFD marker (avian-associated) was evaluated by testing
fecal samples from both avian and non-avian host groups.
Using qPCR, mean concentrations of GFD marker were
found to be two orders of magnitude higher in avian
samples than non-avian samples. GFD marker was
detected in water samples collected in both Brisbane,
Australia and Kissimmee, Florida. The authors concluded
the GFD marker is highly specific to avian host groups
and could serve as an effective marker to detect the
presence and amount of avian fecal pollution in
environmental waters. Strong correlations between E. coli
and Enterococcus were observed in water samples from
both sampling locations, however concentrations of these
fecal indicator bacteria did not correlate with the
concentrations of the avian-associated GFD marker in
samples from these locations.

B-4
Study Name
(*Denotes EPA
Authors)
Location
Study Type
(Search Topic)
Contamination
Sources
Water Quality
Metrics
Summaries
Ahmed et al.
(2016b)
Samples
collected in
USA, Canada,
Singapore,
France,
Bangladesh,
Kenya, and
Belgium
MST (PCR,
qPCR)
Human, cattle,
deer, swine,
canine
Molecular markers:
HF183, HF134,
HuBac, BacHum-
UCD, BacH,
Human-Bac1,
HumM2, HumM3,
B.
thetaiotaomicron,
α-1.6-mannanase,
gyrB
Available research on MST methods that utilize
Bacteroidales/Bacteroides-associated genes as markers of
sewage pollution were reviewed. The advantages and
disadvantages of associated PCR-based methods used for
MST were discussed. The available literature supports the
use of the HF183 marker as it is well characterized with
respect to host sensitivity and specificity and presence in
sewage, worldwide. The available literature indicates that
HumM2 and HumM3 require more study. Limitations
associated with employing a one-marker-one-assay
approach for MST included challenges tied to using qPCR
for evaluating complex environmental water samples.
Ahmed et al.
(2016c)
Brisbane River,
Australia
Method
development –
qPCR
Wildlife, human
Laboratory seeded
human
adenoviruses
(HAdVs, target
Hexon) and human
polyomaviruses
Recovery efficiencies were compared for human
adenoviruses (HAdVs) and human polyomaviruses
(HPyVs) from 10-L river water samples seeded with raw
human wastewater (100 and 10 mL) using hollow-fiber
ultrafiltration (HFUF) and glass wool filter (GWF)
methods. The HFUF method provided better recovery for
HAdVs and HPyVs compared to the GWF method. The
mean recovery efficiencies using HFUF were 36%
(HAdVs) and 90% (HPyVs), whereas the mean recovery
efficiencies of HAdVs and HPyVs in this study for GWF
ranged from 1.3% to 3.4%. Another advantage of HFUF
is that the filters are readily available (also used in dialysis
treatment of patients).

B-5
Study Name
(*Denotes EPA
Authors)
Location
Study Type
(Search Topic)
Contamination
Sources
Water Quality
Metrics
Summaries
Ahmed et al.
(2018a)
Australia
(recreational
beaches, not
specified)
MST (qPCR);
QMRA
Human
MST markers:
Bacteroides HF183,
Methanobrevibacter
smithii nifH, HAdV,
HPyV, pepper mild
mottle virus
(PMMoV).
Reference
pathogens:
norovirus,
adenovirus
QMRA modeling was used to interpret MST markers.
Fresh untreated and secondary treated sewage were
seeded into filter-sterilized recreational water. Five qPCR
MST markers, Bacteroides HF183, Methanobrevibacter
smithii nifH, HAdV, HPyV, and PMMoV were evaluated
to determine at what concentrations of these MST markers
reflected a significant health risk from exposure to fresh
untreated or secondary treated sewage in beach water.
Using NoV and HAdV as reference pathogens to anchor
the risk level for QMRA, the authors determined the
HF183 concentration above 3.22 x 10^3 genome copies
(GC) in 100 mL samples represents a risk above the GI
illness benchmark value (0.036).
Ahmed et al.
(2018b)
Hillsborough
River, Tampa,
Florida, USA
MST (qPCR)
Human
CrAssphage, HF183
Host sensitivity and specificity was determined for
crAssphage marker in environmental river water seeded
with eight composite human samples (untreated sewage).
Method limit of quantification, process limit of
quantification, and DNA recovery efficiency using
TaqMan qPCR was determined. The mean concentrations
of the crAssphage marker were within the same order of
magnitude as HF183 concentrations. The authors
concluded the crAssphage marker is highly sensitive, but
less specific (cross reacts with poultry litter).
Ahmed et al.
(2019)
Lake
Parramatta,
Sydney,
Australia
MST (qPCR);
Method
development
Human
MST markers:
Bacteroides HF183,
crAssphage
CPQ_056
A duplex qPCR assay that allows for simultaneous
quantification of Bacteroides HF183 and crAssphage
CPQ_056 was developed. Multi-lab validation was
conducted using archived water samples collected from
Lake Parramatta in Sydney, Australia during a dry-
weather event and two storm events with gauged sewage
overflow. The simultaneous quantification of two marker
genes minimized false-negative results. The performance
characteristics of the duplex qPCR assay were similar to
its simplex counterparts. When compared to published
simplex qPCR assay results, the duplex qPCR assay
generated highly reproducible data with high sensitivity
and accuracy.

B-6
Study Name
(*Denotes EPA
Authors)
Location
Study Type
(Search Topic)
Contamination
Sources
Water Quality
Metrics
Summaries
Ahmed et al.
(2020)
Davidson Park,
Gymea Bay,
Hen and
Chicken Bay,
Lake
Parramatta,
Sydney,
Australia
MST (qPCR);
Method
development
Human
Bacteroides HF183,
crAssphage
CPQ_056, and
PMMoV
Interlaboratory agreement on qPCR-based testing for
Bacteroides HF183, crAssphage CPQ_056, and PMMoV
from samples collected from estuarine and freshwater
locations was investigated. An approximately 74%
agreement over qualitative co-detection when compared to
non-co-detection between laboratories was found.
However, laboratories were unable to produce comparable
quantitative results. The authors underscore the
importance of standardized protocols, laboratory
equipment, sample processing strategies, and appropriate
quality controls to further improve the accuracy and
precision of qPCR-based MST tracking of microbial gene
markers.
Arnold et al.
(2016)
United States:
Great Lakes
region;
Southern CA;
Gulf Coast,
Eastern
Seaboard,
Puerto Rico
Epi – multiple
cohort studies
Human; point
source sewage
discharge and
urban runoff
Enterococcus EPA
1600, IDEXX, EPA
1611
This analysis of 13 prospective cohorts pooled from
marine and freshwater beaches in the United States found
increased risk at the highest Enterococcus levels, which
supports their use as monitoring tools. Children under the
age of 10 had higher water exposure, GI risk, and illness
burden. Attributable illness estimates illustrated that
attaining EPA water quality guidelines is protective of
public health.
Arnold et al.
(2017)
San Diego, CA,
USA
Epi – cohort
study
Human; storm
runoff
Enterococcus
species US 1600,
fecal coliforms
9222D, total
coliforms 9222B
A longitudinal cohort study of surfers in CA waters to
evaluate wet weather health impacts was conducted. There
was a consistent increase in acute illness incidence rates
between unexposed, dry-weather, and wet-weather
exposure periods. Fecal indicator bacteria were strongly
associated with illness only during wet weather periods.

B-7
Study Name
(*Denotes EPA
Authors)
Location
Study Type
(Search Topic)
Contamination
Sources
Water Quality
Metrics
Summaries
Augustine et al.
(2020)*
Boquerón
Beach, Puerto
Rico
Epi-cohort
study
Human; Publicly
Owned Treatment
Works (POTW)
Enterococcus
USEPA 1600
A rapid population-based, salivary antibody screening
method was used to monitor hepatitis A virus (HAV)
immunoconversions in a population of beachgoers at
Boquerón Beach, Puerto Rico, where directly monitoring
pathogens in the water was unfeasible. Water samples
indicated good water quality with levels of Enterococcus
below the U.S. EPA RWQC. There were no statistically
significant associations between any of the demographic
or exposure risk factors tested and only 1.43% of the
participants who provided three samples were found to
have HAV immunoconversions. The study did not link
FIB levels to illness.
Aw et al. (2019)*
Michigan, USA
Method
development
(qPCR)
Human
E. coli
The performance of 21 laboratories was reported. The labs
had varying experience in meeting proposed, standardized
data quality acceptance (QA) criteria for EPA's draft
qPCR Method C for E. coli. QA criteria for the method
failed to meet in 24% of the 376 test samples analyzed. Of
these failures, 39% came from two of the “new”
laboratories. Deviations from recommended procedures
for the storage and preparation of reference and control
materials likely contributed to QA failure. The study
demonstrated the feasibility of multiple laboratories
implementing this qPCR method.
Ballesté et al.
(2018)
Spain
MST (qPCR,
culture,
Sewage and
wastewater from
abattoirs (human,
ruminant,
porcine, and
poultry waste)
E. coli, enterococci,
Bifidobacterium
spp. markers:
BifHM, BifCW and
BifPL,
Bacteroidales spp.
markers: HF183,
Rum2Bac, and
Pig2Bac
Decay rates of MST markers for human, ruminant,
porcine, and poultry waste were assessed in dialysis bags
filled with diluted wastewater from different sources and
kept in an outdoor water tank during winter and summer.
A higher variability among T90 values of the different
MST markers in winter was observed, whereas similar
T90 values were detected in summer indicating a stronger
effect of environmental parameters during winter. The
different decay trends for FIB observed in human and
animal faecal pollution sources is a key issue when natural
inactivation rates are used to model the kinetics of the
faecal pollution in a water catchment. Incorporating the
MST molecular markers would facilitate microbial faecal
pollution modeling.

B-8
Study Name
(*Denotes EPA
Authors)
Location
Study Type
(Search Topic)
Contamination
Sources
Water Quality
Metrics
Summaries
Beale et al.
(2017)
Brisbane River
and
Queensland,
Australia
Method
development
(culture, PCR)
Human
E. coli, enterococci,
other freshwater
bacteria identified
using
metagenomics
Water samples from five sites along the Brisbane River,
Queensland, Australia (rural and urban downstream
locations) were collected. The study utilized
metagenomics sequencing and reported the top 17 orders
of bacteria using Venn Diagrams. The metagenomics
output indicated a presence of high levels of freshwater
bacteria such as Burkholdariales and lower levels of
Actinomycetes and Rhodospirillae in the upstream sites.
In contrast, the population levels reversed in downstream
sites, affected by salinity, pH, and oxygen availability
changes. Human interference was indicated by the
increasing populations of Actinomycetes (BR3), including
fecal bacteria and Pseudomonadales (BR4and BR5). The
study also evaluated community metabolomics. The
majority of the identified metabolites were sugars, fatty
acids and amino acids. Secondary metabolites such as
perillyl alcohol, lithocholic acid and phytol were also
observed.
Benjamin-Chung
et al. (2017)
Southern
California,
Alabama, and
Rhode Island,
USA
QMRA/Epi
Human point and
non-point
sources, urban
runoff (varied by
location, some
had no known
contamination
sources)
Male-specific and
somatic coliphage,
enterococci
Six prospective cohort studies at coastal beaches in the
United States using enterococci as water quality indicator
were evaluated. Associations between enterococci levels
and GI illness were observed only under human-impacted
conditions. The cumulative incidence ratios for a 1 log
10
increase of enterococci was 1.21 (95% 1.01, 1.46).

B-9
Study Name
(*Denotes EPA
Authors)
Location
Study Type
(Search Topic)
Contamination
Sources
Water Quality
Metrics
Summaries
Blanch et al.
(2020)
NA – review
article
Method
development
(culture, “fast
culture
methods,”
molecular
methods)
NA – review
article
MSC and Somatic
coliphages
Somatic coliphage and MSC detection and enumeration
methods were reviewed with the aim of outlining
improvements of standardized techniques. Weaknesses in
standardized techniques included long operating times,
complex procedures, and readability of plates. The authors
described several new approaches for fast and feasible
coliphage detection including plaque assay improvements,
lysis detection in liquid media, application of molecular
methods, and electronic sensors. Lysis detection in
selective media was underscored as being a specific, fast,
and feasible approach to enumerating coliphages in
contaminated waters.
Boehm (2019)
Mostly U.S.
sewage
samples
QMRA
Human; untreated
sewage
reference
pathogens:
Salmonella,
Campylobacter, E.
coli 0157:H7,
Cryptosporidium,
Giardia, Norovirus,
Adenovirus;
Indicators: somatic
coliphage, MSC
QMRA was used to estimate the levels of somatic and
MSC in sewage impacted recreational waters that
correspond to the 32/1,000 GI illnesses from exposures to
a suite of bacteria, viruses, and protozoa. The risk-based
water quality threshold for somatic and MSC was 60 PFU
per 100 mL and 30 PFU per 100 mL, respectively, for
fresh sewage contamination. The thresholds decrease as
the contamination ages because, on average, coliphages
decay more quickly than norovirus, the pathogen that
contributes the most to risk.

B-10
Study Name
(*Denotes EPA
Authors)
Location
Study Type
(Search Topic)
Contamination
Sources
Water Quality
Metrics
Summaries
Boehm and Soller
(2020)
Mostly U.S.
wastewaters
QMRA
Human and
animal (fresh and
aged sewage and
gull feces)
Reference
pathogens included
Salmonella (CFU),
Campylobacter
(MPN), E. coli
O157:H7,
Cryptosporidium
(oocysts), Giardia
(cysts), norovirus
(gene copies), and
adenovirus
(infectious unit).
Indicators included
HF183 (gene
copies) and
enterococci (CFU)
QMRA was used to estimate a risk-based water quality
threshold for human marker HF183 in ambient waters
corresponding with the health benchmark of 32
illnesses/1,000 recreators for different contamination
scenarios. The threshold of 525 HF183 copies/100 mL
was estimated for human contamination of unknown age
assuming a mix of aged sewage and in the range of 1 to
525 HF183 copies/100 mL when gull contamination was
present.
Boehm et al.
(2018)
Global
QMRA, review,
meta-analysis
Human;
Untreated sewage
of known age
Reference
pathogens:
Salmonella spp,
Campylobacter, E.
coli O157:H7,
Cryptosporidium,
Giardia, norovirus.
Indicator: HF183
A systematic review, meta-analysis and QMRA were
conducted to evaluate decay rate constants for pathogens
and fecal contamination indicators (HF183). The decay
rate constants were used in a risk assessment to evaluate
ingestion exposure to water polluted with untreated
sewage of different ages, using the human fecal marker
HF183.

B-11
Study Name
(*Denotes EPA
Authors)
Location
Study Type
(Search Topic)
Contamination
Sources
Water Quality
Metrics
Summaries
Bonadonna et al.
(2019)
NA – review
article
Method
development
(culture,
molecular
methods)
NA – review
article
Various – review
article
This review discussed the advantages and disadvantages
of a wide array of analytical methods for monitoring
microbial water quality. These methods include rapid
culture-based methods (chromogenic and fluorogenic
substrates), cultivation-independent detection methods
(ATP cell viability luciferase assay, flow cytometry,
biosensors), microbial identification by mass spectrometry
(matrix-assisted laser desorption ionization time-of-flight
(MALDI-TOF)), and molecular techniques (real-time
PCR, digital PCR, nucleic acid sequence-based
amplification (NASBA), DNA microarray, loop-mediated
isothermal amplification (LAMP), next generation
sequencing (NGS): amplicon sequencing, whole-genome
sequencing and metagenomics). The review concluded
that ideal real-time analysis cannot be routinely achieved,
but recent developments have made it possible to detect
many indicator organisms and pathogens in water within a
few hours or in the same day.
Bortagaray et al.
(2020)
Santa Lucia
River and
Uruguay River
in region
between
Argentina,
Brazil, and
Uruguay
Children
Sewage,
agriculture
activities
(livestock,
horticulture, and
fruit production)
Group A Rotavirus
(RVA)
This study detected, quantified, and assessed risk of
infection and illness for RVA in the watersheds of the
Santa Lucia and Uruguay rivers. The authors performed
qPCR on surface water samples and QMRA for people
that use surface waters from the aforementioned rivers,
including children. Both rivers had comparable risks of
infection and illness, as RVA was consistently detected in
surface waters.
Brooks and Field
(2017)
Corvallis,
Oregon, USA
MST (qPCR)
Cattle
E. coli, MST
markers: GenBac3,
CF128, Rum2Bac,
CowM2, CowM3
Decay profiles for culturable E. coli, Bacteroidales
genetic markers (GenBac3, CF128, Rum2Bac), and cattle
markers (CowM2, CowM3) were generated. DNA
samples were amplified using qPCR to quantify marker
concentrations. The study results are useful for future
work studying the decay of faecal MST markers, e.g.,
identifying the limitations of highly specific but less
prevalent markers like CowM2 and CowM3.

B-12
Study Name
(*Denotes EPA
Authors)
Location
Study Type
(Search Topic)
Contamination
Sources
Water Quality
Metrics
Summaries
Brown et al.
(2017a)
California,
USA
QMRA, MST
(qPCR)
Human, gull
Bacteroides dorei,
MST markers:
HF183, CAT
Human and gull-associated fecal markers were used as
indicators in a QMRA estimating the probability of illness
due to swimming in contaminated recreational water. The
authors detected human markers in most of the treated
effluent samples and at lower concentrations compared to
raw sewage. Given the concentrations of HF183 in treated
effluents, treated effluent is unlikely to be responsible for
HF183 in ambient waters above 10^4 copies per 100 ml.
Brown et al.
(2017b)
California,
USA
QMRA, MST
(qPCR)
animal feces, gull
16S rRNA gene
copies of
Catellicoccus
marimammalium
(CAT) through
qPCR
There are no guidelines for interpreting measured
concentrations of CAT genes in recreational waters. The
authors collected 37 gull fecal samples, measured the
CAT gene concentrations, and integrated the
measurements in a QMRA framework using dose-
response functions from reference pathogens. They
estimated that when the level of CAT surpasses 4 × 10^6
copies per 100 mL of water, the median predicted illness
exceeds three illnesses per 100 swimmers.
Brumfield et al.
(2021)
Creek in urban
watershed,
USA
MST (qPCR,
whole
metagenome
sequencing);
Method
development
Human, avian,
dog, ruminant
E. coli, enterococci,
MST markers:
HF183, BacR287,
Rum2Bac, DG3,
GFD, Entero1a,
EC23S857
Culture for FIB, qPCR amplification of FIB and host-
associated genetic markers, and whole metagenome
sequencing (WMS) were used to detect, identify, and
enumerate bacteria, archaea, fungi, protists, and viruses in
an urban watershed before and after a storm event. Fecal
contamination from multiple sources (human, avian, dog,
and ruminant), as well as FIB, enteric microorganisms,
and antibiotic resistance genes increased demonstrably
after a storm event. The addition of qPCR and WMS to
traditional techniques may provide enhanced
characterization and improved understanding of microbial
pollution sources in ambient waters.

B-13
Study Name
(*Denotes EPA
Authors)
Location
Study Type
(Search Topic)
Contamination
Sources
Water Quality
Metrics
Summaries
Bushon et al.
(2017)
Little Blue
River, Kansas
City, Missouri,
USA
MST (culture,
qPCR)
Human, cats,
chickens, cows,
deer, dogs, geese,
horses, mice,
rabbits, wild
turkeys
E. coli, MST
markers: AllBac,
HF183, BacCan,
BoBac
Culture and qPCR methods were used to analyze water
samples in the Little Blue River in Missouri over a 7-year
period for concentrations of E. coli and human, canine,
and ruminant-associated markers. The authors used
microbiological and hydrological data to rank the streams
contributions of bacteria. The results showed that
stormwater contained high levels of E. coli and, in certain
tributaries, high levels of human, canine, and ruminant
markers. The methodologies used in this study may prove
useful in prioritizing remediation in different sub-basins.
Byappanahalli et
al. (2018)
Sleeping Bear
Dunes National
Lakeshore,
Benzie County,
Michigan, USA
Method
development
(culture, qPCR)
Not reported
E. coli, enterococci
This study compared culture methods (EPA Method 1603
for E. coli and EPA Method 1600 for enterococci) to
qPCR-based methods (modified EPA Method 1611 qPCR
for Enterococcus and Chern et al., 2011 qPCR for E. coli)
at coastal beaches and rivers located at the Sleeping Bear
Dunes National Lakeshore in Michigan. Overall, the
culture-based and qPCR-based results for both indicators
were correlated in this study, leading authors to conclude
that qPCR may be a viable alternative method to the
culture-based method for monitoring water quality on
public lands. The results indicated that qPCR methods
would have resulted in fewer water quality advisories, but
the increased benefit of same-day results provided by
qPCR provides better protection overall.
[Chern, E.C., S. Siefring, J. Paar, M. Doolittle, and R.A.
Haugland. 2011. Comparison of quantitative PCR assays
for Escherichia coli targeting ribosomal RNA and single
copy genes. Lett. Appl. Microbiol. 52:298–306.]
Cao et al. (2016)
USA
Method
development;
MST (qPCR,
duplex dPCR);
Human (spiked)
Enterococcus spp.,
HF183
A method for duplex droplet digital PCR, which measures
enterococci and the human marker HF183 simultaneously,
was reported. They also demonstrated that ddPCR is more
resistant to inhibition by humic acid than qPCR. qPCR
had almost no amplification at 5 ng/uL of humic acid,
whereas ddPCR quantification was still within the 95%
confidence level above 15 ng/uL humic acid.

B-14
Study Name
(*Denotes EPA
Authors)
Location
Study Type
(Search Topic)
Contamination
Sources
Water Quality
Metrics
Summaries
Cao et al.
(2018a)*
Escondido,
Marie Canyon,
and Topanga,
California,
USA
MST (qPCR)
Human (spiked)
HF183, BacR287
A standardized HF183/BacR287 qPCR method was used
to establish a human fecal contamination score (HFS) in
42 samples across three marine surface water sites in
coastal California. The authors showed that site
prioritization with HFS is feasible. They also observed
that sampling intensity and the number of qPCR replicates
affect HFS estimates’ reliability. The effort could help
future users design studies aiming for optimal HFS
performance.
Cao et al. (2018b)
USA
MST (droplet
digital TM
PCR); Method
development
Human (spiked)
Enterococcus spp.,
HF183
This study provided a complete method protocol for
Droplet Digital
TM
PCR, which allows for simultaneous
testing for a general microbial water quality indicator
(Enterococcus spp.) and a human-associated fecal marker
(HF183) in environmental waters from marine and
freshwater beaches. This method offers the opportunity to
maintain qPCR’s advantages while addressing qPCR’s
major imitations, which include the need for standard
curves and the impact of inhibition on method
performance.
Chandler et al.
(2017)
Massachusetts,
USA
Method
development
(RT-PCR)
Fecal
contamination
(source not
specified)
F-specific RNA
(FRNA) coliphages,
enteric viruses
An anion exchange resin-based system to concentrate
FRNA coliphages and enteric viruses, followed by RNA
isolation and RT-PCR detection was developed. The
performance of the anion exchange resin-based
methodology was tested using 65 environmental marine
and freshwater samples considered to be fecally
contaminated. The method facilitated detection of FRNA
coliphages in 61.5% samples, which provides evidence of
viability of this system to concentrate FRNA coliphages
in water. This system provides a rapid, ease to use, and
inexpensive method to concentrate coliphages in water,
and it is particularly useful in places that need frequent
sampling to monitor water status as well as to identify the
contamination sources.

B-15
Study Name
(*Denotes EPA
Authors)
Location
Study Type
(Search Topic)
Contamination
Sources
Water Quality
Metrics
Summaries
Chen et al. (2020)
Beijing, China
MST (qPCR)
Human, dog, bird
Bacteroidetes, MST
markers for dog,
bird, humans (not
specified)
Surface water was sampled near Beijing Normal
University during four rain events. The relationships
between the copy number of fecal microbes and other
environmental factors including the rainfall time, total
amount of rainfall, number of antecedent drying days,
runoff amount, peak flow rate, and duration of rainfall
were assessed. The number of antecedent drying days was
the key factor for dog fecal pollution, while human fecal
pollution was impacted by more factors. The bird source
had a weak correlation with the runoff volume and had no
correlation or even a negative correlation with other
environmental factors.
Chyerochana et
al. (2020)
Tha Chin
River, Thailand
MST (culture,
qPCR); Method
development
Human
Enterococci, E.
faecalis phages
(strains AIM06 and
SR14)
EPA Method 1611 (Enterococcus qPCR) and enterococci
culture method (EPA Method 1600) were used to measure
bacteriophages of E. faecalis strains AIM06 and SR14
from freshwater of the Tha Chin River in Central
Thailand. AIM06 and SR14 phages were present at
similar levels as other human-specific bacteriophages of
E. faecalis isolated and detected in other geographical
regions and in environmental water samples. Both phages
were present in 92.9% of freshwater samples. Culture and
qPCR method results showed strong correlation with
human-specific DNA markers (Kendall’s tau = 0.600) as
well as moderate correlation between each other
(Kendall’s tau = 0.445). The correlation between culture
and qPCR was found to be only moderate due to intrinsic
difference in enterococci specificity. qPCR detected genes
found in all enterococci species, whereas the culture
method is specific to certain species including E. faecalis.
Double-layer agar assay method to detect enterococci
phages indicated continuing fecal pollution with no
significant level of variance between stations. The authors
concluded that AIM06 and SR14 phages are low-cost
MST tools for pollution source identification in freshwater
and coastal water.

B-16
Study Name
(*Denotes EPA
Authors)
Location
Study Type
(Search Topic)
Contamination
Sources
Water Quality
Metrics
Summaries
Cloutier and
McLellan (2017)
Lake Michigan,
Wisconsin,
USA
MST (culture,
qPCR)
Human, gull,
ruminant
E. coli, MST
markers: Entero,
Lachno2, HB
(human
Bacteroides), Gull2,
BacR
Recreational water at six Wisconsin beaches was
assessed. The gull-associated Catellicoccus
marimammalium (Gull2) marker was found in over 80%
of water samples, regardless of E. coli levels. Human-
associated Bacteroides (HB) and Lachnospiraceae
(Lachno2) were detected in only 2.4% of water samples
collected under baseflow and post-rain conditions but
produced a robust signal after a combined sewage
overflow, despite low E. coli concentrations. Microcosm
studies to assess decay found that Gull2, HB, and
Lachno2 qPCR signals were reduced twice as quickly as
those from E. coli and enterococci and approximately
20% faster than signals from culturable E. coli.
Codello et al.
(2021)
Hawkesbury-
Nepean River,
Sydney,
Australia
MST (qPCR)
Human
MST markers: uidA
(E. coli), HF183,
Lachno3
A weight-of-evidence approach was used to identify
untreated sewage contamination in the Hawkesbury-
Nepean River in Sydney, Australia. The authors used a
qPCR method targeting markers for human E. coli,
Bacteroidales, and Lachnospiraceae. They found that
using multiple indicators was more effective than using
individual techniques, overcoming their independent
limitations (e.g., E. coli detection).
Cormier and
Janes (2016)
NA – spiked
samples
Method
development
(qPCR)
NA – spiked
samples
Human adenovirus
(HAV), MS2 phage
A method to concentrate HAV from seawater using
zeolite was developed to aid rapid detection. Artificial
seawater was inoculated with HAV and MS2 and filtered
with zeolite. The viruses were detected using qPCR.
Zeolite was able to concentrate HAV and MS2 from
artificial seawater with 99% and 90% efficiencies,
respectively.

B-17
Study Name
(*Denotes EPA
Authors)
Location
Study Type
(Search Topic)
Contamination
Sources
Water Quality
Metrics
Summaries
Crain et al.
(2021)
San Diego
County,
California,
USA
Method
development
(ddPCR, qPCR,
culture:
Enterolert)
Urban runoff,
human (varied
with location,
some samples
were not
impacted by
known
contamination
sources)
Enterococcus spp.
This study compared a ddPCR method (Cao et al., 2018),
to both an EPA-approved culture method SM9230D
(Enterolert) and an EPA-approved qPCR method (EPA
Method 1609.1). Findings supported the conclusion that
ddPCR readouts align closely with Enterolert MPN for
identifying FIB exceedance levels of Enterococcus spp. in
coastal waters of San Diego, CA. Findings also suggested
that ddPCR and genomic 23S rDNA could be effectively
employed as an alternative indicator for beach
management decisions in Southern California.
Crank et al.
(2019)
Adenovirus
data from
Spain, data for
PMMoV from
multiple
countries. All
other data are
from the United
States
QMRA
Fresh untreated
domestic
wastewater (no
specific study
location)
Reference
pathogens:
Salmonella,
Campylobacter, E.
coli O157:H7,
Cryptosporidium,
and Giardia. Viral
water quality
indicators included:
CrAssphage and
PMMoV
A web-based QMRA model that evaluates adults’ risk of
illness from incidental ingestion of recreational water
polluted by fresh wastewater was developed. CrAssphage
and PMMoV were used as viral indicators of human waste
to estimate the risk of illness in the aforementioned
scenario, comparing indicator concentrations to several
viral pathogens’ concentrations and using the pathogen
dose-response functions. The model is accessible through
a web-based interface and can be adapted to other
indicators or pathogens.
Crank et al.
(2020)
Italy
MST (qPCR);
Method
development
Human
crAssphage
(CPQ56), human
polyomavirus
(HPyV), human
bocavirus (HBoc),
hepatitis E virus
(HepE)
CrAssphage abundance was assessed in 156 Italian
wastewater samples. CrAssphage correlation with other
molecular viral markers (human bocavirus, Hepatitis E
virus, and human polyomavirus) was reported.
CrAssphage was present in 150 out of 156 samples, with a
96% overall detection rate. This is notable, as prior studies
have shown a 100% crAssphage detection rate in
untreated wastewater. Most WWTPs represented in this
study are in populous, urban areas, and so results
presented were representative of a large mixture of diets
and potential fecal pathogens in wastewater.

B-18
Study Name
(*Denotes EPA
Authors)
Location
Study Type
(Search Topic)
Contamination
Sources
Water Quality
Metrics
Summaries
Cui et al. (2017)
Olympic Forest
Park, Beijing,
China
Method
development
(next generation
sequencing,
qPCR)
Human
Various pathogens,
including marker
genes of pathogenic
Aeromonas spp.,
Salmonella spp., M.
Avium, P.
aeruginosa
16S rRNA gene targeted next generation sequencing and
qPCR were used to analyze pathogen diversity and
quantify pathogens in samples collected from waterbodies
in Beijing Olympic Forest Park. The authors used these
methods to provide a more comprehensive picture into the
bacterial pathogen diversity in wastewater. While qPCR
provides a quicker result with better specificity and
sensitivity, its detection is limited to the scope and targets
of its surveyors and does not capture the total complexity
of the environment.
DeFlorio-Barker
et al. (2018)*
Multiple
locations in the
U.S.:
California,
Alabama,
Mississippi,
South Carolina,
Indiana,
Michigan,
Ohio, Maine
Children;
Epi/Exposure
Not explicitly
defined
Did not target
microorganisms or
genes; estimated
recreational water
exposure through
ingestion
Data from 12 prospective cohorts with a total sample size
of 68,685 subjects were pooled. Stochastic modeling was
conducted to estimate the range of incidental water
volume ingestion by beachgoers (children and adults).
The authors identified that children are more likely to
have greater exposures by spending more time in the
water and direct contact with algae and sand (compared to
adults). Children, specifically ages 6 to 12 years, swallow
more water per swimming event than any other age group
(median of 36 mL; 90th percentile = 150 mL). In
comparison, adults ≥35 years old swallow 9 mL (90th
percentile = 64 mL) per swimming event. Additionally, on
average, males swallow more water than females per
event. This puts these individuals at a higher risk for
becoming ill after swimming at recreational beaches
compared to adults.

B-19
Study Name
(*Denotes EPA
Authors)
Location
Study Type
(Search Topic)
Contamination
Sources
Water Quality
Metrics
Summaries
Derx et al. (2021)
Vienna, Austria
MST; QMRA
Human, pig,
ruminant, duck
Cryptosporidium,
Giardia, E. coli,
unspecified MST
markers for pig,
ruminant, human,
duck, cow, bird
QMRA was conducted using measured concentrations of
human, ruminant, pig, and bird-associated MST markers
as well as E. coli in a Danube wetland area. The scenarios
investigated included: (i) the impact of river discharges
into the backwater channel (allochthonous sources), (ii)
the resuspension of pathogens from animal fecal deposits
in inundated areas, and (iii) the pathogen release from
animal fecal deposits after rainfall (autochthonous
sources). The study is an integrative modeling approach
for determining the transfer rates of pathogens from
diverse
fecal sources into alluvial wetlands during storm events
and
floods. Although surface water was considered, the focus
was safe drinking water supply.
Deshmukh et al.
(2016)
NA – review
article
Method
development
(various)
NA – review
article
E. coli, Shigella
spp., Salmonella
spp., Vibrio
parahaemolyticus,
Pseudomonas
aeruginosa,
Bacillus anthracis,
Brucella abortus, C.
botulinum, Coxiella
burnetii,
Francisella
tularensis,
Rickettsia
prowazekii, C.
perfringens,
Staphylococcus
aureus, V. cholerae,
Vibrio
alginolyticus,
Yersinia pestis
This review summarized the current state of rapid
methods for the monitoring and detection of waterborne
bacterial pathogens. The nucleic acid-based, immunology-
based, and biosensor-based detection methods studied in
this review were found to be sensitive, specific, time-
effective, and important in prevention and diagnosis of
waterborne bacterial diseases.

B-20
Study Name
(*Denotes EPA
Authors)
Location
Study Type
(Search Topic)
Contamination
Sources
Water Quality
Metrics
Summaries
Devane et al.
(2019)
Avon River,
Christchurch,
New Zealand
MST (culture,
qPCR)
Human, dog,
wildfowl
E. coli, coliphage,
Campylobacter
measured using
culture; MST
markers: GenBac3,
HumM3, B. adol,
HumBac, HF183,
Bac708R primers,
canine-associated
sources (referred to
as a dog marker, not
specified)
MST markers (GenBac3, HumM3, HumBac (HF183-
Bac708R); Bifidobacterium adolescentis, wildfowl and
canine-associated markers), fecal indicator bacteria (E.
coli), and pathogens (Campylobacter) were detected in
sewage-contaminated river water collected in New
Zealand. Human-associated qPCR markers were found in
water samples collected after known sewage discharge
events. The authors reported that the concentration of the
B. adol and HumBac PCR markers were in general,
tenfold lower than the general fecal PCR marker,
GenBac3, and the HumM3 PCR marker was
approximately two orders of magnitude lower than the
other two human PCR markers.

B-21
Study Name
(*Denotes EPA
Authors)
Location
Study Type
(Search Topic)
Contamination
Sources
Water Quality
Metrics
Summaries
Dorevitch et al.
(2017)
Lake Michigan,
Chicago,
Illinois, USA
Method
development
(culture, qPCR)
Noted that the
Chicago River
system protects
Lake Michigan
and its beaches
from point
sources of fecal
pollution
E. coli,
Enterococcus
faecalis
Water samples (n = 1,796) collected in 2015 and 2016 at
nine Lake Michigan beaches in Chicago, Illinois were
analyzed by E. coli culture (Colilert method) and
Enterococcus qPCR (EPA Methods 1609 and 1609.1).
Unlike many other studies that compare culture and qPCR
results from the same water sample (same day), this study
was interested in what data would actually be available for
beach management decisions. From the standpoint of
deciding whether qPCR or culture methods should be
used for daily public notification purposes, it is the
agreement between Day0 culture BAV and Day1 qPCR
BAV (not Day0 vs. Day0) that matters. This study looked
at the two pieces of information available to beach
managers on a given day: Enterococcus qPCR results of
samples collected that morning and E. coli culture results
of samples collected the previous day. The study found
that if the Day1 qPCR result not been available, Day0
culture results would have triggered unnecessary beach
advisories 24% of the time (“false alarms”). And, on 4.7%
of the beach-days, Day0 culture results would have
resulted in a “failure to act” (meaning, failure to trigger
advisories) when advisories were needed (based on
exceedance of the Day1 qPCR BAV). At one beach,
“failure to act” would have occurred 6.8% of the time.
Overall, Day0 culture results were more likely to result in
a “failure to act” (n = 32) than a “correct advisory”
(n = 13). Additionally, this study took chance into
account. The 71.3% concordance of Day1 qPCR and
Day0 culture beach actions can be explained entirely by
chance. The authors conclude that no meaningful
agreement was observed between beach management
actions driven by Enterococcus qPCR results versus E.
coli culture results and that there is little scientific
rationale for continued E. coli culture testing of beach
water in this setting.

B-22
Study Name
(*Denotes EPA
Authors)
Location
Study Type
(Search Topic)
Contamination
Sources
Water Quality
Metrics
Summaries
Duan et al.
(2016)
Three rivers
(Jialing River
in Chongqing
and the Han
River in
Hanzhong and
The Wenxian
River in
Jiaozuo), a
pond in Beibei,
Chongqing, and
seawater in
Yantaim, China
MST
swine, human,
chicken, duck,
cow, dog
Faecalibacterium
16SrRNA gene
sequences
qPCR detection of genetic markers within the 16S rRNA
gene of Faecalibacterium were used for the identification
of swine fecal contamination in surface waters in China.
Using 454 pyrosequencing, the authors obtained a total of
over 146,000 bacterial sequences and assessed their
specificity to swine fecal waste. Two PCR primer sets,
PFB-1 and PFB-2, had no cross-reaction with other
animal samples, though PFB-2 was more sensitive than
PFB-1. PFB-2 was detected in concentrations ranging
from 100 to 10,000 copies per 100 mL of water in sites
near swine farms.
Dufour et al.
(2021)*
NA
Review
NA
NA
This review summarizes methods used for enumeration of
fecal indicators in recreational water from the 1930s to
2021.
D'Ugo et al.
(2019)
Castel
Giubileo,
Mezzocamino,
Albano Lake,
Aniene,
Pertusillo Lake,
Rome, Italy
MST (RT-PCR,
qPCR); Method
development
Human,
unspecified
agricultural
animals
Human adenovirus
(ADVTot = 51
types of human
adenoviruses,
ADV41, ADV40);
human enterovirus,
HEV, HAV;
mammalian
orthoreoviruses;
norovirus (GI, GII)
qPCR was used to analyze samples collected from lakes
and rivers in Italy. The study targeted significant enteric
viruses and results were compared to poliovirus 1 as a
reference virus. No evidence of inhibition was observed in
the samples. All samples showed the presence of at least
one viral species, with the most frequently detected being
ADVTot and ADV41. Human enterovirus was not
detected in any water samples. From the study, it was
suggested that human fecal contaminants are prevalent in
all kinds of surface waters. While controls showed
recovery of MS2 phage (similar in size to Human
Enteroviruses) in spiked water samples, caution must be
taken when concentrating human enteroviruses such as
poliovirus, coxsackievirus, and echovirus because they
might be too small to be recovered in the filtration
processes.

B-23
Study Name
(*Denotes EPA
Authors)
Location
Study Type
(Search Topic)
Contamination
Sources
Water Quality
Metrics
Summaries
Egorov et al.
(2018)*
Lawrence,
Massachusetts,
USA
Children
Not reported,
presumed human
sewage
NA
This study found no association between infection with
noroviruses and swimming in natural waterbodies (rivers,
lakes, or oceans). Swimming in natural waterbodies was
associated with 2.33 adjusted odds ratio (95% confidence
interval 0.35; 15.4) of immunoconversion to
Cryptosporidium. An association was not found between
immunoconversion to norovirus (GI or GII) and
swimming in natural waterbodies. Swimming in all types
of natural waterbodies was only reported among 3% of the
study participants (n= 165) likely because the survey was
administered in the fall. The authors concluded that the
salivary antibody assay can serve as a valuable tool for
assessing these risk factors.
Eregno et al.
(2016)
Norway
QMRA
Human, sewer
overflows
Indicators: E. coli.
Reference
pathogens: Giardia,
Cryptosporidium,
Norovirus,
Salmonella, and
Campylobacter
A hydrodynamic water quality model was coupled with
QMRA to estimate the risk of infection from swimming in
marine waters following a rainfall event with combined
sewer overflows. Of the simulated bacteria, protozoa and
virus infection risks, the virus risk dominated, as
represented by norovirus, and exceeded the infection
health benchmark of 19 illnesses per 1,000 recreators in
the days after the rainfall event.
Fan et al. (2017)
Southeastern
China
MST (qPCR)
Human, swine,
cow, goat, sheep,
chicken, duck,
goose, dog
HF-183
This study sought to identify unique genetic sequences for
bacteria that were specific to swine fecal sources present
in natural water samples collected in southeastern China.
Genome fragment enrichment (GFE) was determined to
be useful in differentiating between different fecal
metagenomes. Two GFE sequences in Bacteriodales-like
organisms were determined to be strongly swine-specific
for sources of fecal contamination when testing natural
waters with qPCR for type of organism responsible for
fecal contamination.

B-24
Study Name
(*Denotes EPA
Authors)
Location
Study Type
(Search Topic)
Contamination
Sources
Water Quality
Metrics
Summaries
Fang et al. (2018)
Beijing, China
Method
development
(next generation
sequencing,
qPCR)
Human
E. coli, Salmonella
enterica,
Aeromonas spp.,
Mycobacterium
avium,
Pseudomonas
aeruginosa
Next generation sequencing was used to determine the
diversity of genera containing pathogens, and qPCR was
used to assess the presence of genes from E. coli,
Salmonella enterica, Aeromonas spp., Mycobacterium
avium, and Pseudomonas aeruginosa. The authors
recommended using combinations of culture-dependent
methods in order to provide comprehensive information
concerning pathogen diversity and distribution.
Farkas et al.
(2018)
North Wales,
United
Kingdom
Method
development
(RT-qPCR,
qPCR, PGM-
MB)
Human
Adenovirus type 40,
JC and BK
polyomavirus,
Norovirus genotype
I and II, Sapovirus
genotype I,
Hepatitis A and E
viruses
A variety of enteric viruses were monitored by RT-qPCR,
qPCR, and porcine gastric mucinconjugated magnetic
beads (PGM-MB) at freshwater and marine shores in the
United Kingdom. PCR-based approaches do not address
viral integrity and infectivity, hence the need to evaluate
viral degradation using the PGM assay. The results were
consistent in diluted nucleic acid extracts, hence inhibition
during detection was unlikely. The study used EPA
Method 1615 for the quantification of enteric viruses and
the authors concluded that their data can be applied to
inform predictive models for the transport of enteric
viruses in water and to improve current viral risk
assessment.
Federigi et al.
(2019)
Global
Review/QMRA
Human and
multiple animals
(not specified)
various
PRISMA (Preferred Reporting Items for Systematic
Reviews and Meta-Analyses) guidelines were used to
review QMRAs of recreational waters published between
2003 and 2018. Found research gaps: 1) lack of
epidemiological data on the main pathogens of concern in
specific geographic areas; 2) lack of site-specific water
quality data; 3) little consideration of recovery efficiency
or infectivity of pathogens; 4) lack of consideration of
host-specific factors (like previous immunity or
immunodeficient); 5) lack of QMRA model validation.

B-25
Study Name
(*Denotes EPA
Authors)
Location
Study Type
(Search Topic)
Contamination
Sources
Water Quality
Metrics
Summaries
Feng and
McLellan (2019)
Milwaukee,
Wisconsin,
USA
MST (qPCR);
Method
development
Humans, pigs,
dogs, cats, deer,
cows, gulls,
chickens, human
Bacteroides
qPCR of the V6 and V4V5 regions of Bacteroides were
used to specifically target sewage-derived Bacteroides.
The V6 and V4V5 regions contained human fecal
sequences that were not part of the HF183 cluster, making
these two regions appropriate for detecting sewage-based
Bacteroides. The authors also suggested that qPCR and
next generation sequencing could be used together for
source tracking.
Ferguson et al.
(2019)
Florida and
Texas, USA
Children
Not reported,
focus was on oil
contaminants
NA
The behavior of children ages 1 to 6 years old was studied
at recreational marine beaches in Florida and Texas. The
study found that age was the most relevant factor in how
and where children play (i.e., dry sand, intertidal area,
water) and that hygiene practices like washing hands
before eating at the beach varied by region. The data from
this study can give insight into microbial exposures
children might be exposed to in these locations.
Ferguson et al.
(2021)
Florida and
Texas, USA
Children
Not reported,
focus was on oil
contaminants
NA
A virtual timing device was used to collect very detailed
activity data for children at beaches. The authors found
that children (1 to 6 years) spend the most time at the
beach in the seawater through videotaping children and
observing their beach activities. Children were observed
to spend the majority of time wading in the intertidal zone
and seawater. The time spent wading was 38.9% for
children 0 to 24 months, 37.6% for children 25 to 48
months, and 45.1% for children greater than 48 months.
Older children were observed to spend more time digging
and running and less time sitting than the younger
children.

B-26
Study Name
(*Denotes EPA
Authors)
Location
Study Type
(Search Topic)
Contamination
Sources
Water Quality
Metrics
Summaries
Fu et al. (2021)
Singapore
Method
development
(MPN-LAMP,
qPCR)
Fecal
contamination
(source not
specified)
Enterococcus spp.,
E. coli
A rapid on-chip gene quantification method was
developed based on loop-mediated isothermal
amplification (LAMP) PCR and using a polymethyl
methacrylate (PMMA) microchip. This method does not
require the use of expensive qPCR instruments. Using the
MPN-LAMP assay, target genes of fecal indicator
bacteria (Enterococcus and E. coli) were quantified in
environmental water samples collected from a beach and a
freshwater reservoir in Singapore. Results obtained using
the MPN-LAMP approach and qPCR were correlated,
demonstrating that the MPN-LAMP method can be used
for microbial quality monitoring in areas that have limited
resources.
García-Aljaro et
al. (2019)
NA – not
environmental
sampling
MST (qPCR)
Human,
ruminant,
porcine, poultry
NA – review article
This review article summarized the types of microbes
found in sewage and used for MST. Detection of sewage
microbes was often done using qPCR, and microbes such
as Bacteroidales, Biffidobacterium spp., enterococci,
Firmicutes, Proteobacteria, human adenovirus, and
bacteriophages were detected. In addition, this review
summarized detection of FIB that were used as surrogates
for pathogens in testing sewage. It was noted that
composition of microbes in sewage was dependent upon
factors such as geographic location and ambient
temperature. Detection of the origins of fecal
contamination using both MST molecular methods and
live organisms by culture-based methods were advocated
in many water management situations.
Gibson et al.
(2017)
Beaver Lake
Watershed and
Beaver Lake
Reservoir,
Arkansas, USA
MST (qPCR)
Human, cow,
poultry
E. coli; MST
markers: HumM2,
CL
Detection of host-associated markers by qPCR was
compared with E. coli concentrations from several
locations within the Beaver Lake Watershed in Arkansas.
While the HumM2 (human) and CL (poultry) markers
correlated with E. coli concentrations, they did not
indicate conclusively the fecal contamination source
needed for MST. The location of sampling, rain events,
and the season were important variables affecting host-
associated marker detection. The authors suggest that

B-27
Study Name
(*Denotes EPA
Authors)
Location
Study Type
(Search Topic)
Contamination
Sources
Water Quality
Metrics
Summaries
similar trends could exist for other human-made
reservoirs.
Gitter et al.
(2020)
Brazos River
Basin, Texas,
USA
MST; QMRA
Human, cattle,
other wildlife
E. coli
Human, cattle, and wildlife MST markers were measured
in a freshwater tributary in Texas. QMRA was conducted
to estimate pathogen dose using MST to identify the
proportion of E. coli load from each source. Human health
risk contributed the most risk despite contributing the
least to the bacterial concentration.
Gonçalves et al.
(2018)
Bay of Koper
Gulf of Trieste,
Adriatic Sea
Method
development
(RT-qPCR)
Human
Rotaviruses,
noroviruses
Enteric viruses from coastal waters were concentrated
using methacrylate monolithic chromatographic and
quantified using RT-qPCR. Rotaviruses and noroviruses
were monitored in an area in the northern Adriatic Sea
impacted by human fecal sources and at a nearby bathing
area. The study demonstrated that CIM C4 hydrophobic
interaction columns combined with RT-qPCR provide an
efficient and consistent tool to monitor enteric viruses
from coastal environments.

B-28
Study Name
(*Denotes EPA
Authors)
Location
Study Type
(Search Topic)
Contamination
Sources
Water Quality
Metrics
Summaries
Gosselin-
Théberge et al.
(2016)
Southern
Quebec,
Canada
Method
development
(qPCR, dPCR)
Avian
Campylobacter spp.
This review evaluated real-time (qPCR) assays targeting
Campylobacter, a significant public health concern
associated with recreational water exposure in developing
countries. The authors compared nine published qPCR
assays: three for thermotolerant Campylobacter spp.
targeting the 16S rRNA and six for C. jejuni targeting
different genes. Campylobacter spp. isolates collected
from recreational water samples in Quebec, Canada were
used. Three C. jejuni-specific assays demonstrated good
specificity and sensitivity when tested. The authors
selected two assays targeting Campylobacter spp. and C.
jejuni to compare DNA concentration estimation, using
spectrophotometry and digital PCR (dPCR), in order to
calibrate standard curves (SC) for greater accuracy of
qPCR-based quantification. Differences in the
quantification of Campylobacter isolates between qPCR
assays were observed and method-specific bias in
standard curve preparation was also identified.
Graciaa et al.
(2018)
35 U.S. states
and 1 territory
(Guam)
Outbreaks
Human, non-
human
NA
This review summarized the outbreaks associated with
untreated recreational water voluntarily reported to CDC
during 2000-2014 from 35 U.S. states and Guam.
Outbreaks resulted in at least 4,958 cases of disease and
two deaths. Among the 95 outbreaks with a confirmed
infectious etiology, enteric pathogens caused 80 (84%);
21 (22%) were caused by norovirus, 19 (20%) by E. coli,
14 (15%) by Shigella, and 12 (13%) by Cryptosporidium.

B-29
Study Name
(*Denotes EPA
Authors)
Location
Study Type
(Search Topic)
Contamination
Sources
Water Quality
Metrics
Summaries
Green et al.
(2019)
Onondaga
Creek, New
York, USA
MST (qPCR,
culture)
Human,
ruminant, dog
Fecal coliforms,
MST markers:
HF183, Rm2Bac,
DG3, Entero1
Samples were collected during dry-weather conditions in
the Onondaga Creek in New York. The authors used
qPCR-based assays targeting human (HF183), ruminant
(Rum2Bac), and canine (DG3) markers. qPCR was used
to enumerate Enterococcus (Entero1) and culture was
used to enumerate fecal coliforms to assess overall fecal
contamination. The authors reported that ruminant
contaminants are likely not major contributors to the high
levels of observed FIB in urban areas during dry weather
and these contaminants were likely significantly degraded,
diluted, and deposited during in-stream transport. Instead,
Enterococcus and human marker concentrations
dominated urban locations.
Gutiérrez-
Cacciabue et al.
(2016)
Vaqueros River
and La Caldera
River,
Argentina
Method
development
(culture, qPCR)
spiked river water
E. coli, enterococci
(E. faecalis)
E. coli and enterococci were spiked into water samples
collected from two Argentinian rivers and enumerated by
culture methods and qPCR. The primary focus of this
study was to assess the impact of sunlight inactivation on
indicator bacteria present in freshwater. E. faecalis
detection by qPCR showed that the persistence of DNA
was higher than that of culturable cells. Sunlight also
accelerated DNA decay and solid particles present in the
water column provided a protective role, thereby reducing
decay.
Hata et al. (2016)
Japan
Method
development
(IC-RT-PCR-
MPN)
Human, animal
Male-specific RNA
phages
Male-specific RNA phages genotypes were characterized
using integrated culture (IC)-RT-PCR-MPN. The method
described allows quantitative analysis of male-specific
RNA phages combining culture and molecular techniques
(through MPN) and was tested in different seasons. The
method differentiates between infectious and non-
infectious viral indicators, which may improve risk
assessments. Surface water samples were evaluated by
three different methods: the IC-RT-PCR–MPN method,
conventional RT-qPCR, and conventional plaque assays.

B-30
Study Name
(*Denotes EPA
Authors)
Location
Study Type
(Search Topic)
Contamination
Sources
Water Quality
Metrics
Summaries
Haugland et al.
(2021)*
Michigan, USA
Method
development
(culture, qPCR)
Human
E. coli
A statewide survey was conducted in Michigan to
compare culture-based methods and EPA Draft Method C
for enumeration of E. coli in a variety of locations and
water sources. This large-scale comparison analyzed
6,965 samples collected from 101 recreational sites. The
hypothetical exercise to evaluate the frequency of water
impairments based on theoretical qPCR thresholds
corresponding to the E. coli water quality standard for
culture methods suggested that the methods may provide
the same beach notification outcomes over 90% of the
time. Results from this study suggest that a statewide,
multi-site threshold value may be feasible for Method C in
Michigan despite site-to-site variability in its relationship
to E. coli culture methods. The authors also indicate that
confirmation is needed to determine whether
corresponding theoretical Method C thresholds utilized in
the study represent an equivalent public health protection
compared to established culture E. coli water quality
standards.
He et al. (2016)
Qianhuang and
Xueyan, China
MST (qPCR,
PCR)
Human, pig,
bovine, goat, dog,
chicken, duck,
goose, cormorant
MST markers: H-
ND6, H-ND5,
BacH, HF183, B.
adolescentis, Pig-2
Bac, L. amylovorus,
P-CytB, P-ND5
The sensitivities and specificities of microbial and
mitochondrial DNA (mtDNA) markers were evaluated
using PCR and qPCR methods. The study collected fecal
samples from humans, pigs, and seven other species and
surface water samples from the Taige River and Taihu
Lake. The authors concluded that human-associated
microbial DNA markers were inferior indicators
compared to the human mtDNA markers. The results
suggest the use of H-ND6, H-ND5, and Pig-2-Bac for
better fecal source tracking.

B-31
Study Name
(*Denotes EPA
Authors)
Location
Study Type
(Search Topic)
Contamination
Sources
Water Quality
Metrics
Summaries
Hofstra et al.
(2019)
Global
QMRA
Unspecified
Cryptosporidium
An analysis framework that includes large-scale
waterborne pathogen concentrations, burden of diarrheal
disease, and modeled global burden of disease for
waterborne cryptosporidiosis was presented. Results
included a global heat map of cryptosporidiosis and fecal
coliform levels in surface waters and disease burden
expressed in DALYs. Although the scenario was drinking
surface water and not recreational exposures, the study is
innovative because two large-scale models, WorldQual
for fecal coliform and GloWPa for Cryptosporidium, were
used for modeling loads and in-stream concentrations.
These concentration data were used with exposure and
dose-response information to estimate risk and burden of
disease. Gaps and opportunities for the further
development of the framework were highlighted.
Holcomb and
Stewart (2020)
NA – review
article
Method
development
NA – review
article
NA – review article
This is a review of indicators and methods for assessing
water quality. Methods were classified into three stages of
development, late, middle, and early. Culture-based
methods for E. coli and enterococci, coliphages, and
Bacteroides bacteriophages were classified as late-stage
development, meaning they are the most developed.
Molecular detection of E. coli and enterococci,
Bacteroides HF183, HumM2, PMMoV, crAssphage,
BacCow, BacCan, avian GFD, Pig-2-Bac, Noroviruses,
rotaviruses, Salmonella spp., Campylobacter spp., and
Cryptosporidium spp. were classified as middle stage
development. Antimicrobial resistant bacteria and
resistance genes were classified as early-stage
development.

B-32
Study Name
(*Denotes EPA
Authors)
Location
Study Type
(Search Topic)
Contamination
Sources
Water Quality
Metrics
Summaries
Hsu et al. (2017)
Olentangy
River Wetland
Research Park,
Columbus, OH,
USA
MST (qPCR,
culture)
Human, avian,
ruminants
E. coli, Arcobacter,
Campylobacter,
Shiga toxin-
producing E. coli,
antibiotic resistance
(tetracycline, tetQ
and sulfonamide,
sul1), MST
markers: HF183,
GFD, Rum2Bac
Water samples were collected across the urban wetlands
in central Ohio from June 2013 to June 2014 and tested
with culture-based (E. coli) and qPCR methods. No
significant reductions in antibiotic resistance genes across
the wetlands were observed in this study. The wildlife
activity, specifically the presence of Canada Geese and
White-Tailed Deer, contributed to potential bacterial
pathogens in the water. At two impoundments where
geese management was applied, total and fecal coliforms
levels were three times lower compared to an unmanaged
site. Reductions in fecal indicator E. coli from inflow to
outflow showed a seasonal difference.
Jennings et al.
(2018)
San Francisco,
California,
USA
MST (qPCR,
culture)
Human
Enterococci, MST
markers: HF183,
BacR287
Traditional (culturable enterococci, cENT) and molecular
(qPCR-enterococci, qENT and human-associated marker,
HF183/BacR287) methods were used to investigate
marine waters. All three indicators showed seasonal
variation. The current California standard for cENT
yielded nearly twice as many exceedances as the qENT
standard given in USEPA’s 2012 RWQC. Combined
sewer discharges were not important predictors of
indicator levels typically measured in weekly monitoring
samples. Precipitation and solar insolation were predictors
of cENT in weekly samples, while precipitation and water
temperature were predictors of HF183/BacR287 and
qENT.
Jiang et al. (2018)
Waller Creek,
Austin, Texas,
USA
Method
development
(LAMP)
Human
Bacteroides HF183
A field-ready nucleic acid diagnostic platform, which
relied on loop-mediated isothermal amplification (LAMP)
of human-associated Bacteroides HF183 genetic markers
and oligonucleotide strand exchange (OSD) probes was
developed. The platform was tested using environmental
water samples (i.e., local creeks and ponds) in Texas and
spiked with lab-grown recombinant E. coli pHF183,
primary filtered raw sewage, or fresh canine/feline feces.
The authors concluded the platform could detect as few as
17 copies/mL within 80 minutes. The assay was
concluded to be a cheap, easy-to-use, and rapid method to

B-33
Study Name
(*Denotes EPA
Authors)
Location
Study Type
(Search Topic)
Contamination
Sources
Water Quality
Metrics
Summaries
identify human fecal contamination (Bacteroides HF183)
without off-target signals from canine/feline feces.
Jikumaru et al.
(2020)
Japan
Method
development
(dPCR)
Not reported
STEC, C. jejuni, E.
coli, Shigella,
Salmonella
Water samples from nine rivers in Japan were collected
and evaluated by dPCR for stx 1 and stx2 of STEC, hipO
of C. jejuni, ipaH of Shigella/EIEC, invA of Salmonella,
and uidA of E. coli. Among the five pathogen genes
tested, only stx2 of STEC and hipO of C. jejuni were
detected in the water samples. The other genes (stx1of
STEC, ipaH of Shigella/EIEC, and invA of Salmonella)
were not detected. The authors found that the abundance
of fecal indicator bacteria such as E. coli does not
necessarily correlate with the density of pathogens.
Jogi et al. (2020)
Anne Canal,
Estonia
Method
development
(immunosensor,
culture, qPCR)
Human
E. coli, total
coliforms
An E. coli immunosensor was compared to culture and
qPCR methods using samples from bathing water (Anne
Canal, Estonia). The median biosensor results were about
four times higher than the qPCR results and 40 times
higher than the results of microbiological cultivation.
Joosten et al.
(2017)
Netherlands
Epi/cohort
study
Human, sewer
overflows
E. coli, enterococci,
norovirus G1 and
G2
A prospective cohort study was conducted to assess risk
factors for health complaints (GI illness, respiratory
illness, and skin) associated with an acute gastrointestinal
illness outbreak after a canal swimming event in two cities
in the Netherlands in 2015. An increased risk of acute
gastrointestinal illness was found for swimmers compared
to non-swimmers in canal waters recently contaminated
by heavy rainfall. Five out of seven stool samples tested
positive for norovirus G1 and one of three water samples
tested positive for norovirus G1. Two water samples
tested positive for norovirus G2. E. coli and enterococci
levels were below EU thresholds days prior to the event
and the morning of the event, however in the afternoon on
the day of the even E. coli levels exceeded the EU
threshold.

B-34
Study Name
(*Denotes EPA
Authors)
Location
Study Type
(Search Topic)
Contamination
Sources
Water Quality
Metrics
Summaries
Kabiri et al.
(2016)
Salt River,
Verde River,
Central
Arizona Project
canal system,
Gilbert,
Arizona, USA
MST (PCR)
Human, cat, cow,
dog, duck, fish,
gull, pig
Bacteroides spp.
(including B. dorei)
Bacteroides 16S rRNA was detected by PCR in water
samples. After constructing cladograms of the data, B.
dorei of human origin was clearly present and separated
from other Bacteroides species from other animal origins.
The authors claim that B. dorei is a strong marker for
MST for human fecal contamination. They further state
that this method provides another piece to a “toolbox”
approach at MST of ambient surface waters.
Kinzelman et al.
(2020)
Racine,
Wisconsin,
USA
MST (culture,
qPCR)
Human
E. coli, enterococci
FIB along North Beach (Racine, Wisconsin, United
States) were evaluated using culture-based and molecular
methods. The authors concluded that emerging
technologies, like 16S rRNA gene sequencing, are needed
to characterize contaminant sources in urban
environments. The sequencing helped to identify different
point sources like the retention/infiltration storm water
basin.
Kolm et al.
(2017)
Austria
Method
development
(qPCR)
Human, animal
Enterococcus (15
specific strains)
An Enterococcus helicase-dependent amplification
(HDA) assay using a set of 15 enterococcal target strains
and 15 non-enterococcal reference strains was evaluated
using environmental surface water samples. The assay
produced results with a high level of agreement with EPA
qPCR Methods 1611 and 1609. The authors concluded
that the assay is a suitable candidate for additional
development and can be performed on a simple heating
block and without the use of a thermocycler.

B-35
Study Name
(*Denotes EPA
Authors)
Location
Study Type
(Search Topic)
Contamination
Sources
Water Quality
Metrics
Summaries
Kolm et al.
(2019)
Austria
MST (qPCR),
Method
development
Cattle, sheep,
goat, deer, and
other ruminant
and non-
ruminants
BacR
This study analyzed 20 single ruminant (target) and 20
single non-ruminant (non-target) fecal suspensions in
surface water from the Danube River. The authors
evaluated a BacR helicase-dependent amplification
(HDA) assay and a BacR strip test to compare to qPCR
methods. They concluded that their HDA assay resulted in
comparable sensitivity, specificity, and limits of detection
to qPCR. Unlike qPCR the strip test requires only a
heating block to amplify the target gene. The reaction
mixture is applied directly to the test strip which detects
and displays the amplification products by marker-
specific hybridization probes via an on-strip colorimetric
reaction. The entire assay takes 2 hours, does not require
extensive practical training, and is low cost.
Kongprajug et al.
(2019)
Tha Chin
River, Thailand
MST (PCR,
qPCR)
Human, animal
Markers: GenBac3,
HF183/BFDrev,
Pig-2-Bac, Bac3
PCR and qPCR results were compared from 48 water
samples from 12 sampling stations along the Tha Chin
River. Universal markers (both PCR and qPCR) were
detected in all samples, indicating persistent and
continuing fecal contamination. There was 87.5%–100%
agreement between the MST-PCR and MST-qPCR. The
authors suggest that traditional presence/absence PCR is a
screening step that requires less technical skills and has
lower costs.
Kongprajug et al.
(2020)
Tha Chin
River, Thailand
MST (qPCR);
Method
development
Human, animal
Markers: GenBac3,
HF183/BFDrev,
CPQ-056, Pig-2-
Bac, Bac3qPCR
Five qPCR assays, GenBac3, human-associated
HF183/BFDrev and CPQ-056, swine-associated Pig-2-
Bac, and cattle-associated Bac3qPCR, were evaluated
using the LinRegPCR model. Freshwater samples from
the Tha Chin River in Thailand were spiked with human
sewage and non-human fecal samples. The LinRegPCR
approach improved identification of sources. Overall, the
authors emphasized the need for a standardized data
analysis protocol for interlaboratory consistency and
comparability. The LinRegPCR model is a standard
curve-independent qPCR analysis, which does not involve
the limit of detection (LOD), limit of quantification
(LOQ), or did not quantify (DNQ). Non-detection by this
method is deemed zero when no or poor amplification

B-36
Study Name
(*Denotes EPA
Authors)
Location
Study Type
(Search Topic)
Contamination
Sources
Water Quality
Metrics
Summaries
efficiency is observed, thus eliminating the incorporation
of nondetected data. LinRegPCR analysis has a
demonstrated advantage for analyzing data obtained at
low target concentrations.
Korajkic et al.
(2019)*
Tampa and
Tierra Verde,
Florida, USA
MST (qPCR)
Animal
Markers: GenBac3,
Entero1a,
EC23S857,
Rum2Bac, CowM2,
CowM3
qPCR was used to estimate decay of general fecal
indicator bacteria (GenBac3, Entero1a, EC23S857) and
MST markers (Rum2Bac, CowM2, CowM3). The study
found that water type is the most influential factor for
their decay. The higher the salinity, the faster the bacteria
decayed. Discrepancies in water type decay suggested that
osmotic stress induced by increased marine salinity and
associated ionic content had a similar influence on all
fecal indicators measured in this study, irrespective of the
analytical technique (e.g., cultivation or qPCR).
Kuhn et al.
(2018)
Denmark
Children;
Epi/case-control
Not reported
NA
A prospective case-control study was conducted among
Danish persons ages 1 to 30 years old to identify risk
factors for campylobacteriosis. For all cases (adults and
children; N = 556 cases, 2,117 controls) the model
showed an increased risk of infection with bathing in fresh
water (OR = 5.1), contact to beach sand (OR = 1.8),
owning a pet dog with diarrhea (OR = 4.6), and eating
minced beef (OR = 2.6) or chicken (OR = 2.5). The model
for children ages 1 to 5 (N = 125 cases, 321 controls)
showed increased risk of infection with bathing in
paddling pool (OR = 13.6).

B-37
Study Name
(*Denotes EPA
Authors)
Location
Study Type
(Search Topic)
Contamination
Sources
Water Quality
Metrics
Summaries
Lane et al. (2020)
Michigan
Method
development
(qPCR)
Not reported
E. coli
This study compared EPA Draft Method C results
between 10 Michigan labs and EPA’s National Exposure
Research Laboratory across 82 recreational sites. The
beach management decision (i.e., remain open or issue an
advisory or closure) agreed in 94% of the samples
analyzed. The authors also note that one important finding
from this study is that EPA Draft Method C can be
effectively taught to and implemented by lab personnel
with minimal qPCR experience. When individual years
were assessed, the Michigan labs’ precision became
progressively better in consecutive years, indicating
increasing skill with the method. The study also suggested
that further research on inhibition mitigation is needed.
Although the inhibition rate overall was low (217 out of
the 3,580 paired data points (6%) had quality control
failures), samples from one laboratory were unusable
throughout most of the 2017 summer because of
inhibition. The study also compared qPCR-based beach
management decisions to Colilert-18
®
-based decisions.
Michigan determined that 1.863 log
10
target gene copies
per reaction is equivalent to 300 E. coli per 100 mL,
which is referred to as the ‘regulatory equivalent’ value
(EV). This value is used for beach notifications. Utilizing
EPA Draft Method C about 7% of samples (240 of 3,166)
exceeded the EV. This was marginally higher than the 4%
of samples reported by the Michigan Department of
Environment, Great Lakes and Energy that exceeded the
Colilert-18
®
Michigan recreational water quality criteria
between 2016 and 2018.

B-38
Study Name
(*Denotes EPA
Authors)
Location
Study Type
(Search Topic)
Contamination
Sources
Water Quality
Metrics
Summaries
Lapen et al.
(2016)
Canada
QMRA
Human (treated
wastewater),
agriculture
Cryptosporidium
Cryptosporidium oocyst concentration and
species/genotype data were collected from three surface
water surveillance programs in two river basins in
Ontario, Canada. QMRA estimated that the risk of
infection was one order of magnitude lower when only the
human infectious species/genotypes were included in the
modeling. Cryptosporidium seasonality in water samples
appeared to match the seasonality of human infections
from Cryptosporidium in the study regions.
Leifels et al.
(2016)
Germany
Method
development
(qPCR)
Human
Human adenovirus,
enterovirus,
rotavirus
This study assessed the utility of pretreatment using
ethidium monoazide (EMA) and propidium monoazide
(PMA), two dyes that are capable of penetrating the
damaged or compromised capsid of inactivated viruses
and binding to viral nucleic acids. The authors collected
urban river water samples in Germany and assessed
whether dye treatment is a suitable approach to improve
the ability of qPCR to distinguish between infectious and
non-infectious human adenovirus, enterovirus, and
rotavirus A. The authors found that pretreatment EMA-
/PMA-qPCR succeeded in removing false positive results
and that this approach could provide a tool for improving
the efficacy of molecular quantification methods and
reduce overestimation of viral load in environmental
samples.

B-39
Study Name
(*Denotes EPA
Authors)
Location
Study Type
(Search Topic)
Contamination
Sources
Water Quality
Metrics
Summaries
Li et al. (2016)
Tampa and St.
Petersburg,
Florida, USA
MST
Human, cow,
sheep, swine,
avian
E. coli,
Enterococcus,
Bacteroidales, S.
aureus, S. enterica,
human
polyomavirus,
human adenovirus,
human norovirus
An MST microarray was used to simultaneously detect
genes for multiple pathogens and indicators of fecal
pollution in freshwater, marine water, sewage-
contaminated freshwater and marine water, and treated
wastewater. A linear relationship between gene copies
determined by qPCR versus microarray fluorescence was
found, indicating the semiquantitative nature of the MST
microarray. Whole-genome amplification (WGA)
increased gene copies from ultrafiltered samples thus
increasing the sensitivity of the microarray. These results
indicate that ultrafiltration coupled with WGA provides
sufficient recovery of nucleic acids for detection of
viruses, bacteria, protozoa, and antibiotic resistance genes
by the microarray in applications ranging from beach
monitoring to risk assessment.
Li et al. (2018)*
Ohio, USA
MST (qPCR)
Human
MST markers:
EC23S857, Entero1
GenBac3, HF183,
HumM2
This study assessed the capacity of periphyton (complex
mixture of algae, microbes, inorganic sediment, and
organic matter that is attached to submerged surfaces in
most flowing freshwater systems) to trap genetic markers
from the water column. After wastewater effluent was
pumped into the periphyton mesocosm, genetic markers
were detected in periphyton at frequencies up to 100%
(EC23S857, Entero1, and GenBac3), 59.4% (HF183), and
21.9% (HumM2) confirming sequestration from the water
column.

B-40
Study Name
(*Denotes EPA
Authors)
Location
Study Type
(Search Topic)
Contamination
Sources
Water Quality
Metrics
Summaries
Li et al. (2019)*
Trask, Kilchis,
and Tillamook
rivers and
tributaries,
Oregon, USA
MST (qPCR);
Method
development
Human, cow,
canine, avian
E. coli, Markers:
HF183/BacR287,
HumM2, CowM2,
CowM3, Rum2Bac,
DG3, DG37, GFD
A large-scale field study in the Tillamook Bay Watershed,
Oregon was conducted to assess the feasibility of
implementing standardized fecal source identification
qPCR methods with established data acceptance metrics.
A total of 602 water samples were collected over a 1-year
period at 29 sites along the Trask, Kilchis, and Tillamook
rivers and tributaries. Using HF183/BacR287 and
HumM2 human-associated qPCR results, geographic
information system land use, and E. coli monitoring data,
the authors concluded that elevated E. coli levels may be
linked to specific pollution sources and land use activities
in the Tillamook Bay Watershed. The study also revealed
key issues regarding qPCR data interpretation including
the importance of confirming method performance with
local reference fecal samples and utilizing appropriate
censored data analysis strategies.
Li et al. (2021)*
Ohio,
Wisconsin,
Indiana, USA
MST (qPCR),
Method
development
Human,
ruminant, canine,
avian
Somatic and male-
specific coliphage,
enterococci, E. coli,
MST markers:
HF183/BacR287,
HumM2, Rum2Bac,
DG3, GFD
Previously reported paired measurements of cultivated
somatic and male-specific coliphage, enterococci, E. coli,
and genetic markers indicative of human, ruminant, avian,
and canine fecal sources were used in this analysis. Due to
a large number of non-detection and below-detection data
points, the authors determined weighted fecal scores,
which is a Bayesian censored data analysis approach. It
compares the average density of a host-associated genetic
marker between two groups of samples using all data
measurements without the need to fabricate any Cq
values. A limitation of the fecal score approach is the
requirement to group samples together which prevents
higher resolution assessment of temporal or site-specific
variability.
Liang et al.
(2021)
Beijing, China
MST (qPCR,
FEAST)
Human, swine,
canine, equine,
donkey, bovine,
sheep, goat,
chicken, duck,
goose, pigeon,
fish
MST markers:
HF183-1, HF183-2,
BacH, BacHum,
Pig2-Bac, Rum-2-
Bac, AV4143
MST methods based on molecular markers and machine
learning programs were applied together to distinguish the
fecal inputs from multiple sources. Along with qPCR, the
community-based FEAST (fast expectation–maximization
microbial source tracking) program was applied to
estimate multiple potential sources and the relative
contributions of various fecal inputs at the same time.

B-41
Study Name
(*Denotes EPA
Authors)
Location
Study Type
(Search Topic)
Contamination
Sources
Water Quality
Metrics
Summaries
Liao et al. (2016)
Virginia, USA
QMRA
Human, non-
human
Indicator: E. coli.
Reference
pathogens:
Campylobacter
jejuni, E. coli
O157:H7,
Cryptosporidium
spp., Giardia
lambia, Salmonella
enterica, and
Norovirus
This study linked a watershed-scale FIB fate and transport
model frequently used in existing TMDL processes
Hydrologic Simulation Program–Fortran (HSPF, version
12.2) with QMRA for comparison of estimated risks to
regulatory benchmarks. The results indicate that total
human illness risks were consistently higher than the
regulatory benchmark of 36 illnesses per 1,000 recreators
for the study watershed, even when the predicted FIB
levels were in compliance with the E. coli geometric mean
standard of 126 CFU/100 mL. Sanitary sewer overflows
were associated with the greatest risk of illness.
Lim et al. (2017)
Baby Beach in
Dana Point,
Orange County,
California
QMRA
Human, seagull,
pig, cow
Enterococci
Historical enterococci measurements at Baby Beach in
Dana Point, Orange County, California were used to
evaluate risks from before and after improvements were
made in dry-weather runoff diversion and installation of a
media filtration system near the main storm drain. A
source-apportionment QMRA based on parameters from
previously published QMRAs was used. During dry
weather, the median recreational waterborne illness risk at
this beach was below the U.S. EPA recreational water
quality criteria (RWQC) of 36 illness cases per 1,000
bathers. During wet weather, the median recreational
waterborne illness risk predicted by the QMRA depended
on the assumed level of human waste associated with
stormwater; the risk was below the EPA RWQC illness
risk benchmark 100% of the time provided that <2% of
the FIB in stormwater are of human origin.
Linke et al.
(2021)
National Park
Lake Neusiedl,
Austria
Method
development
(qPCR)
Human
Markers:
Enterococcus,
HF183/BacR287
qPCR methods targeting a Enterococcus genetic marker
and the human-associated MST assay HF183/BacR287,
were used to test the performance of a modified DNA
extraction protocol on freshwater samples from an
Austrian lake. The authors concluded that no universal
DNA extraction protocol or general rule to improve
extraction efficiency can be provided. The amount of
suspended material and its composition affect the amount
of additives required to improve the extraction efficiency.

B-42
Study Name
(*Denotes EPA
Authors)
Location
Study Type
(Search Topic)
Contamination
Sources
Water Quality
Metrics
Summaries
Liu et al. (2017)
NA
MST; Method
development
raw sewage
Bacteroidales, E.
coli
This study included microcosm experiments to evaluate
microbial decay in untreated seawater. Data from a
previous publication that evaluated decay of cow fecal
markers was compared to human fecal bacteria decay
rates. The authors concluded that tracking of the relative
concentration between Bacteroidales DNA markers and
culturable E. coli can differentiate pollution that is
relatively fresh from one that has aged and could be useful
in episodic pollution events.
Martzy et al.
(2017)
Austria
Method
development
(LAMP, qPCR)
Human, animal
Enterococcus spp.
The authors developed the LAMP assay as an alternative
to qPCR in identifying microbial contamination in fresh
water sources such as springs and surface water. They
found that their assay was equally sensitive, more
specific, and faster at identifying microbes in water
sources than qPCR methods.
Mattioli et al.
(2021)*
Pennsylvania,
USA
MST (qPCR,
culture, genome
sequencing)
Human
MSC and Somatic
coliphages, total
coliforms, E. coli,
enterococci, MST
markers: HF 183/
BFDrev, genome
sequencing:
norovirus GI and
GII
An overloaded septic system at a Pennsylvania event
center and campground was the source of human fecal
contamination in a nearby creek and swimming area that
resulted in several guests developing GI illnesses after
exposure. This is a real-word example of how MST could
be used to track fecal contamination and make
recommendations to protect public health.
McGinnis et al.
(2018)
Cobbs and
Tacony Creek,
Philadelphia,
Pennsylvania,
USA
MST (qPCR
and RT-qPCR)
Human
human
polyomavirus
(HPoV), PMMoV,
adenovirus,
enterovirus,
norovirus
genogroups I and II,
Campylobacter,
Salmonella,
enterohemorrhagic
E. coli, fecal
coliforms, HF183
Fecal indicator organisms, human fecal markers (HF183),
and pathogens were measured at two sites in a freshwater
urban creek. The authors distinguished which organisms
and/or markers could be used as indicators of recent
combined sewage overflows (CSOs). In particular, human
Bacteroides was found to be the most specific, and total
coliforms were found to be the most sensitive indicators.
E. coli, PMMoV, and HPoV did not show consistent
correlations with recent CSO and rainfall events.

B-43
Study Name
(*Denotes EPA
Authors)
Location
Study Type
(Search Topic)
Contamination
Sources
Water Quality
Metrics
Summaries
Ming et al.
(2020)
Xinghai, China
QMRA, MST
(qPCR)
Human non-point
source
Pathogens:
Aeromonas
hydrophila, Listeria
monocytogenes,
Pseudomonas,
Clostridium
perfringens,
Salmonella,
Shigella dysentery,
Campylobacter
jejuni, E. coli
O157:H7,
Streptococcus
faecalis, Aeromonas
caviae, S. aureus,
Vibrio vulnificus,
Vibrio cholera (not
O1), Vibrio
arahaemolyticus,
Vibrio
alginolyticus.
Indicator: human
Bacteroidales
Human Bacteroides and 15 typical bacterial pathogens
were measured using culture-dependent and independent
methods in surface seawater samples from Xinghai, China
beaches. The authors concluded that enterococci may not
accurately indicate fecal source pollution, and human
Bacteroides should be proposed for beach monitoring.
Monteiro et al.
(2021)
Lisbon,
Portugal
MST (culture,
qPCR)
Humans, pig,
poultry, cattle,
dog
E. coli, enterococci,
coliphages,
Markers: CWMit,
PLMit, PGMit,
HMMit and HAdV,
GB-124
Fecal contamination sources were determined by
analyzing fecal indicators (E. coli, enterococci, and
coliphages) as well as culture, and culture-independent,
source-associated markers. Sampling sites were highly
impacted by fecal contamination from both human and
livestock sources. There were also seasonal and
physicochemical parameters that influenced correlations
between fecal indicators.

B-44
Study Name
(*Denotes EPA
Authors)
Location
Study Type
(Search Topic)
Contamination
Sources
Water Quality
Metrics
Summaries
Mosnier et al.
(2018)
French Guiana
Children;
Outbreaks
human
NA
A cryptosporidiosis outbreak among children aged
between 4.5 and 38 months living in a remote area along
the Maroni River in French Guiana was reported. The
clinical features, epidemiology, and state of current
investigations for the largest documented outbreak of
cryptosporidiosis in French Guiana were described. Stool
samples were screened for pathogens. Bacterial culture
and turbidity analysis of water distribution sources of the
area was performed. The outbreak reflected multifactorial
dynamics of waterborne disease among children cases
including human behavior such as playing, bathing, and
eating, and probable immunity.
Napier et al.
(2017)*
Cleveland,
Ohio, St.
Joseph
Michigan,
Michigan City
and Portage,
Indiana,
Fairhope,
Alabama,
Warwick,
Rhode Island,
USA
MST (culture,
qPCR)
Human
Bacteroides MST
markers: BsteriF1,
BuniF2, HumM2,
HF183
The presence of four human-associated Bacteroides
markers (HF183, BsteriF1, BuniF2, and HumM2) were
assessed for association with self-reported gastrointestinal
illness, diarrhea, and respiratory illnesses. This study used
data from the NEEAR study, which involved 12,060 adult
visitors of six beaches across the United States. Less
reliable associations were observed between illness and
the presence of Bacteroides markers than expected. The
authors concluded that quantitative measures of fecal
pollution using Bacteroides, rather than presence-absence,
may be necessary to accurately assess human risk specific
to the presence of human fecal pollution.
Napier et al.
(2018)*
Cleveland, OH,
Portage, IN,
Michigan City,
IN, St. Joseph,
MI, Biloxi,
MS, Fairhope,
AL, Warwick,
RI, USA
Method
development
(qPCR)
Human
Enterococcus spp.
This analysis using data from the NEEAR study involving
12,060 adult visitors to six U.S. beaches reported that with
the exception of bisphenol A and cholesterol, no
chemicals were found to be consistently associated with
an increase of GI illness. Interaction contrast estimates
were imprecise, particularly for chemicals that were
infrequently detected (e.g., acetaminophen).

B-45
Study Name
(*Denotes EPA
Authors)
Location
Study Type
(Search Topic)
Contamination
Sources
Water Quality
Metrics
Summaries
Nappier et al.
(2019)*
NA – review
article
Method
development
(qPCR)
NA – review
article
E. coli,
Enterococcus spp.
This review article included information on qPCR
methods interference controls reported in 16 papers for
Enterococcus spp. and 13 papers for E. coli. EPA Method
1609, EPA Method 1611, EPA Method A, and Scorpion
methods were reported in studies to detect and quantify
Enterococcus spp., and EPA Method C, Scorpion method,
and other methods were applied for E. coli. Low levels of
interference were reported except in tropical marine
waters in Hawaii. Use of E. coli qPCR should be
considered on a site-specific basis, whereas use of EPA
Method 1609 for Enterococcus spp. detection is
appropriate when the required and suggested controls are
employed.
Nieuwkerk et al.
(2020)*
NA – review
article
Method
development
(isothermal
amplification
methods)
NA – review
article
NA – review article
This review article summarized applications and
performance metrics for most common isothermal
amplification methods (IAMs) including helicase-
dependent amplification (HAD), loop-mediated
isothermal amplification (LAMP), recombinase
polymerase amplification (RPA), nucleic acid sequence-
based amplification (NASBA), and rolling circle
amplification (RCA). The sensitivity, specificity, LOD,
and comparison with PCR and inhibition were reported
for each IAMs. The authors concluded that some reported
advantages of IAMs may lack empirical evidence and
more head-to-head studies of qPCR and IAM methods
that include performance metrics and assess susceptibility
to inhibition in environmental samples are needed. IAMs
may be superior tools to inform spatiotemporal
distributions of undesirable microorganisms in the
environment.

B-46
Study Name
(*Denotes EPA
Authors)
Location
Study Type
(Search Topic)
Contamination
Sources
Water Quality
Metrics
Summaries
Olds et al. (2018)
Milwaukee,
Wisconsin,
USA
MST (culture,
qPCR)
Human, ruminant
E. coli, enterococci,
total fecal
coliforms, MST
markers: HB,
Lachno2, other
ruminant-specific
markers
E. coli, enterococci, and total fecal coliforms using human
and ruminant-associated indicators were tested at
freshwater (estuary and river) locations in Milwaukee,
Wisconsin. The authors found that contamination in
surface waters were at levels above the acceptable risk for
recreational use. Their findings quantified hazards in
exposure pathways from rain events and illustrated the
additional stress that climate change may have on urban
water systems.
Oliveira et al.
(2016)
Rio de Janeiro,
Brazil
MST (culture,
PCR, qPCR)
Human
E. coli,
Methanobrevibacter
smithii (nifH gene)
The authors enumerated E. coli using Colilert, detected
Methanobrevibacter smithii by conventional PCR, and
detected and quantified the nifH gene of M. smithii by
TaqMan-based qPCR. Forty-six percent of sampled
beaches presenting E. coli levels in compliance with
Brazilian water quality guidelines showed nifH gene
between 5.7 × 10^9 to 9.5 × 10^11 copies, thus revealing
poor correlation between the two approaches. The authors
suggest that the nifH gene could be used, in combination
with other markers, for source tracking studies to measure
the quality of marine ecosystems.
Oliveira et al.
(2020)
Girona, Spain;
Jena, Germany
Method
development
(LAMP, PCR,
qPCR)
Human, animal,
non-impacted
genetic markers:
lacZ (total coliform
detection) and uidA
(E. coli marker)
A multiplex LAMP coupled to an Au-nanoprobe
colorimetric assay was used for water quality assessment.
The approach was validated in 22 impacted and non-
impacted lake and river water samples collected in Spain
and Germany using standard PCR and qPCR detecting
and quantifying two specific marker genes – lacZ and
uidA. The integration of mLAMP and Au-nanoprobe
colorimetric methods revealed good sensitivity and
specificity without adding to assay complexity. This
methodology significantly reduced the reaction time while
allowing for an easier translation to field context and
simple screening of on-site contaminations.

B-47
Study Name
(*Denotes EPA
Authors)
Location
Study Type
(Search Topic)
Contamination
Sources
Water Quality
Metrics
Summaries
Osunmakinde et
al. (2018)
NA – review
article
Method
development
NA – review
article
NA – review article
This paper reviewed metagenomic techniques for
quantifying human enteric viruses as a method for
monitoring water quality in Africa. Both qPCR and NGS
were highly useful in monitoring water quality but factors
such as cost, training, and equipment necessary for these
techniques present challenges to some African countries.
The authors suggest more efforts should be directed at
instituting water quality monitoring programs that utilize
technologies such as qPCR and NGS in countries with
emerging economies.
Pawar et al.
(2019)
Pune district,
Satara district,
Raigad district,
Maharashtra,
India
MST (RT-
PCR); Method
development
Avian
Avian influenza
virus
RT-PCR was conducted on two avian influenza viruses
(human pathogenic H5N1) spiked in reservoir and sea
water, from Maharashtra India. The authors compared
precipitation with potassium aluminum sulphate and milk
powder to a virus concentration method using
erythrocytes. The precipitates were positive for the
presence of the virus using RT-PCR and viable as shown
by the growth of virus in embryonated chicken eggs. The
method might be suitable for the detection of avian
influenza viruses from different environmental water
sources and can also be applied during outbreak
investigations.
Pedrosa de
Macena et al.
(2021)
Rio de Janeiro,
Brazil
Method
development
(qPCR)
Human
35 enteric
pathogens (using 71
target genes)
The TaqMan array card (TAC) method was used to assess
35 enteric pathogens (using 71 target genes)
simultaneously in a lagoon system in Rio de Janeiro,
Brazil. TAC results identified 17 enteric pathogens
including 4/5 viral species investigated, 8/15 bacteria, 4/6
protozoa and 1/7 helminths. The authors concluded the
TAC methodology is a useful molecular tool for the rapid
screening of microbiological contamination.

B-48
Study Name
(*Denotes EPA
Authors)
Location
Study Type
(Search Topic)
Contamination
Sources
Water Quality
Metrics
Summaries
Pendergraph et al.
(2021)
Absaroka
Beartooth
Wilderness,
Montana, USA
MST (culture,
ddPCR);
Method
development
Human, animal
Bacteroides, E. coli,
total coliforms
Water from 23 wilderness lake outlets and streams in
Montana were assessed with culture-based FIB and
ddPCR for a general Bacteroides marker and a human
Bacteroides marker. The highest E. coli CFUs were found
in lake outlets that are popular recreational water sources
and accessible to stock animals and human foot traffic. In
some cases, samples tested positive for the presence of E.
coli, but human-derived Bacteroides were not detected.
The authors concluded that the results point to other
possible sources of fecal contaminants, including animals
(wild or domesticated).
Petcharat et al.
(2020)
Thailand
MST (qPCR);
Method
development
Human
crAssphage
This study spiked phosphate buffered solution (PBS),
seawater, freshwater, and influent and effluent from a
wastewater treatment plant with crAssphage to evaluate
recovery by qPCR. Recovery of crAssphage in PBS was
0.36. The river and one beach had no indigenous
crAssphage detected. A second beach had low levels of
indigenous crAssphage. Environmental water with no or
low background of crAssphage showed no loss in the
recovery process. Evaluating recovery efficiencies in
samples with high crAssphage backgrounds posed a
challenge due to the inability to prepare crAssphage
spiked samples with high concentrations.

B-49
Study Name
(*Denotes EPA
Authors)
Location
Study Type
(Search Topic)
Contamination
Sources
Water Quality
Metrics
Summaries
Phelan et al.
(2019)
New Jersey,
USA
MST (qPCR);
Method
development
Human, animal
(horse, goose,
dog)
Markers: HF18328,
BacHum29,
HoF597
The results of amplicon sequencing were compared to
established qPCR methods for fecal markers in library and
field samples from the Navesink River in New Jersey to
determine the sources of fecal pollution in samples with
elevated culturable indicator organisms. To determine the
microbial community present in the fecal library and
surface water samples, amplicon sequencing (Illumina
MiSeq, 300 bp, paired end) was performed targeting the
V3–V4 region of the 16S rRNA gene. A separate fecal
library from researchers in Australia was created using the
115 fecal sequences from human or sewage (human feces,
septic, raw sewage, secondary sewage), avian (bird,
common miner, chicken, duck), dog, horse, cat, bat,
agricultural mammals (cow, goat, pig, sheep), and wild
mammals (possum, rabbit, rat). SourceTracker (a
Bayesian statistical technique that relates ‘sink’ microbial
community structure to ‘source’ libraries) was run without
tuning using the Australian fecal library as sources and the
Navesink fecal library and surface water samples as sinks.
Results of this study indicate that fecal indicator
organisms were elevated in the Navesink river at the time
of study and sources include human/sewage, avian, and
horse manure.

B-50
Study Name
(*Denotes EPA
Authors)
Location
Study Type
(Search Topic)
Contamination
Sources
Water Quality
Metrics
Summaries
Polkowska et al.
(2018)
Western
Finland
Children;
Outbreaks
Human
Indicators: E. coli,
intestinal
enterococci,
cyanobacteria,
algae.
Pathogens:
norovirus, and
adenovirus
A retrospective cohort study was conducted using an
internet-based survey to study an outbreak of acute
gastroenteritis in Tampere Finland. Cases were from the
general population and included children who visited
beaches at four separate lakes. Risk factors included
getting water into mouth while swimming and playing at
the beach. Norovirus was found in 19 stool samples. All
water samples from implicated beaches had acceptable
values of FIB and were negative for norovirus. Closure of
swimming beaches in Tampere, advice on hygienic
precautions and rapid outbreak alerts were effective at
controlling swimming in areas affected by the norovirus
outbreak. The results suggest a need for new indicators of
water quality for norovirus and development of evidence-
based recommendations regarding timing of safe reopen
of recreational water venues associated with outbreaks.
Poma et al.
(2019)
Argentina
QMRA
Unspecified
Pathogens: Human
enterovirus and
norovirus
The risk of infection was calculated using: (a) two
methodological approaches to find the distributions that
best fit the data sets, (b) four different exposure scenarios
(primary contact for children and adults and secondary
contact by spray inhalation/ingestion and hand-to-mouth
contact), and (c) five alternatives for treating censored
data. The risk of infection for norovirus GII was much
higher than enterovirus. The authors suggest that in most
cases the use of the half-LOD approach is appropriate for
QMRA modeling.
Powers et al.
(2020)
Corpus Christi,
Texas, USA
MST (ddPCR)
Human, animal
(gull and canine)
Enterococcus,
HF183
Three host-associated markers tested in this study were
detected in all of the water samples. Gull was the most
prevalent, followed by human, then canine. This study
also found that there was no correlation between
enterococci and source-associated human and animal
markers, meaning the use of elevated enterococci
concentrations to inform beach advisories may not be best
practice in urbanized subtropical bays.

B-51
Study Name
(*Denotes EPA
Authors)
Location
Study Type
(Search Topic)
Contamination
Sources
Water Quality
Metrics
Summaries
Prado et al.
(2018)
Sao Paulo,
Brazil
MST (culture)
Human
Bacteroides fragilis
GB-124
bacteriophages
A culture-based, double-layer agar technique was used to
enumerate Bacteroides fragilis GB-124 bacteriophages in
wastewater, secondary effluent, and reclaimed water.
Lower concentrations of bacteriophages were detected in
reclaimed water but they were still detectable. The authors
indicated that monitoring for bacteriophages was low cost
and relatively simple to perform, so bacteriophage
quantification was encouraged for MST of a variety of
water types.
Purnell et al.
(2020)
Southeast
England
QMRA
Wastewater
effluent, without
disinfection
Adenovirus,
Salmonella,
Cryptosporidium
The authors indicate that advanced treatment to remove
adenovirus, Salmonella, and Cryptosporidium would be
required to avoid further deterioration of water quality at
the studied river sites. The QMRA model results
demonstrated the potential for pathogen reduction through
augmentation with reclaimed water in rivers heavily
impacted by de facto reuse practices.
Rachmadi et al.
(2016)
NA – review
article
MST
Human
NA
Human polyomaviruses were human-associated and
concentrations did not vary seasonally. In addition, the
density of human polyomaviruses correlated positively
with the density of other enteric viruses in water samples.
While there were some limitations involved (human
pathogenicity, dsDNA, and persistence in the
environment), the authors suggested that human
polyomaviruses were a useful tool for MST of human
fecal contamination in water.
Raj et al. (2020)
Ghana
Children
Human
E. coli
The SaniPath Exposure Assessment Tool is an open
source online tool (https://tool.sanipath.org/) where users
enter behavioral survey data and E. coli quantification
data for up to up to nine pathways (drinking water,
bathing water, surface water, ocean water, open drains,
floodwater, raw produce, street food, and public or shared
toilets). The tool uses Bayesian analyses to estimate the
percentage of the population exposed and the mean dose
of fecal exposure for children and adults. The tool
provides visual representations of the output. The
SaniPath Tool supports public health evidence-based

B-52
Study Name
(*Denotes EPA
Authors)
Location
Study Type
(Search Topic)
Contamination
Sources
Water Quality
Metrics
Summaries
decision-making for urban sanitation policies and
investments.
Reses et al.
(2018)
Minnesota and
Colorado, USA
Epi/case-control
Not Reported,
suggested human
and animal fecal
contamination
NA
This study provides evidence for transmission of Giardia
via contaminated water or food. Drinking water from a
river, lake, stream, or spring was identified as a strong risk
factor for giardiasis. Waterborne outbreaks and sporadic
cases of giardiasis have been previously associated with
the consumption of untreated water from natural bodies of
water including streams and rivers.
Reyneke et al.
(2017)
South Africa
and Namibia
Method
development
(qPCR)
Not reported
L. pneumophila, S.
typhimurium, P.
aeruginosa
This study optimized ethidium monoazide (EMA) and
propidium monoazide (PMA) concentrations for
pretreatment of qPCR reactions. Water samples spiked
with five waterborne bacterial pathogens were used. 6 uM
EMA and 50 uM PMA were identified as the optimal dye
concentrations for the rapid identification and
quantification of multiple intact opportunistic pathogens
in ambient water.

B-53
Study Name
(*Denotes EPA
Authors)
Location
Study Type
(Search Topic)
Contamination
Sources
Water Quality
Metrics
Summaries
Rocha et al.
(2019)
USA
Method
development
(culture, qPCR)
Human
Total coliforms,
fecal coliforms,
enterococci, E. coli,
markers: gadAB,
uidA, blaCTX-M,
and intI1
This study evaluated whether antibiotic resistance genes
could be used as measures of FIB. Total coliforms, fecal
coliforms, and enterococci were quantified by culture
methods and enterococci (16S rRNA and 23S rRNA) and
E. coli (uidA and gadAB) by qPCR along with antibiotic
resistance genes sul1, sul2, tet(A), tet(O), bla-OXA-1, bla-
CTX-M, and intl1. Regression analyses comparing the
abundance values of cultivable bacteria and gadAB, uidA,
blaCTX-M, and intI1 indicated that these genes may be
suitable biomarkers for total coliforms, beta-lactam
resistant subpopulations, and their possible role in the
dissemination of antibiotic resistance in the environment.
Rosiles-González
et al. (2019)
Mexico
Health Study
Not Reported
Somatic and MSC,
norovirus strains GI
and GII, and human
adenovirus
This study provided a baseline of the distribution,
concentrations, and diversity of noroviruses and human
adenoviruses in aquifers from Mexico. During rainy
season, 70%–80% of the samples contained somatic
coliphages. MSC were detected in 80% of the samples.
During the dry season, 60% of samples had detectable
somatic coliphages using E. coli bacterial strains. MSC
were detected in 30% of the samples during the dry
season. The highest concentration of somatic and MSC
was observed during the rainy season at site 2 which was
a freshwater sinkhole located in Cancun. The enteric
viruses tested were found to be more prevalent during the
rainy season and during the rainy season 70% of the
sampling sited resulted in detection of at least one type of
virus. This is compared to only 40% during the dry
season. Overall, norovirus was more frequently detected
than human adenoviruses.
Rothenheber et
al. (2018)
Wells, Maine,
USA
MST
Human, animal
(gull, dogs, birds,
ruminants)
Enterococci
Elevated enterococcal levels were reflective of a
combination of increased fecal source input,
environmental sources, and environmental conditions.
Enterococci densities in the estuarine and marine waters
were strongly influenced by particle-associated
enterococci and mammalian fecal sources. For freshwater
systems, sediments acted as a reservoir for enterococci.

B-54
Study Name
(*Denotes EPA
Authors)
Location
Study Type
(Search Topic)
Contamination
Sources
Water Quality
Metrics
Summaries
For these reasons, the authors suggested using an
encompassing approach to MST.
Rudko et al.
(2020)
Michigan, USA
Method
development
(qPCR)
Human, animal
18S avian
schistosomes,
toxigenic (mcyE
gene)
cyanobacteria,
HF183
Community-based monitoring (CBM) of freshwater for
avian schistosomes (18S), toxigenic cyanobacteria (mcyE
gene), and HF183 Bacteroides was compared to
laboratory performed qPCR for the same samples. CBM
partners were provided with training video and a written
protocol detailing all steps from DNA extraction to results
analysis. In addition, two in-person trainings were
provided. Water samples were collected in Michigan and
portions were shipped to Alberta for secondary laboratory
analysis for comparisons. CBM partners ran 985 qPCR
samples over 2 years. The field equipment, Open qPCR
thermocycler, has a higher limit of detection than the
laboratory Applied Biosystems (ABI) thermocyclers.
Although HF183 was detected in 54/237 samples by the
lab and only detected in one field assay, the level of
HF183 was between 15-35 copies/5 μL. This level is
below the HF183 gene copy number (210 GC/5 μL) that
would exceed the EPA benchmark for GI illness. The
authors concluded that the detection limit of the Open
qPCR thermocycler is sufficient for potential outbreak
scenarios but may not be sufficiently low for detection of
leaking septic or other source tracking efforts. The authors
indicated that it is important to work with the CBM
partners to understand their specific monitoring questions
and critically appraise if CBM qPCR is capable and
appropriate to answer the questions.

B-55
Study Name
(*Denotes EPA
Authors)
Location
Study Type
(Search Topic)
Contamination
Sources
Water Quality
Metrics
Summaries
Saeidi et al.
(2018)
Singapore
MST (RT-
qPCR)
Human
PMMoV
PMMoV was characterized using RT-qPCR and viral
metagenomics at seven freshwater waterbody locations
with varied land use in Singapore between 2014–2016.
They found a correlation between PMMoV and urban
land use weightage (rho=.728, p < 0.01) in comparison to
FIB (rho = 0.583; p < 0.01). The authors also observed a
correlation between their PMMoV qPCR data and viral
metagenomics data (0.588 < rho < 0.879; p < 0.01),
suggesting that viral metagenomics could be used to
estimate specific microbial indicators during water quality
monitoring.
Sagarduy et al.
(2019)
France, South
Atlantic Coast
Method
development
(reverse
transcription
qPCR)
Human
E. coli, enterococci
Microbial decay experiments were conducted in
controlled microcosms and in situ transparent dialysis
bags in sea water, estuarine water, and freshwater
locations along the South Atlantic Coast of France. The
decay of sewage-sourced enterococci and E. coli
culturable cells and their associated molecular markers
(16S rRNA) was quantified by reverse transcription
qPCR. Decay rate models were also developed based on
the laboratory experiments. The decay rate model
predicted quantification compared reasonably well with
the in situ observed quantification. Sunlight had the
largest influence on the culturable bacteria although it did
not influence decay of the genetic markers.
Saingam et al.
(2018)
Hawaii, USA
Method
development
invA gene of
Salmonella
False-negative PCR and qPCR reactions were created
using serial dilutions of laboratory-prepared Salmonella
genomic DNA and then analyzed directly by NGS. NGS
was able to detect invA sequences in false-negative PCR
and qPCR reactions. The ability of PCR-NGS to identify
false negatives was confirmed under more
environmentally relevant conditions using Salmonella-
spiked stream water and sediment samples. The PCR-
NGS approach was applied to 10 urban stream water
samples and detected invA sequences in eight samples
that would be otherwise deemed Salmonella negative
using PCR or qPCR.

B-56
Study Name
(*Denotes EPA
Authors)
Location
Study Type
(Search Topic)
Contamination
Sources
Water Quality
Metrics
Summaries
Schang et al.
(2016)
Australia
Method
development
(culture, qPCR)
Human
E. coli, coliforms
IDEXX methods for enumeration of E. coli and coliform
densities were compared with the results of three
alternative approaches for monitoring fecal indicator
organisms: the TECTA™ system, U.S. EPA Method 1611
(a qPCR method for enumerating enterococci), and next
generation sequencing (NGS) in surface waters. When
comparing methods, the authors considered cost and
analysis time in addition to how the enumeration results
were correlated. All of the alternative approaches
produced statistically significant correlations with IDEXX
results, but there were differences in cost and time. U.S.
EPA Method 1611 had significant disadvantages such as
highly technical analysis and higher operational costs
(330% of IDEXX) and even higher costs (3,000% of
IDEXX) and analysis time (300% of IDEXX) were found
for NGS.
Schets et al.
(2018)
The
Netherlands
Outbreaks
Human
Indicator: E. coli.
Pathogens:
Norovirus,
Campylobacter
A recreational water-associated outbreak of norovirus
infections affecting at least 100 people in the Netherlands
occurred in August 2012. Patients, mostly children,
became ill with gastroenteritis 1–6 days (median 2 days)
after exposure. The results of this investigation, combined
with detection of norovirus GI in stool samples of patients
and sandy samples collected at the lake, suggested that
exposure to the recreational lake resulted in the outbreak.
The most likely source of contamination was determined
to be an infected human and the authors concluded that
active communication about human shedding of viruses
during and after diarrheal is needed along with guidance
to refrain from swimming when contamination is
suspected.
Schets et al.
(2011)
The
Netherlands
Children
NA
NA
This study evaluated 742 bathing related outbreaks
(between 1991–2007) involving 5,623 patients at all 641
official bathing sites in the Netherlands. Health endpoints
included gastroenteritis, skin, ear, eye, leptospirosis, and
other. This paper indicated that skin conditions and
gastroenteritis were the two most common health

B-57
Study Name
(*Denotes EPA
Authors)
Location
Study Type
(Search Topic)
Contamination
Sources
Water Quality
Metrics
Summaries
endpoints in outbreaks in the Netherlands between 1991
and 2007.
Schoen et al.
(2020)
United States
QMRA
human point
source
crAssphage
(CPQ_056),
Bacteroides spp.
(HF183/BacR287
and HumM2), and
human
polyomavirus
(HPyV)
The QMRA predictions in this study provided useful
information to compare potential indicators and to explore
different recreational water quality monitoring scenarios
based on a series of assumptions and uncertainties. The
methods used in this work can be used to establish risk-
based thresholds for alternative indicators.
Seidel et al.
(2017)
North Rhine-
Westphalia and
Lower Saxony,
Germany
MST (qPCR);
Method
development
Human, cow,
dog, pig, deer,
badger
Markers: AllBac,
HF183, BacCow
This study targeted host-specific markers in Bacteroidales
bacteria for subgroups associated with human and
ruminant fecal matter. Two amplicon sizes were
compared and applied to water samples from three
different freshwater sites in western Germany. The
proportion of intact cells dropped by up to 38% when the
longer sequence was targeted. The B-57addition of
dimethyl sulfoxide (DMSO) improved the efficiency of
PMA treatment. The authors concluded that the
comparison of signal decay by qPCR reactions targeting
sequences of different lengths might present a
methodological tool to shed more light on the role of
DNA disintegration and DNA damage in MST signal
decay.

B-58
Study Name
(*Denotes EPA
Authors)
Location
Study Type
(Search Topic)
Contamination
Sources
Water Quality
Metrics
Summaries
Seruge et al.
(2019)
Island of
O’ahu, Hawaii,
USA
Method
development
(culture, qPCR)
Human
Enterococci,
Clostridium
perfringens
Culture (EPA Method 1600 and Enterolert) and qPCR
(EPA Method 1611) methods were tested at beaches and
canals in Hawaii. Method 1611 was compromised due to
coralline sand which interfered with DNA extraction.
Sample process controls indicated that the PCR reaction
was not inhibited. In addition to enterococci, the study
also assessed Clostridium perfringens enumerated using
culture methods. There was good agreement among
methods for beach management decisions when qPCR
samples were not compromised. The authors concluded
that samples for EPA Method 1611 should not be
collected close to shore where particles are suspended or
there are visible milky plumes.
Shanks et al.
(2016)*
Ohio, USA
MST (qPCR);
Method
development
Human
HF183/BacR287,
HumM2
This study specified conditions for data acceptance
derived from a multiple laboratory data set using
standardized procedures for human-associated fecal
source identification quantitative real-time PCR. The
validity of qPCR data acceptance criteria for measurement
of genetic marker concentrations in reference DNA
material and freshwater sources using standardized
HF183/BacR287 and HumM2 qPCR protocols was
assessed. Lab-to-lab, replicate testing within a lab, and
random error for amplification inhibition and sample
processing controls were specified. Other data acceptance
measurements included extraneous DNA contamination
assessments and calibration model performance
(correlation coefficient, amplification efficiency, and
lower limit of quantification). This work is important for
the transition of qPCR methods from a research tool into a
standardized protocol with a high degree of confidence in
data quality across laboratories.

B-59
Study Name
(*Denotes EPA
Authors)
Location
Study Type
(Search Topic)
Contamination
Sources
Water Quality
Metrics
Summaries
Sheth et al.
(2016)
Door County,
Wisconsin,
USA
Method
development
(culture, qPCR)
Human, animal
E. coli, enterococci
Concentrations of Enterococcus in samples collected
along freshwater beaches along Lake Michigan in Door
County, WI were evaluated using qPCR and compared
with E. coli detected using culture-based methods. The
most differences were seen for qPCR E. coli compared to
E. coli culture-based beach notification decisions.
Detection of numbers of enterococci were closer for
Enterolert and Enterococcus qPCR (EntTaq), but there
was still disagreement of numbers between both methods.
The authors suggested studying what conditions might
affect the difference in numbers of enterococci and E. coli
between Colilert/Enterolert and qPCR, including ambient
conditions and sanitary surveys. The authors noted that
molecular techniques such as qPCR can be done
successfully in small laboratories with personnel with
minimal molecular biology training but cautioned that
each beach community should consider the impact of
utilizing rapid methods for beach management decisions
prior to adoption.
Shoults et al.
(2021)
Borden Park,
Edmonton,
Canada
QMRA
Human non-point
source (accidental
fecal
contamination)
Spiked water with
MS2 bacteriophage,
E. faecalis, and
baker’s yeast
surrogates and E.
coli. Reference
pathogens:
norovirus,
Campylobacter,
Cryptosporidium,
Giardia
A reverse QMRA was conducted for a natural swimming
pool. Of the four reference pathogens used (norovirus,
Campylobacter, Cryptosporidium, and Giardia), only
norovirus exceeded the median risk benchmark.
Campylobacter was the only other reference pathogen that
exceeded the risk benchmark at the estimated 95th
percentile.

B-60
Study Name
(*Denotes EPA
Authors)
Location
Study Type
(Search Topic)
Contamination
Sources
Water Quality
Metrics
Summaries
Shrestha and
Dorevitch (2019)
Lake Michigan,
Chicago,
Illinoi, USA
Method
development
(culture, qPCR)
Not reported
E. coli, enterococci
EPA draft Method C (E. coli qPCR) was compared to E.
coli culture (Colilert) and Enterococcus qPCR (EPA
Method 1609.1) in 288 water samples collected from eight
of Chicago’s Lake Michigan beaches. Method C
demonstrated acceptable performance characteristics, but
was prone to low level DNA contamination, possibly
originating from assay reagents derived from E. coli
bacteria. The study found that lag E. coli culture results,
e.g., samples collected Monday with results available
Tuesday, were not associated with either E. coli culture
results (r = 0.12; p = 0.08) or Enterococcus qPCR results
(r = 0.09; p = 0.17) for Tuesday. This indicates that E. coli
culture results available to a beach manager on a given
day (from samples cultured the prior day) are not
predictive of current water quality. In contrast, Method C
was most strongly statistically significantly associated
with same-day Colilert
®
results (r = 0.83; p < 0.0001).
And, in addition, statistically significant correlations were
also observed between Enterococcus qPCR and Method C
and between Enterococcus qPCR and same-day Colilert
®
results (both r = 0.67, p < 0.0001). The study also utilized
two different approaches for estimating potential E. coli
qPCR BAV thresholds: 1) EPA’s Site-Specific
Alternative Recreational Criteria Technical Support
Materials for Alternative Indicators and Methods
(Alternative Methods TSM) and 2) a receiver operating
characteristic (ROC) analysis. Potential BAV thresholds
differed substantially, ranging from 200.9 calibrator cell
equivalents (CCE)/100 mL (ROC analysis, Enterococcus
qPCR BAV as the reference) to 1,000 CCE/100 mL
(Alternative Methods TSM analysis, Enterococcus qPCR
BAV as the reference). The authors suggest that ROC
analysis, which optimizes sensitivity and specificity and
generates a dichotomous cut point should be given
consideration for generating BAVs, rather than relying on
the linear relationship between the two sets of continuous

B-61
Study Name
(*Denotes EPA
Authors)
Location
Study Type
(Search Topic)
Contamination
Sources
Water Quality
Metrics
Summaries
variables as is currently provided by the Alternative
Methods TSM.

B-62
Study Name
(*Denotes EPA
Authors)
Location
Study Type
(Search Topic)
Contamination
Sources
Water Quality
Metrics
Summaries
Shrestha and
Dorevitch (2020)
Great Lakes,
USA
Methods
(qPCR)
NA
Enterococcus
This article describes a qPCR-based beach monitoring
program in which a central laboratory tested water
samples from up to 20 beaches per day, seven days per
week, and reported the results to the public by noon.
Sips et al. (2020)
The
Netherlands
Children;
Outbreaks
Human,
waterfowl
E. coli, enterococci,
cyanobacteria,
Norovirus GI and
GII
A norovirus outbreak in a natural playground in the
Netherlands was investigated. The Public Health Service
received 21 case notifications via the national online
reporting system and an additional 100 cases (mostly
children) were identified following an online query of the
local health-related Facebook platform. This approach
indicated that public health monitoring could serve as an
early warning system. Water samples and fecal samples
from humans and birds were tested. The results indicated
that human introduction of norovirus was the most likely
cause of the outbreak. Although waterfowl feces could
have contributed to the outbreak, no evidence for
transmission via waterfowl was found.
Sivaganesan et al.
(2019)
NA – spiked
samples
Method
development
(qPCR)
Not reported
E. coli
This study included 21 laboratories that evaluated
standardized water samples, to determine data quality
acceptance criteria values for laboratories wishing to
establish their own comparable laboratory-specific criteria
for Draft EPA Method C or other qPCR methods used in
monitoring programs. After evaluating a total of 18 shared
surface water samples, including ambient, low spike, and
high spike for six different locations, the quality
acceptance criteria for Draft EPA Method C method
resulted in a 24% failure rate. The two newest laboratories
included in the study were responsible for 39% of the
failures. It was determined that the quality acceptance
failures were due to inconsistencies in storage and
preparation of reference materials. Variability between
laboratories was the greatest contributor to overall method
variability. The study concluded that it is technical
feasible for multiple laboratories to implement Draft
Method C or other qPCR water quality monitoring
methods with similar data quality acceptance criteria, but

B-63
Study Name
(*Denotes EPA
Authors)
Location
Study Type
(Search Topic)
Contamination
Sources
Water Quality
Metrics
Summaries
additional practice and/or assistance may be valuable,
even for some more generally experienced qPCR
laboratories.
Soller et al.
(2016)
Puerto Rico
QMRA
WWTP and
recreators
Adenovirus
(culture),
Enterovirus
(culture),
Cryptosporidium
Giardia, Salmonella
enterica, E. coli
0157:H7 (qPCR)
A QMRA was conducted to estimate the GI Illness at
Boquerón Bay, Puerto Rico to improve interpretation of a
recreational water epidemiological study. The QMRA
estimated an event risk of two illnesses per 1,000
recreation events, which was below the level that the
epidemiological study was designed to detect. The QMRA
findings provided a feasible explanation for the lack of
relationship between fecal indicator organisms and
swimming-related illness during the study.
Soller et al.
(2017)
California,
USA
QMRA
Urban river
discharges
Norovirus,
Adenovirus,
Campylobacter
jejuni, Salmonella
enterica
QMRA was used to predict the GI illness risk to surfers at
marine beaches in Southern California impacted by urban
stormwater. The predicted GI illness was dominated by
norovirus risk and varied between 0.6 and 36.0 illnesses
per 1,000 recreators, depending on the norovirus dose-
response selected. The QMRA results were in broad
agreement with epidemiological results from the same
location.
Somnark et al.
(2018)
Thailand
MST (PCR)
Human, cattle,
swine
Bacteroidales
(BacUni EP,
HF183/BFDrev EP,
Pig-2-Bac EP,
Bac3)
The performance of modified endpoint PCR assays in
detecting Bacteroidales in agricultural watersheds in
central Thailand was evaluated. The BacUni EP,
HF183/BFDrev EP,Pig-2-Bac EP, and Bac3 assays
demonstrated potential for MST of general and human-,
swine-, and cattle-derived fecal pollution.
Staley and Edge
(2016)
Toronto,
Canada
MST (culture,
qPCR, PCR)
Human,
ruminant, gull,
dog
E. coli, MST
markers: genBac,
Bac32, HF183,
CowM2, DG37,
Gull2, qGull4,
CF128
MST markers for human, cow, gull, and dog were used to
identify sources of fecal contamination at beaches in the
Toronto region. They found that the human (HF183), cow
(CowM2), and dog (DG37) markers had good host-
specificity, but the gull markers (Gull2 and qGull4) and
ruminant endpoint PCR marker (CF128) amplified other
species.

B-64
Study Name
(*Denotes EPA
Authors)
Location
Study Type
(Search Topic)
Contamination
Sources
Water Quality
Metrics
Summaries
Staley et al.
(2016)
Toronto,
Canada
MST (culture,
qPCR)
human, ruminant,
gull, dog
E. coli, MST
markers: Bac32,
GenBactF3, HF183,
CF128, CowM2,
Gull2, DH37, DG37
E. coli and ampicillin resistant E. coli as well as MST
markers for general Bacteroidales spp. (Bac32,
GenBactF3), human (HF183), ruminant/cow (CF128,
CowM2), gull (Gull2), and dog (DH37, DG37) were
assessed in the Humber River and one of its tributaries in
Toronto, Canada. Human and gull fecal source markers
were detected at all sampled sites. The authors also
observed a higher correlation between the presence of
ampicillin resistant E. coli and human qPCR marker than
culturable E. coli, suggesting that ampicillin resistant E.
coli might act as a better indicator of human sewage
contamination in this watershed.
Staley et al.
(2018a)
Toronto,
Canada
MST (culture,
qPCR)
Human, gull
E. coli, HF183,
qGull4
MST, chemical source tracking (CST), and environmental
DNA (eDNA) sequencing were used to confirm that storm
water was an important source of human fecal
contamination. However, the authors indicated that MST-
qPCR methods have limitations in that each individual
assay can only detect a singular known target. The authors
concluded that eDNA could be a good supplement to
MST-qPCR and CST for profiling sources of fecal
contamination.
Staley et al.
(2018b)
Great Lakes,
Canada
MST (dPCR,
qPCR); Method
development
Human, gull
HF183, qGull4
qPCR for human HF183 marker and the gull qGull4
marker were compared to digitalPCR duplexed to assess
both markers at the same time. The authors found that the
dPCR multiplex assay was more sensitive and capable of
detecting fecal contamination than was undetected by
qPCR assays. The cost per multiplexed dPCR was
equivalent to the cost of running single-target qPCRs for
two targets, making dPCR a cost-effective alternative to
qPCR.

B-65
Study Name
(*Denotes EPA
Authors)
Location
Study Type
(Search Topic)
Contamination
Sources
Water Quality
Metrics
Summaries
Stokdyk et al.
(2016)
NA – spiked
samples
Method
development
(qPCR)
Not reported
Salmonella,
adenovirus,
poliovirus
This study developed an approach for determining qPCR
LOD. The approach limited the number of analyses
required and was amenable to testing multiple genetic
targets simultaneously (i.e., spiking a single sample with
multiple microorganisms). An LOD determined this way
can facilitate study design, guide the number of required
technical replicates, aid method evaluation, and inform
data interpretation.
Sunger et al.
(2019)
United States,
hypothetical
freshwater
beach
QMRA
Treated
wastewater
effluent
Adenovirus,
Norovirus,
Cryptosporidium,
Giardia,
Salmonella,
Campylobacter
jejuni
QMRA and literature values of pathogen densities in raw
sewage were used to simulate the risk of illness from
swimming in freshwater impacted by secondarily treated
wastewater effluent aged 1 day. The estimated average
total risk due to the reference pathogens was similar to
FIB-based estimates and was dominated by viral risk. The
risk evaluated captured the likelihood of gastrointestinal
illnesses only and did not address the overall health risk
potential of recreational waters with respect to other
disease endpoints.
Symonds et al.
(2016)
Miami-area,
Florida, USA
MST (qPCR,
RT-qPCR,
culture)
Human (point
and non-point
sources)
Enterococci, MST
markers: BacHum,
CowM2, DogBact,
HF183, HPyV,
PMMoV
Surface water samples were collected from inlets and
coastal sites in Florida. Human sources of fecal pollution
were detected at all sampling sites following analysis of
culturable enterococci and a suite of MST markers
(BacHum, CowM2, DogBact, HF183, HPyV, PMMoV)
detected using qPCR and RT-qPCR. All sites met the
2012 U.S. EPA RWQC, despite detection of these
wastewater-associated MST markers. PMMoV were only
correlated with human-associated MST markers,
indicating that PMMoV RT-qPCR can act as an efficient
human-associated marker and should be included in the
MST toolbox.

B-66
Study Name
(*Denotes EPA
Authors)
Location
Study Type
(Search Topic)
Contamination
Sources
Water Quality
Metrics
Summaries
Symonds et al.
(2017)
Gulf of
Nacoya, Costa
Rica
MST (culture,
RT-qPCR)
Human, gull,
cow, dog, bird,
horse
E. coli, enterococci,
MST markers:
BacCow, BacCan,
DogBac, PMMoV,
GFD, Gull2,
HorseBac, HoF,
HF183, HPyV, PF
Surface water samples were collected and analyzed from a
shellfish harvesting area in the Gulf of Nacoya, Costa
Rica. E. coli was enumerated from these samples using
culture and RT-qPCR. The authors also evaluated the
sensitivity and specificity of 11 MST-PCR assays,
associated with cows (BacCow), dogs (BacCan, DogBac),
domestic wastewater (PMMoV), general avian (GFD),
gulls (Gull2), horses (HorseBac, HoF), humans (HF183,
HPyV), and pigs (PF). Human/domestic wastewater-
associated markers and low concentrations of FIB were
determined using molecular methods, indicating sufficient
microbial water quality for shellfish harvesting. The
authors found that E. coli was always detected using MPN
methods, and that E. coli and Entero1a were only detected
by qPCR in 81% and 41% of the samples, respectively.
Symonds et al.
(2018)
USA
MST
NA
PMMoV
This literature review discusses PMMoV as an indicator
for fecal MST. The review determined that there are needs
for new indicators closely related to enteric viruses that
could replace or supplement current monitoring tools.
According to the authors, PMMoV is a proven domestic
wastewater tracer and a promising indicator and index
virus for enteric viruses which makes PMMoV a good
candidate for efficient molecular-based microbial water
quality monitoring.
Thulsiraj et al.
(2017)
Tijuana,
Mexico
MST (qPCR)
Human, gull, dog,
horse
MST markers:
HF183, Gu112,
DogBac, HoF597
Propidium monoazide (PMA) was used to inhibit
amplification of DNA from dead cells during PCR. The
modified MST approach was used to identify fecal
contamination from humans, dogs, horses, and gulls in
freshwater creek and coastal seawater. The technique was
successful in inhibiting amplification of dead cells’ DNA
in freshwater that receives treated wastewater effluent.
Horse- and gull-associated markers were detected in 4%
and 8% of samples tested, respectively. The human- and
dog-associated markers were positive in 74% and 63% of
watershed samples and 92% and 75% of storm drain
samples, respectively.

B-67
Study Name
(*Denotes EPA
Authors)
Location
Study Type
(Search Topic)
Contamination
Sources
Water Quality
Metrics
Summaries
Toubiana et al.
(2021)
Marseille,
France
MST (qPCR)
Human, gull, dog,
horse
Catellicoccus
marimammalium,
MST markers:
HF183, DF475,
HoF597
Seawater samples were collected from a busy beach at
Marseille, France and MST was used to confirm that
humans were the main fecal source, while horses, dogs,
and gulls have little contribution to fecal pollution at the
study site. The authors indicated that high attendance at
beaches is an important factor to be considered to prevent
public health risks.
Unno et al.
(2018)
NA – review
article
MST (qPCR,
next generation
sequencing);
Method
development
NA – review
article
NA
This review summarized the use of qPCR as a traditional
MST method and discussed the potential of next
generation sequencing (NGS)-based MST approaches.
One NGS method defines operational taxonomic units
(OTUs) that are specific to a fecal source, (e.g., humans
and animals or shared among multiple fecal sources) to
determine the magnitude and likely source of fecal
pollution. The other method uses SourceTracker, a
program using a Bayesian algorithm, to determine which
OTUs have contributed to an environmental community
based on the composition of microbial communities in
multiple fecal sources. The geographical variability of the
fecal material should be taken into account when using
SourceTracker. For example, fecal sources from Florida
could not be identified when tested against libraries from
Minnesota, California, and Australia. However, including
sources from other geographic regions in the library did
not compromise the detection of sources in spiked
samples once the native sources were included in the
source library.
Urban et al.
(2021)
Cambridge,
United
Kingdom
Method
development
(16S rRNA
sequencing)
NA
Not specified
This study sequenced water samples from the River Cam
in Cambridge, UK. The study indicates that portable
nanopore sequencing technology is simple, fast, and cost-
effective. The study complemented DNA analyses with
physicochemical measurements to depict the hydrological
core microbiome and fine temporal gradients.

B-68
Study Name
(*Denotes EPA
Authors)
Location
Study Type
(Search Topic)
Contamination
Sources
Water Quality
Metrics
Summaries
Vadde et al.
(2019a)
Tiaoxi River
and Taihu
watershed,
China
MST (next
generation
sequencing);
Method
development
Human, chicken,
cow, dog, duck,
goose, pig
Bacteroides,
Prevotella, Blautia,
Faecalibacterium,
Dorea,
Acinetobacter,
Aeromonas,
Arcobacter,
Brevundimonas,
Enterococcus,
Escherichia-
Shigella,
Streptococcus
The microbial population of the Tiaoxi River water was
characterized using next generation sequencing (NGS)
paying particular attention to detection of bacteria of fecal
origin. For water samples (n = 45), a total of 39,192 OTUs
were generated ranging from 564 to 1292 OTUs. The
Ribosomal Database Project classifier categorized all the
OTUs of water into 20 bacterial phyla, however, their
relative abundance varied with the type of sample. NGS
was shown to be useful for preliminary investigations of
water to detect bacteria of fecal origin including potential
pathogens. Shared OTUs between water samples and
chicken, pig, and human fecal samples ranged from 4.5%
to 9.8% indicating the presence of avian, pig, and human
fecal contamination in the Tiaoxi River. The authors
suggested that Faecalibacterium could be used as a
human-associated target for MST using qPCR. NGS was
shown to be useful for preliminary investigations of water
to detect bacteria of fecal origin including potential
pathogens. NGS could be used in conjunction with other
methods for MST including qPCR.
Vadde et al.
(2019b)
Tiahu Lake,
Tiaoxi River,
and Changxing
River, China
MST (qPCR)
Human, pig,
chicken, cow,
duck, goose,
industrial
E. coli,
Bacteroidales, C.
jejuni, Leptospira
spp., Shigella, MST
markers: acUni,
GenBac, BacHum,
HF183, Pig-2-Bac,
GFD, AV4143,
mapA, LipL32,
ipaH, stx2, eae
The use of MST for fecal contamination was evaluated in
the Taihu watershed which was impacted by human,
animal, and industrial pollution. The study indicated that
BacUni, HF183, Pig-2-Bac, and GFD markers as detected
by qPCR were best at identifying the source of
contamination and differentiating between human and
fecal contamination. Five bacterial pathogens were
monitored. Of these pathogens, Campylobacter jejuni was
found in avian fecal contamination, Shigella in human
fecal contamination, and E. coli (STEC) in either human
or pig fecal contamination. These pathogen results
correlated with the fecal marker data obtained using
qPCR.

B-69
Study Name
(*Denotes EPA
Authors)
Location
Study Type
(Search Topic)
Contamination
Sources
Water Quality
Metrics
Summaries
Van Abel et al.
(2017)
Gauteng
province, South
Africa
QMRA
Not reported
norovirus
A QMRA was conducted to estimate risk of swimming,
boating, and playing in river waters in South Africa using
norovirus as reference hazard. Risks varied based on
recreational use (swimming, boating, or playing) and on
the dose-response model selected for the modeling.
Vergara et al.
(2016)
Singapore
QMRA
Urban non-point
sources
norovirus,
adenovirus
A QMRA was conducted to estimate the GI illness risk
from primary and secondary contact recreation in an urban
catchment in Singapore using field measurements of
norovirus and human adenovirus. The higher prevalence
and illness risk of norovirus supported the use of
norovirus as a reference pathogen for QMRA in
recreational waters in Singapore.
Verhougstraete et
al. (2020)
Sao Paulo,
Brazil
QMRA;
Children
Poorly treated
sewage
enterococci
Adjusted risk difference models (excess gastrointestinal
illness per swimming event) were determined for children
(<10 years of age) and non-children (≥10 years of age)
across five Brazil beaches using epidemiological data
collected in the UK and Brazil. The risk models indicated
that children in Brazil have twice the risk of
gastrointestinal illness than non-children. Elevated
enterococci levels resulted in an excess of 96 cases of
NGI cases per 1,000 swimming children. Enterococci
levels are higher in Brazil than in the primary UK study
(Kay et al., 1994) that informed WHO beach guideline
values. The study found that distinct enteric pathogen
profiles exist in tropical settings as well as in settings with
minimal wastewater treatment, highlighting the
importance of regionally specific guideline development.
Vincent-Hubert et
al. (2021)
France
Method
development
(qPCR)
Human, animal
Viruses, bacteria
The authors used passive water sampling and qPCR to
detect viruses and bacteria from two sites in an estuary of
the French Atlantic coast. The method allowed for
detection of microorganisms at low or variable
concentrations, and observation of the seasonality of
microorganisms.

B-70
Study Name
(*Denotes EPA
Authors)
Location
Study Type
(Search Topic)
Contamination
Sources
Water Quality
Metrics
Summaries
Vital et al. (2017)
Vietnam
Method
development
(qPCR)
Human, animal
E. coli
E. coli enumerated by qPCR in urban canal samples were
significantly lower than E. coli enumerated by
conventional culture methods for the wet season. The
authors conclude that qPCR may be impacted by sources
of runoff based on surveyed samples.
Wade et al.
(2018)
Puerto Rico
Epi/cohort
study
Treated
wastewater,
recreators, diffuse
sources
norovirus GI.1
(Norwalk virus) and
GII.4 (VA387
variant)
Saliva samples were collected as part of the U.S. EPA
Boquerón Beach epidemiology study to tested for IgG
responses to norovirus GI.1 and GII.4. There was a strong
association between swimming and asymptomatic
norovirus infection. The findings implicate wastewater
impacted recreational water as potentially important
transmission pathway for norovirus infection.
Walker et al.
(2017)
England
Method
development
(qPCR)
NA
E. coli: ybbW gene
A new quantitative real-time PCR assay was developed to
detect the ybbW gene sequence, which was found to be
100% exclusive and inclusive (specific and sensitive) for
E. coli. Of the 87 E. coli strains tested, 100% were found
to be ybbW positive, 94.2% were culture positive, 100%
were clpB positive and 98.9% were uidA positive.
Wang et al.
(2016)
Northern
California,
USA
Method
development
(dPCR, qPCR,
culture)
Human, animal
Enterococci
This study used dPCR, qPCR, and culture methods to
evaluate chip-based dPCR for quantifying enterococci.
The authors took 24 samples from multiple surface waters
in California (marine and freshwater) and observed a
consistent quantification capability in dPCR compared to
qPCR. They also observed that at realistic concentrations,
dPCR had lower variability (narrower 95% CI) than
qPCR. There were similar levels of inhibition by humic
acid, but less inhibition by calcium for dPCR compared to
qPCR.
World Health
Organization
2018
EU
Review
NA
Enterococci and E.
coli
The European Union Bathing Water Directive
(2006/7/EC) was reviewed, and it was recommended that
the two indicators in use (enterococci and E. coli) as well
as the four levels within the current classification system
(excellent, good, sufficient, and poor) should be retained.

B-71
Study Name
(*Denotes EPA
Authors)
Location
Study Type
(Search Topic)
Contamination
Sources
Water Quality
Metrics
Summaries
Wu et al. (2017)
Cuihu Lake in
Kunming,
China
MST (culture,
qPCR)
Human, birds,
cat, rabbit, pig,
goat, horse, dog,
squirrel,
hedgehog
Catellicoccus
marimamalium, E.
coli, Bacteroidales,
Campylobacter
spp., Helicobacter
A novel genetic marker targeting Catellicoccus
marimamalium was tested for MST of seagull fecal
contamination. Higher levels of the C. marimamalium
marker correlated with overwintering of seagulls at Cuihu
Lake, China. Levels of C. marimamalium dropped when
seagulls were not present, but a detectable amount of the
marker remained after seagull migration. An MST scheme
involving the C. marimamalium marker, detected using
qPCR, with human-associated Bacteroidales and FIB
could be useful for assessing potential human health risks.
Wu et al. (2020)
NA
QMRA/MST
(qPCR)
raw sewage,
secondary treated
effluent WWTP,
cattle, seagulls
Markers:
Catellicoccus assay,
BacCow, HF183,
Enterococcus.
Pathogens:
norovirus,
adenovirus (40/41),
Campylobacter,
Salmonella, E. coli
O157:H7,
Cryptosporidium,
Giardia
Risk-based thresholds (RBT) (corresponding with 36
illnesses per 1,000 swimmers) were determined for a
hypothetical waterbody contaminated by a continuous
loading of both human and non-human fecal
contamination. Results indicated that a larger difference in
decay rates between pathogen and indicator leads to an
increased miscalculation of gastroenteritis risk compared
to calculations that do not account for differential decay.
Given the continuous loading scenario, the difference in
RBT between fresh and aged contamination was less for
human marker HF183 in the human contamination
scenarios than for animal markers in the animal
contamination scenarios. The median RBT for human
contamination was roughly 3.5 to 3.7 log
10
gene
copies/100 mL for HF183.

B-72
Study Name
(*Denotes EPA
Authors)
Location
Study Type
(Search Topic)
Contamination
Sources
Water Quality
Metrics
Summaries
Wymer et al.
(2021)
Four Great
Lakes and three
marine beaches
(NEEAR
study)
Method
development
(qPCR, culture)
one or more
sources of treated
sewage effluents
which were
generally in
compliance with
local and federal
water quality
guidelines
enterococci
The variability of enterococci measured by culture and
qPCR was assessed for the NEEAR beaches. The 27-hour
variance of changes in CFU was significantly higher than
the three-hour variance of changes in CCE. This
illustrated the inherent magnitude of uncertainty in
predictability of next-day CFU results compared to a
faster measurement method (qPCR). The authors
concluded that even if it were possible to obtain culture
results within 3 hours, as it is for qPCR, CFUs would be a
less reliable predictor of conditions later in the day than
would be CCE. The levels of the molecular indicator were
more consistent throughout the day between 8:00 am and
3:00 pm.
Xie et al. (2017)
Baltic Sea,
India, United
Kingdom,
USA, Israel,
China,
Portugal,
Poland, South
Africa
Method
development
(qPCR)
NA – review
article
Salmonella, Vibrio,
Staphylococcus,
Candida, Hepatitis
A virus, among
other targets
This study reviewed multiple techniques to detect
microbial contamination in coastal waters, including
qPCR. The authors discussed interference on the success
of qPCR and the importance of having high-quality
template for qPCR assays.
Xue et al. (2018)
Lake
Pontchartrain,
Louisiana,
USA
MST (culture,
qPCR)
Human, cow
E. coli, total
coliforms,
Enterococcus, MST
markers: HF183,
CowM3
Surface water samples from Lake Pontchartrain,
Louisiana were evaluated to identify sources of fecal
contamination. Fecal indicator bacteria (i.e., E. coli, total
coliforms, and Enterococcus) were measured using
culture and qPCR-based assays and the genetic markers
HF183 and CowM3 were enumerated using qPCR. E. coli
was detected in 90.6% (culture) and 97.5% (qPCR) of
water samples. Enterococcus was detected in 95.8%
(culture) and 91.8% (qPCR) of water samples. The
relationship between E. coli and Enterococcus results
observed for both qPCR and culture methods was
statistically significant. The HF183 marker was detected
in 94.3% of water samples (149 of 158). Daily
precipitation levels were graphed with microbial results
and the authors concluded that rainfall events might
introduce large amounts of fecal bacteria into the lake.

B-73
Study Name
(*Denotes EPA
Authors)
Location
Study Type
(Search Topic)
Contamination
Sources
Water Quality
Metrics
Summaries
Xue et al. (2019)
Alabama, USA
MST (qPCR)
Cow
CowM2, CowM3
Two cattle-associated Bacteroidales fecal markers,
CowM2 and CowM3, were used to assess MST in
ambient water. CowM3 had a lower limit of detection
(LLOD) and lower limit of quantification (LLOQ) than
CowM2, suggesting CowM3 was a more reliable marker
to use for MST. The method developed was useful in
MST studies and standardizing comparison of different
labs’ results.
Young 2016
Epi/Review
Review
various
NA
This study reviewed epidemiological evidence for the
association between marine bathing and infectious
disease. Numerous studies demonstrated an increased risk
of gastrointestinal illness associated with marine
swimming compared to non-swimming. An association
between levels of FIB and illness among swimmers was
found in some studies while other studies indicated that
traditional FIB may not be predictive of human health
impacts when human waste is not the predominant source
of pathogens.
Yuan et al.
(2018)
Missouri, USA
Method
development
(qPCR)
NA – spiked
samples
E. coli
Freshwater samples were collected from multiple sources
around Missouri and spiked with fecal E. coli strains.
Prior to qPCR, the spiked samples were treated with
propidium monoazide (PMA), a dye that can inhibit the
amplification of DNA from dead cells, allowing for the
detection and quantification of only viable cells present in
the sample. The authors reported no significant
differences among the PMA-qPCR assay and two other
standard culture-based methods for detection of viable E.
coli (including EPA Method 1603).

B-74
Study Name
(*Denotes EPA
Authors)
Location
Study Type
(Search Topic)
Contamination
Sources
Water Quality
Metrics
Summaries
Zeki et al. (2021)
Istanbul,
Turkey
MST (culture,
qPCR)
Human
Fecal coliforms,
enterococci,
Bacteroides (BT-a)
An MST study was conducted on an urban estuary,
Golden Horn, Istanbul, Turkey, which was subject to
decades of industrial and urban pollution and then years of
rehabilitation. The study compared membrane filtration
results to qPCR results, and both methods confirmed that
despite rehabilitation of the estuary, there was still sewage
intrusion. The Bacteroides marker (BT-a) was detected in
30% of the samples and had a positive correlation with
culture and qPCR-based enterococci concentrations.
Zhang and Ishii
(2018)
Sapporo, Japan,
Minneapolis,
Minnesota,
USA
Method
development
(qPCR)
NA
Pseudogulbenkiania
spp., E. coli
O157:H7, S.
Typhimurium,
Campylobacter
jejuni, Listeria
monocytogenes
A novel sample process control (SPC) strain was created
by genetically engineering a Pseudogulbenkiania sp.
strain. And a new TaqMan qPCR assay was developed to
quantify the strain. Environmental water samples were co-
spiked with four different pathogens, including E. coli
O157:H7, and the SPC strain. The estimated pathogen
densities were not significantly different from the
inoculated pathogen densities. This approach could be
used to quantify FIB, multiple pathogens, and the SPC
strain simultaneously in high throughput in environmental
water samples.
Zhang et al.
(2020)
China
MST (qPCR)
Studied fecal
samples collected
from humans and
various animals
BacH, BacHum,
SYBR-HF183,
Hum2, Hum163,
CPQ_056,
CPQ_064; Pig-q-
Bac, Pig-2-Bac,
Lamylovorus,
P.ND5, Rum-2-Bac,
Bac708, BacCow,
GFD
The sensitivity, specificity, and magnitude of a variety of
human, ruminant, and poultry MST markers in fecal
samples in China was tested. The authors found evidence
that a higher mean concentration of markers increased the
likelihood of observing true positive signals for target
hosts (above the limit of detection). Bacteroidales
markers exhibited greater sensitivity and higher
concentrations compared to other bacterial/viral markers,
but had low specificity; thus, multiple markers might be
necessary.
Zimmer-Faust et
al. (2018)
Northwestern
Baja California,
Mexico
MST (culture,
qPCR, Inv-
IMS/ATP)
Human, non-
human
Enterococci, MST
markers: HF183, B.
theta
An inversely coupled (Inv-IMS/ATP) viability-based
assay for rapid evaluation and screening of surface water
was developed. There was a high correlation between the
human genetic marker, B. theta, measured by Inv-
IMS/ATP and measurements of this marker and HF183
using qPCR (r=0.76 for HF183, and r=0.82 for B. theta).

B-75
Study Name
(*Denotes EPA
Authors)
Location
Study Type
(Search Topic)
Contamination
Sources
Water Quality
Metrics
Summaries
Zimmer-Faust et
al. (2021)
Punta Bandera
to Silver Strand
State Beach,
California,
USA and
Mexico
MST (culture,
ddPCR);
Method
development
Human
Enterococci, MST
markers: HF183,
dENT, Lachno3
Water samples were collected along the coast of the
United States-Mexico border. The authors used culture
(Enterolert) and molecular methods (ddPCR for gene
quantification, and next generation sequencing for
microbial community analysis) as MST tools. A
significant gradient of human fecal contamination from
the San Antonio de los Buenos wastewater treatment plant
was observed. This work increases the understanding of
the fate and transport of wastewater treatment plant
pollution.
References in Table B-1
Abello, J.J.M., Malajacan, G.T., Labrador, K.L., Nacario, M.A.G., Galarion, L.H., Obusan,
M.C.M., Rivera, W.L. 2021. Library-independent source tracking of fecal contamination in
selected stations and tributaries of Laguna Lake, Philippines. Journal of Water and Health,
19: 846-854.
Abia, A.L.K., Ubomba-Jaswa, E., Genthe, B., Momba, M.N.B. 2016. Quantitative microbial risk
assessment (QMRA) shows increased public health risk associated with exposure to river
water under conditions of riverbed sediment resuspension. Science of the Total
Environment, 566: 1143-1151.
Ahmad, F., Stedtfeld, R.D., Waseem, H., Williams, M.R., Cupples, A.M., Tiedje, J.M.,
Hashsham, S.A. 2017. Most probable number – loop mediated isothermal amplification
(MPN-LAMP) for quantifying waterborne pathogens in <25min. Journal of
Microbiological Methods, 132: 27-33.
Ahmed, W., Harwood, V.J., Nguyen, K., Young, S., Hamilton, K., Toze, S. 2016a. Utility of
Helicobacter spp. associated GFD markers for detecting avian fecal pollution in natural
waters of two continents. Water Research, 88: 613-622.
Ahmed, W., Hughes, B., Harwood, V.J. 2016b. Current status of marker genes of Bacteroides
and related taxa for identifying sewage pollution in environmental waters. Water, 8(6):
231.
Ahmed, W., Gyawali, P., Toze, S. 2016c. Evaluation of glass wool filters and hollow-fiber
ultrafiltration concentration methods for qPCR detection of human adenoviruses and
polyomaviruses in river water. Water, Air, and Soil Pollution, 227: 327.
Ahmed, W., Hamilton, K. A., Lobos, A., Hughes, B., Staley, C., Sadowsky, M.J., Harwood, V. J.
2018a. Quantitative microbial risk assessment of microbial source tracking markers in
recreational water contaminated with fresh untreated and secondary treated sewage.
Environmental International, 117: 243-249.
Ahmed, W., Lobos, A., Senkbeil, J., Peraud, J., Gallard, J., Harwood, V.J. 2018b. Evaluation of
the novel crAssphage marker for sewage pollution tracking in storm drain outfalls in
Tampa, Florida. Water Research, 131: 142-150.
Ahmed, W., Payyappat, S., Cassidy, M., Besley, C. 2019. A duplex PCR assay for the
simultaneous quantification of Bacteroides HF183 and crAssphage CPQ_056 marker genes
in untreated sewage and stormwater. Environmental International, 126: 252-259.
Ahmed, W., Payyappat, S., Cassidy, M., Harrison, N., Besley, C. 2020. Interlaboratory accuracy
and precision among results of three sewage-associated marker genes in urban
environmental estuarine waters and freshwater streams. Science of the Total Environment,
741: 140071.
Arnold, B.F., Wade, T.J., Benjamin-Chung, J.S., K.C., Griffith, J.F., Dufour, A.P., Weisberg,
S.B., Colford, J.M., Jr. 2016. Acute gastroenteritis and recreational water: Highest burden
among young U.S. children. American Journal of Public Health, 106: 1690-1697.
Arnold, B.F., Schiff, K.C., Ercumen, A., Benjamin-Chung, J., Steele, J.A., Griffith, J.F.,
Steinberg, S.J., Smith, P., McGee, C.D., Wilson, R., Nelsen, C., Weisberg, S.B., Colford,
J.M., Jr. 2017. Acute illness among surfers after exposure to seawater in dry- and wet-
weather conditions. American Journal of Epidemiology, 186: 866-875.
Augustine, S.A.J., Eason, T.N., Simmons, K.J., Griffin, S.M., Curioso, C.L., Ramudit, M.K.D.,
Sams, E.A., Oshima, K.H., Dufour, A., Wade, T.J. 2020. Rapid salivary IgG antibody
screening for hepatitis A. Journal of Clinical Microbiology, 58(10): e00358-00320.
B-77
Aw, T.G., Sivaganesan, M., Briggs, S., Dreelin, E., Aslan, A., Dorevitch, S., Shrestha, A., Isaacs,
N., Kinzelman, J., Kleinheinz, G., Noble, R., Rediske, R., Scull, B., Rosenberg, S.,
Weberman, B., Sivy, T., Southwell, B., Siefring, S., Oshima, K., Haugland, R. 2019.
Evaluation of multiple laboratory performance and variability in analysis of recreational
freshwaters by a rapid Escherichia coli qPCR method (Draft Method C). Water Research,
156: 465-474.
Ballesté, E., García-Aljaro, C., Blanch, A.R. 2018. Assessment of the decay rates of microbial
source tracking molecular markers and faecal indicator bacteria from different sources.
Journal of Applied Microbiology, 125(6), 1938-1949.
Beale, D.J., Karpe, A.V., Ahmed, W., Cook, S., Morrison, P.D., Staley, C., Sadowsky, M.J.,
Palombo, E.A. 2017. A community multi-omics approach towards the assessment of
surface water quality in an urban river system. International Journal of Environmental
Research and Public Health, 14(3): 303.
Benjamin-Chung, J., Arnold, B.F., Wade, T.J., Schiff, K., Griffith, J.F., Dufour, A.P., Weisberg,
S.B., Colford, J.M., Jr. 2017. Coliphages and gastrointestinal illness in recreational waters:
Pooled analysis of six coastal beach cohorts. Epidemiology, 28(5): 644-652.
Blanch, A.R., Lucena, F., Muniesa, M., Jofre, J. 2020. Fast and easy methods for the detection of
coliphages. Journal of Microbiological Methods, 173: 105940.
Boehm, A.B. 2019. Risk-based water quality thresholds for coliphages in surface waters: Effect
of temperature and contamination aging. Environmental Science: Processes Impacts,
21(12): 2031-2041.
Boehm, A.B., Soller, J.A. 2020. Refined ambient water quality thresholds for human-associated
fecal indicator HF183 for recreational waters with and without co-occurring gull fecal
contamination. Microbial Risk Analysis, 16.
Boehm, A.B., Graham, K.E., Jennings, W.C. 2018. Can we swim yet? Systematic review, meta-
analysis, and risk assessment of aging sewage in surface waters. Environmental Science &
Technology, 52: 9634-9645.
Bonadonna, L., Briancesco, R., La Rosa, G. 2019. Innovative analytical methods for monitoring
microbiological and virological water quality. Microchemical Journal, 150: 104160.
Bortagaray, V., Girardi, V., Pou, S., Lizasoain, A., Tort, L.F.L., Spilki, F.R., Colina, R., Victoria,
M. 2020. Detection, quantification, and microbial risk assessment of group A rotavirus in
rivers from Uruguay. Food and Environmental Virology, 12(2), 89-98.
Brooks, L.E., Field, K.G. 2017. Global model fitting to compare survival curves for faecal
indicator bacteria and ruminant-associated genetic markers. Journal of Applied
Microbiology, 122: 1704-1713.
Brown, K.I., Graham, K.E., Soller, J.A., Boehm, A.B. 2017a. Estimating the probability of
illness due to swimming in recreational water with a mixture of human- and gull-associated
microbial source tracking markers. Environmental Science: Processes Impacts, 19: 1528-
1541.
Brown, K.I., Graham, K.E., Boehm, A.B. 2017b. Risk-based threshold of gull-associated fecal
marker concentrations for recreational water. Environmental Science & Technology
Letters, 4(2): 44-48.
Brumfield, K.D., Cotruvo, J.A., Shanks, O.C., Sivaganesan, M., Hey, J., Hasan, N.A., Huq, A.,
Colwell, R.R., Leddy, M.B. 2021. Metagenomic sequencing and quantitative real-time
PCR for fecal pollution assessment in an urban watershed. Frontiers in Water, 3: 626849.
B-78
Bushon, R.N., Brady, A.M., Christensen, E.D., Stelzer, E.A. 2017. Multi-year microbial source
tracking study characterizing fecal contamination in an urban watershed. Water
Environment Research, 89: 127-143.
Byappanahalli, M.N., Nevers, M.B., Shively, D.A., Spoljaric, A., Otto, C. 2018. Real-time water
quality monitoring at a Great Lakes National Park. Journal of Environmental Quality, 47:
1086-1093.
Cao, Y., Raith, M.R., Griffith, J.F. 2016. A duplex digital PCR assay for simultaneous
quantification of the Enterococcus spp. and the human fecal-associated HF183 marker in
waters. Journal of Visualized Experiments (109): 53611.
Cao, Y., Sivaganesan, M., Kelty, C.A., Wang, D., Boehm, A.B., Griffith, J.F., Weisberg, S.B.,
Shanks, O.C. 2018a. A human fecal contamination score for ranking recreational sites
using the HF183/BacR287 quantitative real-time PCR method. Water Research, 128: 148-
156.
Cao, Y., Raith, M.R., Griffith, J.F. 2018b. Testing of general and human-associated fecal
contamination in waters. Methods Molecular Biology, 1768: 127-140.
Chandler, J.C., Pérez-Méndez, A., Paar, J.R., Doolittle, M.M., Bisha, B., Goodridge, L.D. 2017.
Field-based evaluation of a male-specific (F+) RNA coliphage concentration method.
Journal of Virological Methods, 239: 9-16.
Chen, L., Zhang, X., Zhi, X., Dai, Y., Zhang, P., Xiao, Y., Shen, Z. 2020. Tracking faecal
microorganisms using the qPCR method in a typical urban catchment in China.
Environmental Monitoring and Assessment, 192: 158.
Chyerochana, N., Kongprajug, A., Somnark, P., Leelapanang Kamphaengthong, P., Mongkolsuk,
S., Sirikanchana, K. 2020. Distributions of enterococci and human-specific bacteriophages
of enterococci in a tropical watershed. International Journal of Hygiene and Environmental
Health, 226: 113482.
Cloutier, D.D., McLellan, S.L. 2017. Distribution and differential survival of traditional and
alternative indicators of fecal pollution at freshwater beaches. Applied and Environmental
Microbiology, 83(4): e02881-02816.
Codello, A., McLellan, S.L., Steinberg, P., Potts, J., Scanes, P., Ferguson, A., Hose, G.C.,
Griffith, M., Roguet, A., Lydon, K.A., Maher, W.A., Krikowa, F., Chariton, A. 2021. A
weight-of-evidence approach for identifying potential sources of untreated sewage inputs
into a complex urbanized catchment. Environmental Pollution, 275: 116575.
Cormier, J., Janes, M. 2016. Concentration and detection of hepatitis A virus and its indicator
from artificial seawater using zeolite. Journal of Virological Methods, 235: 1-8.
Crain, C., Kezer, K., Steele, S., Owiti, J., Rao, S., Victorio, M., Austin, B., Volner, A., Draper,
W., Griffith, J., Steele, J., Seifert, M. 2021. Application of ddPCR for detection of
Enterococcus spp. in coastal water quality monitoring. Journal of Microbiological
Methods, 184: 106206.
Crank, K., Petersen, S., Bibby, K. 2019. Quantitative microbial risk assessment of swimming in
sewage impacted waters using CrAssphage and pepper mild mottle virus in a customizable
model. Environmental Science & Technology Letters, 6(10), 571-577.
Crank, K., Li, X., North, D., Ferraro, G.B., Iaconelli, M., Mancini, P., La Rosa, G., Bibby, K.
2020. CrAssphage abundance and correlation with molecular viral markers in Italian
wastewater. Water Research, 184: 116161.
Cui, Q., Fang, T., Huang, Y., Dong, P., Wang, H. 2017. Evaluation of bacterial pathogen
diversity, abundance and health risks in urban recreational water by amplicon next-
B-79
generation sequencing and quantitative PCR. Journal of Environmental Sciences, 57, 137-
149.
DeFlorio-Barker, S., Arnold, B.F., Sams, E. A., Dufour, A. P., Colford, J. M., Jr Weisberg, S. B.,
Schiff, K. C., Wade, T. J. 2018. Child environmental exposures to water and sand at the
beach: Findings from studies of over 68,000 subjects at 12 beaches. Journal of Exposure
Science and Environmental Epidemiology, 28: 93-100.
Derx, J., Demeter, K., Linke, R., Cervero-Aragó, S., Lindner, G., Stalder, G., Schijven, J.,
Sommer, R., Walochnik, J., Kirschner, A., Komma, J., Blaschke, A.P., Farnleitner, A.H.
2021. Genetic microbial source tracking support QMRA modeling for a riverine wetland
drinking water resource. Frontiers in Microbiology, 12, 668778.
Deshmukh, R.A., Joshi, K., Bhand, S., Roy, U. 2016. Recent developments in detection and
enumeration of waterborne bacteria: A retrospective mini review. Microbiology Open, 5:
901-922.
Devane, M.L., Moriarty, E.M., Robson, B., Lin, S., Wood, D., Webster-Brown, J., Gilpin, B.J.
2019. Relationships between chemical and microbial faecal source tracking markers in
urban river water and sediments during and post-discharge of human sewage. Science of
the Total Environment, 651: 1588-1604.
Dorevitch, S., Shrestha, A., DeFlorio-Barker, S., Breitenbach, C., Heimler, I. 2017. Monitoring
urban beaches with qPCR vs. culture measures of fecal indicator bacteria: Implications for
public notification. Environmental Health, 16: 45.
Duan, C., Cui, Y., Zhao, Y., Zhai, J., Zhang, B., Zhang, K., Sun, D., Chen, H. 2016. Evaluation
of Faecalibacterium 16S rDNA genetic markers for accurate identification of swine faecal
waste by quantitative PCR. Journal of Environmental Management. 181: 193-200.
Dufour, A. 2021. A short history of methods used to measure bathing beach water quality.
Journal of Microbiological Methods, 181: 106134.
D'Ugo, E., Sdanganelli, M., Giuseppetti, R., Mancini, L. 2019. Enteric viruses pollution from
aquatic ecosystems of two Italian regions. Fresenius Environmental Bulletin, 28: 4998-
5003.
Egorov, A.I., Griffin, S.M., Ward, H.D., Reilly, K., Fout, G.S., Wade, T.J. 2018. Application of a
salivary immunoassay in a prospective community study of waterborne infections. Water
Research, 142: 289-300.
Eregno, F.E., Tryland, I., Tjomsland, T., Myrmel, M., Robertson, L., Heistad, A. 2016.
Quantitative microbial risk assessment combined with hydrodynamic modelling to estimate
the public health risk associated with bathing after rainfall events. Science of the Total
Environment, 548-549: 270-279.
Fan, L., Shuai, J., Zeng, R., Mo, H., Wang, S., Zhang, X., He, Y. 2017. Validation and
application of quantitative PCR assays using host-specific Bacteroidales genetic markers
for swine fecal pollution tracking. Environmental Pollution, 231: 1569-1577.
Fang, T., Cui, Q., Huang, Y., Dong, P., Wang, H., Liu, W.-T., Ye, Q. 2018. Distribution
comparison and risk assessment of free-floating and particle-attached bacterial pathogens
in urban recreational water: Implications for water quality management. Science of the
Total Environment, 613-614, 428-438.
Farkas, K., Cooper, D.M., McDonald, J.E., Malham, S.K., de Rougemont, A., Jones, D.L. 2018.
Seasonal and spatial dynamics of enteric viruses in wastewater and in riverine and
estuarine receiving waters. Science of the Total Environment, 634, 1174-1183.
B-80
Federigi, I., Verani, M., Donzelli, G., Cioni, L., Carducci, A. 2019. The application of
quantitative microbial risk assessment to natural recreational waters: A review. Marine
Pollution Bulletin, 144: 334-350.
Feng, S., McLellan, S.L. 2019. Highly specific sewage-derived Bacteroides quantitative PCR
assays target sewage-polluted waters. Applied and Environmental Microbiology, 85:
e02696-02618.
Ferguson, A., Del Donno, C., Obeng-Gyasi, E., Mena, K., Altomare, T.K., Guerrero, R., Gidley,
M., Montas, L., Solo-Gabriele, H. M. 2019. Children exposure-related behavior patterns
and risk perception associated with recreational beach use. International Journal of
Environmental Research and Public Health, 16(15): 2783.
Ferguson, A., Dwivedi, A., Adelabu, F., Ehindero, E., Lamssali, M., Obeng-Gyasi, E., Mena, K.,
Solo-Gabriele, H. 2021. Quantified activity patterns for young children in beach
environments relevant for exposure to contaminants. International Journal of
Environmental Research and Public Health, 18(6): 3274.
Fu, J., Chiang, E.L.C., Medriano, C.A.D., Li, L., Bae, S. 2021. Rapid quantification of fecal
indicator bacteria in water using the most probable number – loop-mediated isothermal
amplification (MPN-LAMP) approach on a polymethyl methacrylate (PMMA) microchip.
Water Research, 199: 117172.
García-Aljaro, C., Blanch, A.R., Campos, C., Jofre, J., Lucena, F. 2019. Pathogens, faecal
indicators and human-specific microbial source-tracking markers in sewage. Journal of
Applied Microbiology, 126: 701-717.
Gibson, K. E., Lee, J.A., Jackson, J.M., Smith, L.N., Almeida, G. 2017. Identification of factors
affecting fecal pollution in Beaver Lake Reservoir. Journal of Environmental Quality, 46:
1048-1056.
Gitter, A., Mena, K. D., Wagner, K. L., Boellstorff, D.E., Borel, K. E., Gregory, L. F., Gentry, T.
J., Karthikeyan, R. 2020. Human health risks associated with recreational waters:
Preliminary approach of integrating quantitative microbial risk assessment with microbial
source tracking. Water, 12(2): 327.
Gonçalves, J., Gutiérrez-Aguirre, I, Balasubramanian, MN, Zagorščak, M, Ravnikar, M, Turk, V.
2018. Surveillance of human enteric viruses in coastal waters using concentration with
methacrylate monolithic supports prior to detection by RT-qPCR. Marine Pollution
Bulletin, 128: 307-317.
Gosselin-Théberge, M., Taboada, E, Guy, RA. 2016. Evaluation of real-time PCR assays and
standard curve optimisation for enhanced accuracy in quantification of Campylobacter
environmental water isolates. Journal of Microbiological Methods, 129: 70-77.
Graciaa, D.S., Cope, J.R., Roberts, V.A., Cikesh, B.L., Kahler, A.M., Vigar, M., Hilborn, E. D.,
Wade, T.J., Backer, L.C., Montgomery, S.P., Secor, W.E., Hill, V.R., Beach, M.J.,
Fullerton, K.E., Yoder, J.S., Hlavsa, M.C. 2018. Outbreaks associated with untreated
recreational water – United States, 2000-2014. Morbidity and Mortality Weekly Report,
67(25): 701-706.
Green, H., Weller, D., Johnson, S., Michalenko, E. 2019. Microbial source-tracking reveals
origins of fecal contamination in a recovering watershed. Water, 11(10): 2162.
Gutiérrez-Cacciabue, D., Cid, A.G., Rajal, V.B. 2016. How long can culturable bacteria and total
DNA persist in environmental waters? The role of sunlight and solid particles. Science of
the Total Environment, 539: 494-502.
B-81
Hata, A., Hanamoto, S., Shirasaka, Y., Yamashita, N., Tanaka, H. 2016. Quantitative distribution
of infectious F-Specific RNA phage genotypes in surface waters. Applied and
Environmental Microbiology, 82: 4244-4252.
Haugland, R., Oshima, K., Sivaganesan, M., Dufour, A., Varma, M., Siefring, S., Nappier, S.,
Schnitker, B., Briggs, S. 2021. Large-scale comparison of E. coli levels determined by
culture and a qPCR method (EPA Draft Method C) in Michigan towards the
implementation of rapid, multi-site beach testing. Journal of Microbiological Methods,
184: 106186.
He, X., Liu, P. Zheng, G., Chen, H., Shi, W., Cui, Y., Ren, H., Zhang, X.X. 2016. Evaluation of
five microbial and four mitochondrial DNA markers for tracking human and pig fecal
pollution in freshwater. Scientific Reports, 6: 35311.
Hofstra, N., Vermeulen, L.C., Derx, J., Florke, M., Mateo-Sagasta, J., Rose, J., Medema, G.
2019. Priorities for developing a modelling and scenario analysis framework for
waterborne pathogen concentrations in rivers worldwide and consequent burden of disease.
Current Opinion in Environmental Sustainability, 36: 28-38.
Holcomb, D.A., Stewart, J.R. 2020. Microbial indicators of fecal pollution: Recent progress and
challenges in assessing water quality. Current Environmental Health Reports, 7: 311-324.
Hsu, T.T.D., Mitsch, W.J., Martin, J.F., Lee, J. 2017. Towards sustainable protection of public
health: The role of an urban wetland as a frontline safeguard of pathogen and antibiotic
resistance spread. Ecological Engineering, 108: 547-555.
Jennings, W.C., Chern, E.C., O'Donohue, D., Kellogg, M.G., Boehm, A.B. 2018. Frequent
detection of a human fecal indicator in the urban ocean: Environmental drivers and
covariation with enterococci. Environmental Science: Processes Impacts, 20: 480-492.
Jiang, Y.S., Riedel, T.E., Popoola, J.A., Morrow, B.R., Cai, S., Ellington, A.D., Bhadra, S. 2018.
Portable platform for rapid in-field identification of human fecal pollution in water. Water
Research, 131: 186-195.
Jikumaru, A., Ishii, S., Fukudome, T., Kawahara, Y., Iguchi, A., Masago, Y., Nukazawa, K.,
Suzuki, Y. 2020. Fast, sensitive, and reliable detection of waterborne pathogens by digital
PCR after coagulation and foam concentration. Journal of Bioscience and Bioengineering,
130: 76-81.
Jogi, E., Valing, I., Rinken, T. 2020. Assessment of bathing water quality with an E. coli
immunosensor. International Journal of Environmental Analytical Chemistry: 1-12.
Joosten, R., Sonder, G., Parkkali, S., Brandwagt, D., Fanoy, E., Mughini-Gras, L., Lodder, W.,
Ruland, E., Siedenburg, E., Kliffen, S., van Pelt, W. 2017. Risk factors for gastroenteritis
associated with canal swimming in two cities in the Netherlands during the summer of
2015: A prospective study. PloS One, 12(4): e0174732.
Kabiri, L., Alum, A., Rock, C., McLain, J.E., Abbaszadegan, M. 2016. A tool box strategy using
Bacteroides genetic markers to differentiate human from non-human sources of fecal
contamination in natural water. Science of the Total Environment, 572: 897-905.
Kinzelman, J., Byappanahalli, M.N., Nevers, M.B., Shively, D., Kurdas, S., Nakatsu, C. 2020.
Utilization of multiple microbial tools to evaluate efficacy of restoration strategies to
improve recreational water quality at a Lake Michigan beach (Racine, WI). Journal of
Microbiological Methods, 178: 106049.
Kolm, C., Martzy, R., Brunner, K., Mach, R.L., Krska, R., Heinze, G., Sommer, R., Reischer,
G.H., Farnleitner, A.H. 2017. A complementary isothermal amplification method to the
B-82
U.S. EPA quantitative polymerase chain reaction approach for the detection of enterococci
in environmental waters. Environmental Science & Technology, 51: 7028-7035.
Kolm, C., Martzy, R., Führer, M., Mach, R.L., Krska, R., Baumgartner, S., Farnleitner, A.H.,
Reischer, G.H. 2019. Detection of a microbial source tracking marker by isothermal
helicase-dependent amplification and a nucleic acid lateral-flow strip test. Scientific
Reports, 9: 393.
Kongprajug, A., Chyerochana, N., Somnark, P., Leelapanang Kampaengthong, P., Mongkolsuk,
S., Sirikanchana, K. 2019. Human and animal microbial source tracking in a tropical river
with multiple land use activities. International Journal of Hygiene and Environmental
Health, 222: 645-654.
Kongprajug, A., Chyerochana, N, Mongkolsuk, S, Sirikanchana, K. 2020. Effect of quantitative
polymerase chain reaction data analysis using sample amplification efficiency on microbial
source tracking assay performance and source attribution. Environmental Science &
Technology, 54: 8232-8244.
Korajkic, A., McMinn, B.R., Ashbolt, N.J., Sivaganesan, M., Harwood, V.J., Shanks, O.C. 2019.
Extended persistence of general and cattle-associated fecal indicators in marine and
freshwater environment. Science of the Total Environment, 650: 1292-1302.
Kuhn, K.G., Nielsen, E.M., Mølbak, K., Ethelberg, S. 2018. Determinants of sporadic
Campylobacter infections in Denmark: A nationwide case-control study among children
and young adults. Clinical Epidemiology, 10: 1695-1707.
Lane, M.J., Rediske, R.R., McNair, J.N., Briggs, S., Rhodes, G., Dreelin, E., Sivy, T., Flood, M.,
Scull, B., Szlag, D., Southwell, B., Isaacs, N.M., Pike, S. 2020. A comparison of E. coli
concentration estimates quantified by the EPA and a Michigan laboratory network using
EPA Draft Method C. Journal of Microbiological Methods, 179: 106086.
Lapen, D.R., Schmidt, P.J., Thomas, J.L., Edge, T.A., Flemming, C., Keithlin, J., Neumann, N.,
Pollari, F., Ruecker, N., Simhon, A., Topp, E., Wilkes, G., Pintar, K. D. M. 2016. Towards
a more accurate quantitative assessment of seasonal Cryptosporidium infection risks in
surface waters using species and genotype information. Water Research, 105: 625-637.
Leifels, M., Hamza, I.A., Krieger, M., Wilhelm, M., Mackowiak, M., Jurzik, L. 2016. From lab
to lake – evaluation of current molecular methods for the detection of infectious enteric
viruses in complex water matrices in an urban area. PloS One, 11: e0167105.
Li, X., Harwood, V.J., Nayak, B., Weidhaas, J.L. 2016. Ultrafiltration and microarray for
detection of microbial source tracking marker and pathogen genes in riverine and marine
systems. Applied and Environmental Microbiology, 82: 1625-1635.
Li, X., Peed, L., Sivaganesan, M., Kelty, C.A., Nietch, C., Shanks, O. C. 2018. Evidence of
genetic fecal marker interactions between water column and periphyton in artificial
streams. ACS Omega, 3(8), 10107-10113.
Li, X., Sivaganesan, M., Kelty, C.A., Zimmer-Faust, A., Clinton, P., Reichman, J.R., Johnson,
Y., Matthews, W., Bailey, S., Shanks, O.C. 2019. Large-scale implementation of
standardized quantitative real-time PCR fecal source identification procedures in the
Tillamook Bay Watershed. PloS One, 14: e0216827.
Li, X., Kelty, C.A., Sivaganesan, M., Shanks, O.C. 2021. Variable fecal source prioritization in
recreational waters routinely monitored with viral and bacterial general indicators. Water
Research, 192: 116845.
Liang, H., Yu, Z., Wang, B., Ndayisenga, F., Liu, R., Zhang, H., Wu, G. 2021. Synergistic
application of molecular markers and community-based microbial source tracking methods
B-83
for identification of fecal pollution in river water during dry and wet seasons. Frontiers in
Microbiology, 12: 660368.
Liao, H., Krometis, L.A., Kline, K. 2016. Coupling a continuous watershed-scale microbial fate
and transport model with a stochastic dose-response model to estimate risk of illness in an
urban watershed. Science of the Total Environment, 551-552: 668-675.
Lim, K.Y., Shao, S., Peng, J., Grant, S.B., Jiang, S.C. 2017. Evaluation of the dry and wet
weather recreational health risks in a semi-enclosed marine embayment in Southern
California. Water Research, 111: 318-329.
Linke, R.B., Zeki, S., Mayer, R., Keiblinger, K., Savio, D., Kirschner, A.K.T., Reischer, G.H.,
Mach, R.L., Sommer, R., Farnleitner, A.H. 2021. Identifying inorganic turbidity in water
samples as potential loss factor during nucleic acid extraction: Implications for molecular
fecal pollution diagnostics and source tracking. Frontiers in Microbiology, 12: 660566.
Liu, R.L., Yeung, L.T.C., Ho, P.H., Lau, S.C.K. 2017. Tracking the relative concentration
between Bacteroidales DNA markers and culturable Escherichia coli in fecally polluted
subtropical seawater: Potential use in differentiating fresh and aged pollution. Canadian
Journal of Microbiology, 63: 252-259.
Martzy, R., Kolm, C., Brunner, K., Mach, R.L., Krska, R., Šinkovec, H., Sommer, R.,
Farnleitner, A.H., Reischer, G.H. 2017. A loop-mediated isothermal amplification (LAMP)
assay for the rapid detection of Enterococcus spp. in water. Water Research, 122: 62-69.
Mattioli, M.C., Benedict, K.M., Murphy, J., Kahler, A., Kline, K.E., Longenberger, A., Mitchell,
P.K., Watkins, S., Berger, P., Shanks, O.C., Barrett, C.E., Barclay, L., Hall, A. J., Hill, V.,
Weltman, A. 2021. Identifying septic pollution exposure routes during a waterborne
norovirus outbreak – A new application for human-associated microbial source tracking
qPCR. Journal of Microbiological Methods, 180: 106091.
McGinnis, S., Spencer, S., Firnstahl, A., Stokdyk, J., Borchardt, M., McCarthy, D.T., Murphy,
H.M. 2018. Human Bacteroides and total coliforms as indicators of recent combined sewer
overflows and rain events in urban creeks. Science of the Total Environment, 630: 967-
976.
Ming, H. X., Ma, Y.J., Gu, Y.B., Su, J., Guo, J.L., Li, J.Y., Li, X., Jin, Y., Fan, J.F. 2020.
Enterococci may not present the pollution of most enteric pathogenic bacteria in
recreational seawaters of Xinghai bathing Beach, China. Ecological Indicators, 110:
105938.
Monteiro, S., Ebdon, J., Santos, R., Taylor, H. 2021. Elucidation of fecal inputs into the River
Tagus catchment (Portugal) using source-specific mitochondrial DNA, HAdV, and phage
markers. Science of the Total Environment, 783: 147086.
Mosnier, E., Martin, N., Razakandrainibe, R., Dalle, F., Roux, G., Buteux, A., Favennec, L.,
Brousse, P., Guarmit, B., Blanchet, D., Epelboin, L., Girouin, C., Martin, E., Djossou, F.,
Nacher, M., Demar, M. 2018. Cryptosporidiosis outbreak in immunocompetent children
from a remote area of French Guiana. American Journal of Tropical Medicine and
Hygiene, 98: 1727-1732.
Napier, M.D., Haugland, R., Poole, C., Dufour, A.P., Stewart, J.R., Weber, D.J., Varma, M.,
Lavender, J.S., Wade, T.J. 2017. Exposure to human-associated fecal indicators and self-
reported illness among swimmers at recreational beaches: A cohort study. Environmental
Health, 16(1): 103.
Napier, M.D., Poole, C., Stewart, J.R., Weber, D.J., Glassmeyer, S.T., Kolpin, D.W., Furlong,
E.T., Dufour, A.P., Wade, T.J. 2018. Exposure to human-associated chemical markers of
B-84
fecal contamination and self-reported illness among swimmers at recreational beaches.
Environmental Science & Technology, 52: 7513-7523.
Nappier, S.P., Ichida, A., Jaglo, K., Haugland, R., Jones, K.R. 2019. Advancements in mitigating
interference in quantitative polymerase chain reaction (qPCR) for microbial water quality
monitoring. Science of the Total Environment, 671: 732-740.
Nieuwkerk, D.M., Korajkic, A., Valdespino, E.L., Herrmann, M.P., Harwood, V.J. 2020. Critical
review of methods for isothermal amplification of nucleic acids for environmental analysis.
Journal of Microbiological Methods, 179: 106099.
Olds, H. T., Corsi, S.R., Dila, D.K., Halmo, K.M., Bootsma, M.J., McLellan, S.L. 2018. High
levels of sewage contamination released from urban areas after storm events: A
quantitative survey with sewage specific bacterial indicators. PLoS Medicine, 15:
e1002614.
Oliveira, S.S., Sorgine, M.H.F., Bianco, K., Pinto, L.H., Barreto, C., Albano, R.M., Cardoso,
A.M., Clementino, M.M. 2016. Detection of human fecal contamination by nifH gene
quantification of marine waters in the coastal beaches of Rio de Janeiro, Brazil.
Environmental Science and Pollution Research International, 23: 25210-25217.
Oliveira, B.B., Veigas, B., Carlos, F.F., Sánchez-Melsió, A., Balcázar, J.L., Borrego, C.M.,
Baptista, P.V. 2020. Water safety screening via multiplex LAMP-Au-nanoprobe integrated
approach. Science of the Total Environment, 741: 140447.
Osunmakinde, C.O., Selvarajan, R., Sibanda, T., Mamba, B.B., Msagati, T.A.M. 2018. Overview
of trends in the application of metagenomic techniques in the analysis of human enteric
viral diversity in Africa's environmental regimes. Viruses, 10(8), 429.
Pawar, S.D., Keng, S.S., Tare, D.S., Thormothe, A.L., Sapkal, G.N., Anukumar, B., Lole, K.S.,
Mullick, J., Mourya, D.T. 2019. A virus precipitation method for concentration detection of
avian influenza viruses from environmental water resources its possible application in
outbreak investigations. Indian Journal of Medical Research, 150: 612-619.
Pedrosa de Macena, L.D.G., Castiglia Feitosa, R., Vieira, C.B., Araújo, I.T., Taniuchi, M.,
Miagostovich, M.P. 2021. Microbiological assessment of an urban lagoon system in the
coastal zone of Rio de Janeiro, Brazil. Environmental Science and Pollution Research
International, 28: 1170-1180.
Pendergraph, D., Ranieri, J., Ermatinger, L., Baumann, A., Metcalf, A.L., DeLuca, T.H., Church,
M.J. 2021. Differentiating sources of fecal contamination to wilderness waters using
droplet digital PCR and fecal indicator bacteria methods. Wilderness Environmental
Medicine, 32: 332-339.
Petcharat, T., Kongprajug, A., Chyerochana, N., Sangkaew, W., Mongkolsuk, S., Sirikanchana,
K. 2020. Assessing human-specific CrAssphage recovery after acidification-filtration
concentrating method in environmental water. Water Environment Research, 92: 35-41.
Phelan, S., Soni, D., Medina, W.R.M., Fahrenfeld, N.L. 2019. Comparison of qPCR and
amplicon sequencing based methods for fecal source tracking in a mixed land use estuarine
watershed. Environmental Science: Water Research Technology, 5: 2108-2123.
Polkowska, A., Räsänen, S., Al-Hello, H., Bojang, M. Lyytikäinen, O., Nuorti, J.P., Jalava, K.
2018. An outbreak of norovirus infections associated with recreational lake water in
Western Finland, 2014. Epidemiology and Infection, 146: 544-550.
Poma, H.R., Kundu, A., Wuertz, S., Rajal, V.B. 2019. Data fitting approach more critical than
exposure scenarios and treatment of censored data for quantitative microbial risk
assessment. Water Research, 154: 45-53.
B-85
Powers, N.C., Wallgren, H.R., Marbach, S., Turner, J.W. 2020. Relationship between rainfall,
fecal pollution, antimicrobial resistance, and microbial diversity in an urbanized subtropical
bay. Applied and Environmental Microbiology, 86(19): e01229-01220.
Prado, T., Bruni, A.C., Barbosa, M.R.F., Bonanno, V.M.S., Garcia, S.C., Sato, M.I.Z. 2018.
Distribution of human fecal marker GB-124 bacteriophages in urban sewage and reclaimed
water of São Paulo city, Brazil. Journal of Water and Health, 16: 289-299.
Purnell, S., Halliday, A., Newman, F., Sinclair, C., Ebdon, J. 2020. Pathogen infection risk to
recreational water users, associated with surface waters impacted by de facto and indirect
potable reuse activities. Science of the Total Environment, 722: 137799.
Rachmadi, A.T., Torrey, J.R., Kitajima, M. 2016. Human polyomavirus: Advantages and
limitations as a human-specific viral marker in aquatic environments. Water Research, 105:
456-469.
Raj, S.J., Wang, Y., Yakubu, H., Robb, K., Siesel, C., Green, J., Kirby, A., Mairinger, W.,
Michiel, J., Null, C., Perez, E., Roguski, K., Moe, C.L. 2020. The SaniPath Exposure
Assessment Tool: A quantitative approach for assessing exposure to fecal contamination
through multiple pathways in low resource urban settlements. PloS One, 15: e0234364.
Reses, H.E., Gargano, J.W., Liang, J.L., Cronquist, A., Smith, K., Collier, S.A., Roy, S.L.,
Vanden Eng, J., Bogard, A., Lee, B., Hlavsa, M.C., Rosenberg, E.S., Fullerton, K.E.,
Beach, M.J., Yoder, J.S. 2018. Risk factors for sporadic Giardia infection in the USA: A
case-control study in Colorado and Minnesota. Epidemiology and Infection, 146: 1071-
1078.
Reyneke, B., Ndlovu, T., Khan, S., Khan, W. 2017. Comparison of EMA-, PMA- and DNase
qPCR for the determination of microbial cell viability. Applied Microbiology and
Biotechnology, 101: 7371-7383.
Rocha, J., Fernandes, T., Riquelme, M. V., Zhu, N., Pruden, A., Manaia, C.M. 2019. Comparison
of culture- and quantitative PCR-based indicators of antibiotic resistance in wastewater,
recycled water, and tap water. International Journal of Environmental Research and Public
Health, 16(21).
Rosiles-González, G., Ávila-Torres, G., Moreno-Valenzuela, O.A., Cháidez-Quiroz, C.,
Hernández-Flores, C.I., Acosta-González, G., Brown, J.K., Betancourt, W.Q., Gerba, C.P.,
Hernández-Zepeda, C. 2019. Norovirus and human adenovirus occurrence and diversity in
recreational water in a karst aquifer in the Yucatan Peninsula, Mexico. Journal of Applied
Microbiology, 127: 1255-1269.
Rothenheber, D., Jones, S. 2018. Enterococcal concentrations in a coastal ecosystem are a
function of fecal source input, environmental conditions, and environmental sources.
Applied and Environmental Microbiology, 84(17): e01038-01018.
Rudko, S.P., Reimink, R.L., Peter, B., White, J., Hanington, P.C. 2020. Democratizing water
monitoring: Implementation of a community-based qPCR monitoring program for
recreational water hazards. PloS One, 15: e0229701.
Saeidi, N., Gu, X.Q., Tran, N.H., Goh, S.G., Kitajima, M., Kushmaro, A., Schmitz, B.W., Gin,
K.Y.H. 2018. Occurrence of traditional and alternative fecal indicators in tropical urban
environments under different land use patterns. Applied and Environmental Microbiology,
84(14): e00287-00218.
Sagarduy, M., Courtois, S, Del. Campo, A., Garmendia, J.M., Petrau, A. 2019. Differential decay
and prediction of persistence of Enterococcus spp. and Escherichia coli culturable cells and
B-86
molecular markers in freshwater and seawater environments. International Journal of
Hygiene and Environmental Health, 222: 695-704.
Saingam, P., Li, B., Yan, T. 2018. Use of amplicon sequencing to improve sensitivity in PCR-
based detection of microbial pathogen in environmental samples. Journal of
Microbiological Methods, 149: 73-79.
Schang, C., Henry, R., Kolotelo, P. A., Prosser, T., Crosbie, N., Grant, T., Cottam, D., O'Brien,
P., Coutts, S., Deletic, A., McCarthy, D. T. 2016. Evaluation of techniques for measuring
microbial hazards in bathing waters: A comparative study. PloS One, 11: e0155848.
Schets, F.M., De Roda Husman, A.M., Havelaar, A. H. 2011. Disease outbreaks associated with
untreated recreational water use. Epidemiology and Infection, 139(7): 1114-1125.
Schets, F.M., van den Berg, H., Vennema, H., Pelgrim, M.T.M., Collé, C., Rutjes, S.A., Lodder,
W.J. 2018. Norovirus outbreak associated with swimming in a recreational lake not
influenced by external human fecal sources in The Netherlands, August 2012. International
Journal of Environmental Research and Public Health, 15(11): 2550.
Schoen, M.E., Boehm, A.B., Soller, J., Shanks, O.C. 2020. Contamination scenario matters when
using viral and bacterial human-associated genetic markers as indicators of a health risk in
untreated sewage-impacted recreational waters. Environmental Science & Technology, 54:
13101-13109.
Seidel, L., Strathmann, M., Nocker, A. 2017. The feasibility of improved live-dead distinction in
qPCR-based microbial source tracking. Journal of Microbiological Methods, 140: 23-31.
Seruge, J., Wong, M., Noble, R.T., Blackwood, A.D., Moravcik, P.S., Kirs, M. 2019.
Application of a rapid qPCR method for enterococci for beach water quality monitoring
purposes in Hawaii: Loss of DNA during the extraction protocol due to coral sands. Marine
Pollution Bulletin, 149: 110631.
Shanks, O.C., Kelty, C.A., Oshiro, R., Haugland, R.A., Madi, T., Brooks, L., Field, K.G.,
Sivaganesan, M. 2016. Data acceptance criteria for standardized human-associated fecal
source identification quantitative real-time PCR methods. Applied and Environmental
Microbiology, 82: 2773-2782.
Sheth, N., McDermott, C., Busse, K., Kleinheinz, G. 2016. Evaluation of Enterococcus
concentrations at beaches in Door County, WI (Lake Michigan, USA) by qPCR and
defined substrate culture analysis. Journal of Great Lakes Research, 42: 768-774.
Shoults, D.C., Li, Q.Z., Petterson, S., Rudko, S.P., Dlusskaya, L., Leifels, M., Scott, C.,
Schlosser, C., Ashbolt, N.J. 2021. Pathogen performance testing of a natural swimming
pool using a cocktail of microbiological surrogates and QMRA-derived management goals.
Journal of Water and Health, 19: 629-641.
Shrestha, A., Dorevitch, S. 2019. Evaluation of rapid qPCR method for quantification of E. coli
at non-point source impacted Lake Michigan beaches. Water Research, 156: 395-403.
Shrestha, A., Dorevitch, S. 2020. Slow adoption of rapid testing: Beach monitoring and
notification using qPCR. Journal of Microbiological Methods, 174: 105947.
Sips, G.J., Dirven, M.J.G., Donkervoort, J.T., van Kolfschoten, F.M., Schapendonk, C.M.E.,
Phan, M.V.T., Bloem, A., van Leeuwen, A.F., Trompenaars, M.E., Koopmans, M.P.G., van
der Eijk, A.A., de Graaf, M., Fanoy, E.B. 2020. Norovirus outbreak in a natural
playground: A One Health approach. Zoonoses and Public Health, 67: 453-459.
Sivaganesan, M., Aw, T.G., Briggs, S., Dreelin, E., Aslan, A., Dorevitch, S., Shrestha, A., Isaacs,
N., Kinzelman, J., Kleinheinz, G., Noble, R., Rediske, R., Scull, B., Rosenberg, S.,
Weberman, B., Sivy, T., Southwell, B., Siefring, S., Oshima, K., Haugland, R. 2019.
B-87
Standardized data quality acceptance criteria for a rapid Escherichia coli qPCR method
(Draft Method C) for water quality monitoring at recreational beaches. Water Research,
156: 456-464.
Soller, J.A., Eftim, S., Wade, T.J., Ichida, A.M., Clancy, J.L., Johnson, T.B., Schwab, K.,
Ramirez-Toro, G., Nappier, S., Ravenscroft, J.E. 2016. Use of quantitative microbial risk
assessment to improve interpretation of a recreational water epidemiological study.
Microbial Risk Analysis, 1: 44238.
Soller, J.A., Schoen, M., Steele, J.A., Griffith, J.F., Schiff, K.C. 2017. Incidence of
gastrointestinal illness following wet weather recreational exposures: Harmonization of
quantitative microbial risk assessment with an epidemiologic investigation of surfers.
Water Research, 121: 280-289.
Somnark, P., Chyerochana, N., Mongkolsuk, S., Sirikanchana, K. 2018. Performance evaluation
of Bacteroidales genetic markers for human and animal microbial source tracking in
tropical agricultural watersheds. Environmental Pollution, 236: 100-110.
Staley, Z.R., Edge, T.A. 2016. Comparative microbial source tracking methods for identification
of fecal contamination sources at Sunnyside Beach in the Toronto region area of concern.
Journal of Water and Health, 14: 839-850.
Staley, Z.R., Grabuski, J., Sverko, E., Edge, T.A. 2016. Comparison of microbial and chemical
source tracking markers to identify fecal contamination sources in the Humber River
(Toronto, Ontario, Canada) and associated storm water outfalls. Applied and
Environmental Microbiology, 82: 6357-6366.
Staley, Z.R., Chuong, J.D., Hill, S.J., Grabuski, J., Shokralla, S., Hajibabaei, M., Edge, T.A.
2018a. Fecal source tracking and eDNA profiling in an urban creek following an extreme
rain event. Scientific Reports, 8(1): 14390.
Staley, Z.R., Boyd, R.J., Shum, P., Edge, T.A. 2018b. Microbial source tracking using
quantitative and digital PCR to identify sources of fecal contamination in stormwater, river
water, and beach water in a Great Lakes area of concern. Applied and Environmental
Microbiology, 84(20): e01634-01618.
Stokdyk, J.P., Firnstahl, A.D., Spencer, S.K., Burch, T.R., Borchardt, M.A. 2016. Determining
the 95% limit of detection for waterborne pathogen analyses from primary concentration to
qPCR. Water Research, 96: 105-113.
Sunger, N., Hamilton, K. A., Morgan, P. M., Haas, C. N. 2019. Comparison of pathogen-derived
'total risk' with indicator-based correlations for recreational (swimming) exposure.
Environmental Science and Pollution Research International, 26: 30614-30624.
Symonds, E.M., Sinigalliano, C., Gidley, M., Ahmed, W., McQuaig-Ulrich, S.M., Breitbart, M.
2016. Faecal pollution along the southeastern coast of Florida and insight into the use of
pepper mild mottle virus as an indicator. Journal of Applied Microbiology, 121: 1469-
1481.
Symonds, E.M., Young, S., Verbyla, M.E., McQuaig-Ulrich, S.M., Ross, E., Jimenez, J.A.,
Harwood, V.J., Breitbart, M. 2017. Microbial source tracking in shellfish harvesting waters
in the Gulf of Nicoya, Costa Rica. Water Research, 111: 177-184.
Symonds, E.M., Nguyen, K.H., Harwood, V.J., Breitbart, M. 2018. Pepper mild mottle virus: A
plant pathogen with a greater purpose in (waste)water treatment development and public
health management. Water Research, 144: 1-12.
B-88
Thulsiraj, V., Zimmer-Faust, A.G., Jay, J.A. 2017. Use of viability-based methods for improved
detection of recent fecal contamination in a microbial source tracking study near Tijuana,
Mexico. Water, Air, and Soil Pollution, 228: 63.
Toubiana, M., Salles, C., Tournoud, M.G., Licznar-Fajardo, P., Zorgniotti, I., Tremelo, M.L.,
Jumas-Bilak, E., Robert, S., Monfort, P. 2021. Monitoring urban beach quality on a
summer day: Determination of the origin of fecal indicator bacteria and antimicrobial
resistance at Prophete Beach, Marseille (France). Frontiers in Microbiology, 12: 710346.
Unno, T., Staley, C., Brown, C.M., Han, D., Sadowsky, M.J., Hur, H.G. 2018. Fecal pollution:
New trends and challenges in microbial source tracking using next-generation sequencing.
Environmental Microbiology, 20: 3132-3140.
Urban, L., Holzer, A., Baronas, J.J., Hall, M.B., Braeuninger-Weimer, P., Scherm, M.J., Kunz,
D.J., Perera, S.N., Martin-Herranz, D.E., Tipper, E.T., Salter, S.J., Stammnitz, M.R. 2021.
Freshwater monitoring by nanopore sequencing. eLife, 10.
Vadde, K.K., Feng, Q.L., Wang, J.J., McCarthy, A.J., Sekar, R. 2019a. Next-generation
sequencing reveals fecal contamination and potentially pathogenic bacteria in a major
inflow river of Taihu Lake. Environmental Pollution, 254(Pt B): 113108.
Vadde, K.K., McCarthy, A.J., Rong, R., Sekar, R. 2019b. Quantification of microbial source
tracking and pathogenic bacterial markers in water and sediments of Tiaoxi River (Taihu
Watershed). Frontiers in Microbiology, 10: 699.
Van Abel, N., Mans, J., Taylor, M.B. 2017. Quantitative microbial risk assessment to estimate
the health risk from exposure to noroviruses in polluted surface water in South Africa.
Journal of Water and Health, 15(6): 908-922.
Vergara, G., Rose, J.B., Gin, K.Y.H. 2016. Risk assessment of noroviruses and human
adenoviruses in recreational surface waters. Water Research, 103: 276-282.
Verhougstraete, M.P., Pogreba-Brown, K., Reynolds, K.A., Lamparelli, C.C., Zanoli Sato, M. I.,
Wade, T.J., Eisenberg, J.N.S. 2020. A critical analysis of recreational water guidelines
developed from temperate climate data and applied to the tropics. Water Research, 170:
115294.
Vincent-Hubert, F., Wacrenier, C., Morga, B., Lozach, S., Quenot, E., Mège, M., Lecadet, C.,
Gourmelon, M., Hervio-Heath, D., Le Guyader, F.S. 2021. Passive samplers, a powerful
tool to detect viruses and bacteria in marine coastal areas. Frontiers in Microbiology, 12:
631174.
Vital, P.G., Ha, N.T.V., Tuyet, L.T.H., Widmer, K.W. 2017. Application of quantitative real-
time PCR compared to filtration methods for the enumeration of Escherichia coli in surface
waters within Vietnam. Journal of Water and Health, 15: 155-162.
Wade, T.J., Augustine, S.A.J., Griffin, S.M., Sams, E.A., Oshima, K.H., Egorov, A.I., Simmons,
K.J., Eason, T.N., Dufour, A.P. 2018. Asymptomatic norovirus infection associated with
swimming at a tropical beach: A prospective cohort study. PloS One, 13: e0195056.
Walker, D.I., McQuillan, J., Taiwo, M., Parks, R., Stenton, C.A., Morgan, H., Mowlem, M.C.,
Lees, D.N. 2017. A highly specific Escherichia coli qPCR and its comparison with existing
methods for environmental waters. Water Research, 126: 101-110.
Wang, D., Yamahara, K.M., Cao, Y., Boehm, A.B. 2016. Absolute quantification of
Enterococcal 23S rRNA gene using digital PCR. Environmental Science & Technology,
50: 3399-3408.
B-89
World Health Organization (WHO). 2018. WHO recommendations on scientific, analytical and
epidemiological developments relevant to the parameters for bathing water quality in the
Bathing Water Directive (2006/7/EC) – Recommendations.
Wu, B., Wang, X.C., Dzakpasu, M. 2017. Genetic characterization of fecal impacts of seagull
migration on an urban scenery lake. Water Research, 117: 27-36.
Wu, B.L., Wang, C.W., Zhang, C.M., Sadowsky, M.J., Dzakpasu, M., Wang, X.C.C. 2020.
Source-associated gastroenteritis risk from swimming exposure to aging fecal pathogens.
Environmental Science & Technology, 54(2): 921-929.
Wymer, L.J., Wade, T.J., Sams, E., Oshima, K., Dufour, A.P. 2021. Comparative stability of
assay results of enterococci measured by culture and qPCR over time in bathing beach
waters. Journal of Microbiological Methods, 188: 106274.
Xie, Y., Qiu, N., Wang, G. 2017. Toward a better guard of coastal water safety-microbial
distribution in coastal water and their facile detection. Marine Pollution Bulletin, 118(1-2):
5-16.
Xue, J., Lin, S., Lamar, F.G., Lamori, J.G., Sherchan, S. 2018. Assessment of fecal pollution in
Lake Pontchartrain, Louisiana. Marine Pollution Bulletin, 129: 655-663.
Xue, J., Feng, Y. 2019. Comparison of microbial source tracking efficacy for detection of cattle
fecal contamination by quantitative PCR. Science of the Total Environment, 686: 1104-
1112.
Young, N. 2016. The association between marine bathing and infectious diseases – a review.
Journal of the Marine Biological Association of the United Kingdom, 96: 93-100.
Yuan, Y., Zheng, G., Lin, M., Mustapha, A. 2018. Detection of viable Escherichia coli in
environmental water using combined propidium monoazide staining and quantitative PCR.
Water Research, 145: 398-407.
Zeki, S., Aslan, A., Burak, S., Rose, J.B. 2021. Occurrence of a human-associated microbial
source tracking marker and its relationship with faecal indicator bacteria in an urban
estuary. Letters in Applied Microbiology, 72: 167-177.
Zhang, Q., Ishii, S. 2018. Improved simultaneous quantification of multiple waterborne
pathogens and fecal indicator bacteria with the use of a sample process control. Water
Research, 137: 193-200.
Zhang, Y., Wu, R., Lin, K., Wang, Y., Lu, J. 2020. Performance of host-associated genetic
markers for microbial source tracking in China. Water Research, 175: 115670.
Zimmer-Faust, A.G., Thulsiraj, V., Lee, C.M., Whitener, V., Rugh, M., Mendoza-Espinosa, L.,
Jay, J.A. 2018. Multi-tiered approach utilizing microbial source tracking and human
associated-IMS/ATP for surveillance of human fecal contamination in Baja California,
Mexico. Science of the Total Environment, 640: 475-484.
Zimmer-Faust, A.G., Steele, J.A., Xiong, X., Staley, C., Griffith, M., Sadowsky, M.J., Diaz, M.,
Griffith, J.F. 2021. A combined digital PCR and next generation DNA-sequencing based
approach for tracking nearshore pollutant dynamics along the Southwest United
States/Mexico Border. Frontiers in Microbiology, 12: 674214.
