
224 Volume 40
•
Number 3
•
September 2005
Journal of Athletic Training 2005;40(3):224–245
q by the National Athletic Trainers’ Association, Inc
www.journalofathletictraining.org
National Athletic Trainers’ Association
Position Statement: Management of Asthma
in Athletes
Michael G. Miller*; John M. Weiler†; Robert Baker‡; James Collins§;
Gilbert D’Alonzo\
*Western Michigan University, Kalamazoo, MI; †University of Iowa and CompleWare, Iowa City, IA; ‡Michigan
State University Kalamazoo Center for Medical Studies, Kalamazoo, MI; §San Diego Chargers, San Diego, CA;
\Temple University School of Medicine, Philadelphia, PA
Michael G. Miller, EdD, ATC, CSCS; John M. Weiler, MD; Robert Baker, MD, PhD, ATC; James Collins, ATC; and Gilbert
D’Alonzo, DO, contributed to conception and design; acquisition and analysis and interpretation of the data; and drafting,
critical revision, and final approval of the article.
Address correspondence to National Athletic Trainers’ Association, Communications Department, 2952 Stemmons Freeway,
Dallas, TX 75247.
Objective:
To present guidelines for the recognition, prophy-
laxis, and management of asthma that lead to improvement in
the quality of care certified athletic trainers and other heath care
providers can offer to athletes with asthma, especially exercise-
induced asthma.
Background:
Many athletes have difficulty breathing during
or after athletic events and practices. Although a wide variety
of conditions can predispose an athlete to breathing difficulties,
the most common cause is undiagnosed or uncontrolled asth-
ma. At least 15% to 25% of athletes may have signs and symp-
toms suggestive of asthma, including exercise-induced asthma.
Athletic trainers are in a unique position to recognize breathing
difficulties caused by undiagnosed or uncontrolled asthma, par-
ticularly when asthma follows exercise. Once the diagnosis of
asthma is made, the athletic trainer should play a pivotal role
in supervising therapies to prevent and control asthma symp-
toms. It is also important for the athletic trainer to recognize
when asthma is not the underlying cause for respiratory diffi-
culties, so that the athlete can be evaluated and treated prop-
erly.
Recommendations:
The recommendations contained in this
position statement describe a structured approach for the di-
agnosis and management of asthma in an exercising popula-
tion. Athletic trainers should be educated to recognize asthma
symptoms in order to identify patients who might benefit from
better management and should understand the management of
asthma, especially exercise-induced asthma, to participate as
active members of the asthma care team.
Key Words:
airway hyperresponsiveness, airway obstruc-
tion, exercise-induced asthma, exercise-induced broncho-
spasm, pulmonary function tests, certified athletic trainer
INTRODUCTION
A
sthma is defined as a chronic inflammatory disorder
of the airways characterized by variable airway ob-
struction and bronchial hyperresponsiveness.
1
Airway
obstruction can lead to recurrent episodes of wheezing, breath-
lessness, chest tightness, and coughing, particularly at night or
in the early morning.
1
Asthma can be triggered by many stim-
uli, including allergens (eg, pollen, dust mites, animal dander),
pollutants (eg, carbon dioxide, smoke, ozone), respiratory in-
fections, aspirin, nonsteroidal anti-inflammatory drugs
(NSAIDs), inhaled irritants (eg, cigarette smoke, household
cleaning fumes, chlorine in a swimming pool), particulate ex-
posure (eg, ambient air pollutants, ice rink pollution), and ex-
posure to cold and exercise.
1–5
Airflow limitation is often re-
versible, but as asthma symptoms continue, patients may
develop ‘‘airway remodeling’’ that leads to chronic irreversible
airway obstruction.
6,7
Severe attacks of asthma can also cause
irreversible airflow obstruction that can lead to death.
4,8
The National Heart, Lung, and Blood Institute of the Na-
tional Institutes of Health launched the National Asthma Ed-
ucation and Prevention Program (NAEPP) in March 1989 to
address the increasing prevalence of asthma in the United
States and its economic costs to the society; the program was
updated in 1997 (as NAEPPII).
1
An updated expert panel re-
port from the NAEPP is expected to be released in 2006. The
Global Initiative for Asthma (GINA) was also developed to
provide worldwide guidelines for asthma awareness and man-
agement.
2
These guidelines are extremely comprehensive and
have been regularly updated to reflect advances in the diag-
nosis and management of asthma. Nevertheless, the guidelines
do not describe the role of certified athletic trainers or other
allied health care professionals in recognizing and managing
asthma in an athletic population.
PURPOSE
The purpose of this position statement is to provide athletic
trainers and other allied health care professionals who care for
athletes with information to:
1. Identify the characteristics and diagnostic criteria of asth-

Journal of Athletic Training 225
ma, especially exercise-induced asthma (EIA) or exercise-
induced bronchospasm (EIB).
2. Provide guidelines for referral so that patients with asthma
and those in whom asthma is suspected can receive a thor-
ough evaluation.
3. Describe management plans to prevent attacks and to con-
trol asthma exacerbations when they occur.
4. Educate certified athletic trainers and athletes about phar-
macologic and nonpharmacologic therapies and techniques
to help control asthma.
RECOMMENDATIONS
Based on current research and literature, the National Ath-
letic Trainers’ Association provides the following guidelines
for the identification, examination, management, and prophy-
laxis of asthma, including EIA, and the education of athletes,
parents, coaches, and health care personnel about asthma. Not
all individuals who suffer from asthma present in the same
manner, nor do they all respond to the same management or
treatment plan. Therefore, these recommendations are intended
to provide the certified athletic trainer and other health care
professionals with an overall guide for a better understanding
of the asthmatic condition.
Asthma Identification and Diagnosis
1. All athletes must receive preparticipation screening eval-
uations sufficient to identify the possible presence of asth-
ma.
9–12
In most situations, this evaluation includes only
obtaining a thorough history from the athlete. However,
in special circumstances, additional screening evaluations
(eg, spirometry testing or the challenge testing described
below) should also be performed because the history
alone is not reliable.
10
2. Athletic trainers should be aware of the major signs and
symptoms suggesting asthma, as well as the following as-
sociated conditions
5,13,14
:
a. Chest tightness (or chest pain in children)
b. Coughing (especially at night)
c. Prolonged shortness of breath (dyspnea)
d. Difficulty sleeping
e. Wheezing (especially after exercise)
f. Inability to catch one’s breath
g. Physical activities affected by breathing difficulty
h. Use of accessory muscles to breathe
i. Breathing difficulty upon awakening in the morning
j. Breathing difficulty when exposed to certain allergens
or irritants
k. Exercise-induced symptoms, such as coughing or
wheezing
l. An athlete who is well conditioned but does not seem
to be able to perform at a level comparable with other
athletes who do not have asthma
m. Family history of asthma
n. Personal history of atopy, including atopic dermatitis/
eczema or hay fever (allergic rhinitis)
Note: Although there is a correlation between the presence
of symptoms and EIA, the diagnosis should not be based
on history alone.
5
Rather, these symptoms should serve to
suggest that an athlete may have asthma.
3. The following types of screening questions can be asked
to seek evidence of asthma
13
:
a. Does the patient have breathing attacks consisting of
coughing, wheezing, chest tightness, or shortness of
breath (dyspnea)?
b. Does the patient have coughing, wheezing, chest tight-
ness, or shortness of breath (dyspnea) at night?
c. Does the patient have coughing, wheezing, or chest
tightness after exercise?
d. Does the patient have coughing, wheezing, chest tight-
ness, or shortness of breath (dyspnea) after exposure to
allergens or pollutants?
e. Which pharmacologic treatments for asthma or allergic
rhinitis, if any, were given in the past, and were they
successful?
4. Patients with atypical symptoms, symptoms despite proper
therapy, or other complications that can exacerbate asthma
(such as sinusitis, nasal polyps, severe rhinitis, gastro-
esophageal reflux disease, or vocal cord dysfunction)
should be referred to a physician with expertise in sports
medicine (eg, allergist; ear, nose, and throat physician;
cardiologist; or pulmonologist with training in providing
care for athletes).
15
Testing might include a stress electro-
cardiogram, upper airway laryngoscopy or rhinoscopy,
echocardiogram, or upper endoscopy.
Pulmonary Function Testing
5. Athletes with a history of asthma or of taking a medica-
tion used to treat asthma and those suspected of having
asthma should consult a physician for proper medical
evaluation and to obtain a classification of asthma severity
(Table 1). This evaluation should include pulmonary func-
tion testing.
16–18
6. An exercise challenge test is recommended for athletes
who have symptoms suggestive of EIA to confirm the
diagnosis.
19
7. If the diagnosis of asthma remains unclear after the above
tests have been performed, then additional testing should
be performed to assist in making a diagnosis.
15,20,21
Phy-
sicians should be encouraged, when possible, to test the
athlete using a sport-specific and environment-specific ex-
ercise-challenge protocol, in which the athlete participates
in his or her venue to replicate the activity or activities
and the environment that may serve to trigger airway hy-
perresponsiveness.
20,21
In some cases, athletes should also
be tested for metabolic gas exchange during strenuous ex-
ercise to determine fitness (eg, to assess anaerobic thresh-
old and V
˙
O
2
max), especially to rule out the diagnosis of
asthma or to rule in another diagnosis (eg, pulmonary fi-
brosis) for a patient with an unclear diagnosis.
16
Asthma Management
8. Athletic trainers should incorporate into the existing emer-
gency action plan an asthma action plan for managing and
urgently referring all patients who may experience signif-
icant or life-threatening attacks of breathing difficulties
(Figure 1).
1,2
Immediate access to emergency facilities
during practices and game situations should be available.
For example, athletic trainers should be familiar with ap-
propriate community resources and must have a fully
functional telephone (mobile or cellular) available, pre-
programmed with emergency medical care access num-
bers. A telephone might be the single most important de-

226 Volume 40
•
Number 3
•
September 2005
Table 1. National Asthma Education and Prevention Program II: Classification of Asthma Severity*
1
Clinical Features Before Treatment†
Symptoms‡
Nighttime
Symptoms Lung Function
Step 4
Severe persistent
● Continual symptoms
● Limited physical activity
● Frequent exacerbations
Frequent ● FEV
1
or PEF #60% predicted
● PEF variability .30%
Step 3
Moderate persistent
● Daily symptoms
● Daily use of inhaled short-acting beta
2
-
agonist
● Exacerbations affect activity
● Exacerbations $2 times/wk; may last
days
.1 time/wk ● FEV
1
or PEF .60%–,80% predicted
● PEF variability $30%
Step 2
Mild persistent
● Symptoms .2 times/wk but ,1 time/d
● Exacerbations may affect activity
.2 times/mo ● FEV
1
or PEF $80% predicted
● PEF variability 20–30%
Step 1
Mild intermittent
● Symptoms #2 times/wk
● Asymptomatic and normal PEF be-
tween exacerbations
● Exacerbations brief (from a few hours to
a few days); intensity may vary
#2 times/mo ● FEV
1
or PEF $80% predicted
● PEF variability ,20%
*FEV
1
indicates forced expiratory volume in 1 s; PEF, peak expiratory flow.
†The presence of one of the features of severity is sufficient to place a patient in that category. An individual should be assigned to the most
severe grade in which any feature occurs. The characteristics noted in this figure are general and may overlap because asthma is highly variable.
Furthermore, an individual’s classification may change over time.
‡Patients at any level of severity can have mild, moderate, or severe exacerbations. Some patients with intermittent asthma experience severe
and life-threatening exacerbations separated by long periods of normal lung function and no symptoms.
vice to have on the practice field for a patient who is
experiencing an asthma exacerbation. In addition, athletic
trainers should have pulmonary function measuring de-
vices (such as peak expiratory flow meters [PFMs] or por-
table spirometers) at all athletic venues for athletes for
whom such devices have been prescribed and should be
familiar with how to use these devices.
22
9. Patients who are experiencing any degree of respiratory
distress (including a significant increase in wheezing or
chest tightness, a respiratory rate greater than 25 breaths
per minute, inability to speak in full sentences, uncon-
trolled cough, significantly prolonged expiration phase of
breathing, nasal flaring, or paradoxic abdominal move-
ment) should be referred rapidly to an emergency depart-
ment or to their personal physicians for further evaluation
and treatment. Referral to an emergency room or equiv-
alent facility should be sought urgently if the patient is
exhibiting signs of impending respiratory failure (eg,
weak respiratory efforts, weak breath sounds, uncon-
sciousness, or hypoxic seizures).
10. All patients with asthma should have a rescue inhaler
available during games and practices, and the certified ath-
letic trainer should have an extra rescue inhaler for each
athlete for administration during emergencies. In case of
emergencies, a nebulizer should also be available. With a
metered dose inhaler (MDI), athletes should be encour-
aged to use a spacer to help ensure the best delivery of
inhaled therapy to the lungs.
23
11. Athletic trainers and coaches should consider providing
alternative practice sites for athletes with asthma triggered
by airborne allergens when practical. Indoor practice fa-
cilities that offer good ventilation and air conditioning
should be considered for at least part of the practice if this
can be accomplished, although in most cases it will not
be practical. For example, indoor and outdoor allergens or
irritants, tobacco smoke, and air pollutants might trigger
asthma, and attempts should be made to limit exposure to
these triggers when possible. Another option is to sched-
ule practices when pollen counts are lowest (eg, in the
evening during the ragweed pollen season). Pollen count
information can be accessed from the National Allergy
Bureau at http://www.aaaai.org/nab.
12. Patients with asthma should have follow-up examinations
at regular intervals, as determined by the patient’s primary
care physician or specialist, to monitor and alter therapy.
In general, these evaluations should be scheduled at least
every 6 to 12 months, but they may be more frequent if
symptoms are not well controlled.
Asthma Pharmacologic Treatment
13. Athletic trainers should understand the various types of
pharmacologic strategies used for short- and long-term
asthma control and should be able to differentiate controller
from rescue or reliever medications (Figure 2).
24–30
14. Patients with EIA may benefit from the use of short- and
long-acting b
2
-agonists. Rapid-acting agents can be used
for prophylaxis during practice and game participation.
When the goal is to prevent EIA, a short-acting b
2
-ago-
nist, such as albuterol, should be inhaled 10 to 15 minutes
before exercise. The excessive need (3–4 times per day)
for short-acting b
2
-agonist therapy during practice or an
athletic event should cause concern, and a physician
should evaluate the patient before return to participation.
Long-acting b
2
-agonists should, in general, only be used
for asthma prophylaxis and control and are usually com-
bined with an inhaled corticosteroid. Athletic trainers
should understand the use, misuse, and abuse of short-
acting b
2
-agonists.
15. Patients with asthma may also benefit from the use of

Journal of Athletic Training 227
Figure 1. Sample asthma action plan. Extracted from
Managing Asthma: A Guide for Schools.
National Heart, Lung, and Blood Institute.
Available at: http://www.nhlbi.nih.gov/health/prof/lung/asthma/asthpsch.htm. Accessed June 7, 2005.
leukotriene modifiers, inhaled or parenteral corticoste-
roids, and cromones (such as cromolyn sodium).
16. Pharmacotherapy should be customized for each asthma
patient, and a specialist (an allergist or pulmonologist with
expertise in sports medicine) should be consulted to max-
imize therapy when symptoms break through despite ap-
parently optimal therapy.
17. Patients with past allergic reactions or intolerance to as-
pirin or NSAIDs should be identified and provided with
alternative medicines, such as acetaminophen, as needed.
Asthma Nonpharmacologic Treatment
18. Health care providers should identify and consider non-
pharmacologic strategies to control asthma, including nose
breathing, limiting exposure to allergens or pollutants, and
air filtration systems.
31–33
However, these therapies should
be expected to provide only limited protection from asth-
ma in most circumstances.
19. Proper warm-up before exercise may lead to a refractory
period of as long as 2 hours, which may result in de-

228 Volume 40
•
Number 3
•
September 2005
Figure 1. Continued.
creased reliance on medications by some patients with
asthma.
34
20. Patients who have been diagnosed previously as having
asthma or suspected of having asthma should follow the
recommendations of NAEPPII and GINA for evaluation
and everyday management and control.
1,2
Asthma Education
21. Athletes should be properly educated about asthma, es-
pecially EIA, by health care professionals who are knowl-
edgeable about asthma.
35–52
Athletes should be educated
about the following:
a. Recognizing the signs and symptoms of uncontrolled
asthma.
b. Using spirometry recording devices to monitor lung
function away from the clinic or athletic training room.
c. Methods of limiting exposure to primary and second-
ary smoke and to other recognized or suspicious asth-
ma triggers (eg, pollens, animal allergens, fungi, house
dust, and other asthma sensitizers and triggers). Pa-
tients with asthma who smoke should be provided with

Journal of Athletic Training 229
Figure 1. Continued.
information about smoking cessation and encouraged
to participate in classes to change socialization patterns.
d. The need for increased asthma rescue medication (eg,
short-acting b
2
-agonists) as a signal for asthma flare-
up. Increased use of short-acting b
2
-agonists signals a
need for enhanced treatment with asthma controller
therapy.
e. The proper techniques for using MDIs, dry powder in-
halers, nebulizers, and spacers to control asthma symp-
toms and to treat exacerbations. Health care profes-
sionals should periodically check the patient’s
medication administration techniques and should ex-
amine medication compliance.
f. Asthma and EIA among competitive athletes. These
conditions are common, and athletic performance need
not be hindered if the patient takes an active role in
controlling the disease and follows good practice and
control measures.
22. The athletic trainer should also be familiar with vocal cord
dysfunction and other upper airway diseases, which can
sometimes be confused with asthma.
15,53,54
Vocal cord
dysfunction may be associated with dyspnea, chest tight-

230 Volume 40
•
Number 3
•
September 2005
Figure 2. Asthma pharmacologic management.
2
PEF indicates peak expiratory flow.
ness, coughing, wheezing, and inspiratory stridor. In many
cases, the condition is triggered with exercise. Visual in-
spection of the vocal cords by a physician experienced in
examining the upper airway during exercise to differen-
tiate vocal cord dysfunction from asthma is recommended.
23. Patients with asthma should be encouraged to engage in
exercise as a means to strengthen muscles, improve re-
spiratory health, enhance endurance, and otherwise im-
prove overall well-being.
55
24. The athletic trainer should differentiate among restricted,
banned, and permitted asthma medications. Athletic train-
ers should be familiar with the guidelines of the Interna-
tional Olympic Committee Medical Commission, the
United States Anti-Doping Agency, the World Anti-Dop-
ing Agency, and the doping committees of the various
federations.
25. The athletic trainer should be aware of the various Web
sites that provide general information and frequently asked
questions on asthma and EIA. These sites include the
American Academy of Allergy, Asthma and Immunology
(www.aaaai.org); the American Thoracic Society (www.
thoracic.org); the Asthma and Allergy Foundation of Amer-

Journal of Athletic Training 231
ica (www.aafa.org); the American College of Allergy,
Asthma, & Immunology (www.acaai.org); and USA Swim-
ming (http://www.usaswimming.org/USASWeb/pRainbow/
Documents/6c812467-b717-4c16-a32c-a1d9bcc9f444/
Asthma-%20Comprehensive%20Guide%2004%20Nov%
2029.pdf).
BACKGROUND AND LITERATURE REVIEW
Definition and Pathophysiology of Asthma
Asthma is a common condition that has been recognized for
more than 2000 years.
56
Asthma is usually defined operation-
ally as a chronic inflammatory disorder of the airways.
1,2,4,56
In many patients, this chronic inflammation causes an increase
in airway hyperresponsiveness, leading to recurrent episodes
of wheezing, breathlessness, chest tightness (or chest pain in
children), and coughing, particularly at night or in the early
morning and after exercise, especially in cold, dry environ-
ments. These episodes are associated with widespread but var-
iable airflow obstruction that is often reversible, either spon-
taneously or with treatment.
1,2,4
This definition implies that
asthma has multiple causes, and indeed, it is a complex dis-
order.
The chronic inflammatory process causes excess mucus pro-
duction and bronchial smooth muscle constriction,
57–61
which
result from a release of inflammatory mediators that include
histamine, tryptase, prostaglandin, and leukotrienes from mast
cells.
62–66
Airways may also accumulate thick, viscous secre-
tions produced by goblet cells and submucosal glands; more-
over, there is leakage of plasma proteins and accumulated cel-
lular debris.
57–61,67
Although airway narrowing affects the
tracheobronchial tree, small bronchi (2–5 mm in diameter) are
most affected.
68–70
Maximal expiratory flow rate is reduced
and residual lung volumes are increased as air is trapped be-
hind the blocked airways.
71
As a result, during an asthma at-
tack, the respiratory rate increases to compensate for the in-
creased obstruction of the airways and the inability of the
usually elastic lung to recoil (dynamic hyperinflation). The pa-
tient must work harder to breathe as the thorax becomes over-
inflated. With progression of the attack, the diaphragm and
intercostal muscles must compensate and contribute more en-
ergy during respiration.
72
In a severe attack, muscle efficiency
is eventually lost, and the increased breathing rate leads to
respiratory muscle fatigue and physical distress that may result
in death. Indeed, as many as 4200 to 5000 people die from
asthma each year in the United States.
73
Environmental Factors
Environmental factors, such as allergens, air pollution, oc-
cupational sensitizers, and tobacco smoke, may cause or ex-
acerbate asthma.
74–79
These factors are important triggers that
should be considered when evaluating a patient with asth-
ma.
79–83
Recently, concern about indoor air pollution has been
heightened.
79,80,82–84
Indoor ambient air can contain allergens
and pollution that can cause or exacerbate asthma and other
respiratory ailments when susceptible individuals are exposed
to these environments.
85
Many factors should be considered
indoors, such as adequacy of ventilation, humidity level, pres-
ence of allergens, presence of wall-to-wall carpeting and up-
holstered furniture, and types of building materials.
85–87
In-
door animal allergens (such as from cats, dogs, and other pets)
are an important trigger of symptoms in many people.
88
Cock-
roach allergens are commonly seen indoors (such as in swim-
ming pool locker rooms), and the cockroach allergenic mate-
rials can remain for a long period of time, even after
extermination.
89–92
Indoor mold and fungal spores, house dust
mites, and particulate matter, such as aerosols or smoke from
cigarettes, wood, or fossil fuels, also can trigger asthma symp-
toms.
93–107
Tobacco smoke is a risk factor for the development
of asthma, and smoking tobacco appears to increase asthma
severity.
108–110
Other indoor air irritants, such as chlorine, can exacerbate
asthma and cause eye and lung irritation.
111,112
School-age
children who frequently visit chlorinated pools may have an
increased risk of developing asthma, especially if they have
other risk factors for asthma.
113
Even individuals who do not
usually enter the water but who are exposed to indoor chlo-
rinated pools (eg, lifeguards, coaches) may have respiratory
irritation on exposure to chlorine.
114,115
Many actions can be taken to limit indoor allergen exposure,
including prohibiting smoking indoors, using air cleaners
equipped with a high-efficiency particulate air cleaner (HEPA)
filter, washing walls, vacuuming carpets, and cleaning mattress
covers weekly.
116–119
Additional measures include removing
carpets and installing linoleum or wood flooring, washing pets
(dogs and cats) and their bedding twice a week, keeping pets
out of the bedroom or living room at all times to reduce ex-
posure, covering mattresses and pillows, and controlling hu-
midity to help manage dust mites and mold.
81,82,120
Some of
these measures, such as the removal of a family pet from the
home, can be very difficult, so it is necessary to discuss the
effect of these exposures with the asthma patient. Although
air filters might help, the house should be cleaned thoroughly
before their use and regularly thereafter.
33
When inhaled, outdoor air pollution, caused by sulfur diox-
ide, carbon monoxide, nitrogen dioxide, ozone, and particulate
matter, can cause pulmonary function decrements, increased re-
liance on medications, bronchial hyperresponsiveness, and in-
creased asthma symptoms.
121–125
Many pollens (trees, grasses,
and weeds) are inhaled into the bronchi and cause allergen-
induced asthma.
126,127
Tree pollens predominate in the spring,
grass pollens in the late spring or early summer (and fall), and
weed pollens in the late summer and fall in the United States,
depending on geographic location, but may be present at other
times of the year in locations outside of the United
States.
128,129
Information about the pollen seasons in the Unit-
ed States can be accessed from the National Allergy Bureau
Web site.
Although it is virtually impossible to avoid all outdoor pol-
lution and allergens, some practical precautions can be imple-
mented: move indoors and close all windows, close car win-
dows when traveling, limit exposure when pollen is at its
highest levels, monitor local weather stations for allergy re-
ports, and practice indoors if possible when pollen counts are
high.
Diagnosis and Classification of Asthma
Asthma can be difficult to diagnose and classify (see Table
1). Some individuals, especially elite athletes, do not display
consistent signs or symptoms of asthma.
9,10,130
Asthma symp-
toms may be present only during certain times (seasons) of

232 Volume 40
•
Number 3
•
September 2005
the year or only after exercise and may be highly variable,
depending upon the athlete, the environment, and the activi-
ty.
13
The first step in determining whether asthma is present is
to obtain a detailed medical history. Questions regarding past
experiences, symptoms, smoking history, and family history
can help to rule out other respiratory disorders such as chronic
bronchitis, emphysema, bronchiectasis, allergic rhinitis, upper
respiratory infection, congestive heart failure, disorders of the
upper airway (eg, vocal cord dysfunction), and nonrespiratory
conditions such as anxiety. Most importantly, the athletic train-
er should ask general questions as listed in Recommendation
3 to assist in making a proper diagnosis.
13
If a patient appears
to have one or more symptoms suggestive of asthma, then lung
function testing should be performed (see Table 1).
9
However,
it is important to recognize that the history and baseline phys-
ical examination will fail to identify many patients with
EIA.
9,10,12
Lung function tests are essential to assess asthma severity
and airflow limitation and to determine whether the obstruc-
tion is fully reversible with treatment.
16–18,131–133
The most
common measures of airway function are the forced expiratory
volume in 1 second (FEV
1
), the forced vital capacity (FVC),
and the peak expiratory flow rate (PEFR). These tests can be
performed while the patient is at rest or after a challenge. The
FEV
1
measures the volume of air in liters forcefully exhaled
out of the airway in 1 second after a full inspiration. The FVC
measures the total volume of air in liters forcefully exhaled
out of the airway when the breath continues (usually for a
period of 6 or more seconds). The FVC procedure is effort
dependent and requires the patient to fully understand that he
or she needs to inhale a deep breath and then ‘‘blast’’ the air
out of the lungs into the measurement device. The FVC testing
also requires considerable expertise by the technician and the
ability to communicate with the patient. The PEFR measures
the maximal flow rate of air (in L/s or L/min) out of the air-
ways and is easier to perform than an FEV
1
or FVC maneuver,
although PEFR testing is also effort dependent. A flow volume
curve (flow loop) provides a graphic depiction of the breathing
effort in which flow rate is plotted against volume (in L/s) of
air exhaled and inhaled (in L), as shown in Figure 3. A vol-
ume-time curve (called a spirogram) is another way to plot
the breath, in which the volume of air exhaled (in L) is plotted
against time (in seconds). The FEV
1
/FVC ratio is also exam-
ined, along with a variety of measures of flow, such as the
flow between 25% and 75% of the FVC (called the FEF
25–75
).
Asthma is an example of an obstructive lung disease in
which the airways obstruct the outflow of air. In contrast, pul-
monary fibrosis is an example of a restrictive lung disease in
which the functional size of the lungs decreases. In obstructive
lung diseases, the FEV
1
decreases, whereas the FVC remains
relatively normal, so the FEV
1
/FVC ratio decreases (until late
in the disease or with severe exacerbations, when the FVC
may also decrease).
131
In restrictive lung disease, both the
FEV
1
and the FVC decrease proportionally (so that the FEV
1
/
FVC ratio is normal). Nomograms exist to provide guidance
as to normal ranges for FEV
1
and FVC based on age, size and
race.
134,135
It is important to recognize racial differences in the
normal values for these tests. Generally, levels should be at
least 80% of the predicted values to be considered ‘‘normal.’’
The FEV
1
/FVC ratio should also be above 80%. An increase
of 12% or more in FEV
1
after an inhaled bronchodilator (eg,
a b
2
-agonist such as albuterol) suggests reversible airway dis-
ease and may be used as a diagnostic criterion of asthma.
131
A decrease in FEV
1
after a challenge (such as after inhalation
of methacholine or running on a treadmill) suggests that the
airways are reactive to the stimulus.
19
It is important to de-
termine a patient’s personal best FEV
1
, FVC, and PEFR,
which are identified by plotting these values over time. These
values can also demonstrate variability between morning and
evening and over time, which may reflect airway hyperreac-
tivity.
Spirometers are used in the clinic or training room to de-
termine these pulmonary function values.
136
Patients may also
be given a PFM to measure PEFR away from the clinic or
athletic training room (Tables 2 and 3). The PFM is a small,
handheld device that measures maximal flow rate during
forced exhalation.
137–140
Maximal flow rate usually occurs
within the first 120 to 150 milliseconds of a forced exhala-
tion.
140
When used properly, a PFM can be somewhat helpful
in following the course of asthma and might even be useful
in suggesting the presence of asthma. The PFM can also be
used to identify asthma triggers and to monitor medication
changes, and it may help to reduce asthma morbidity.
141–147
The device allows the asthmatic patient to measure lung var-
iability over time to assist in determining when to seek med-
ical attention. The PFM should be used at least daily (in the
morning after awakening) and preferably also in the late af-
ternoon or evening.
1,2,148
At least 2 or 3 trials should be per-
formed at each specified time and the highest value recorded.
The device should be used before taking any medications and
at least 4, but preferably 8, hours after inhalation of a bron-
chodilator, if possible. The device should be used for at least
a 2- to 3-week monitoring period. Some devices record the
PEFR electronically, which can assist the patient in keeping
the data secure and available. Over time, the patient’s personal
best value will be determined. Subsequently, if the PEFR value
is less than 80% of the personal best or if daily variability is
greater than 20% of the morning value, then the patient should
be reevaluated in an attempt to find better control measures.
If the PEFR value is less than 50% of the personal best, the
individual should seek immediate attention (see Table 2).
1
Spi-
rometry, including the use of PFMs, may be especially useful
in patients who do not perceive the severity of their symp-
toms.
149,150
The PFM should be used regularly, even if asthma symp-
toms appear to be well controlled.
132,151–153
Patients who have
good PFM technique (see Table 3) adhere to their treatment
plan and control their symptoms better than those who have
poor technique. The PFM should be available in all athletic
training facilities and on the field in medical kits.
Unfortunately, some reports suggest that PFM recordings
are not always reliable indicators of airflow obstruction.
154–159
In certain cases, elite or well-trained athletes may possess large
lung capacities, which may exceed the measuring capacity of
the PFM. In addition, it appears that PFM values are not con-
sistent from one to another PFM from the same manufacturer,
across different PFM devices, when men and women use
PFMs, with different techniques while using a single PFM,
and at high altitudes.
159–164
The most accurate spirometry test-
ing is performed with office-based spirometry testing equip-
ment.
140
However, as noted above, spirometry testing requires
training
22,165
and may be impractical for everyday asthma
management, especially in primary care settings in which most
patients are not being seen for asthma.
140
If baseline lung function tests are within normal values and
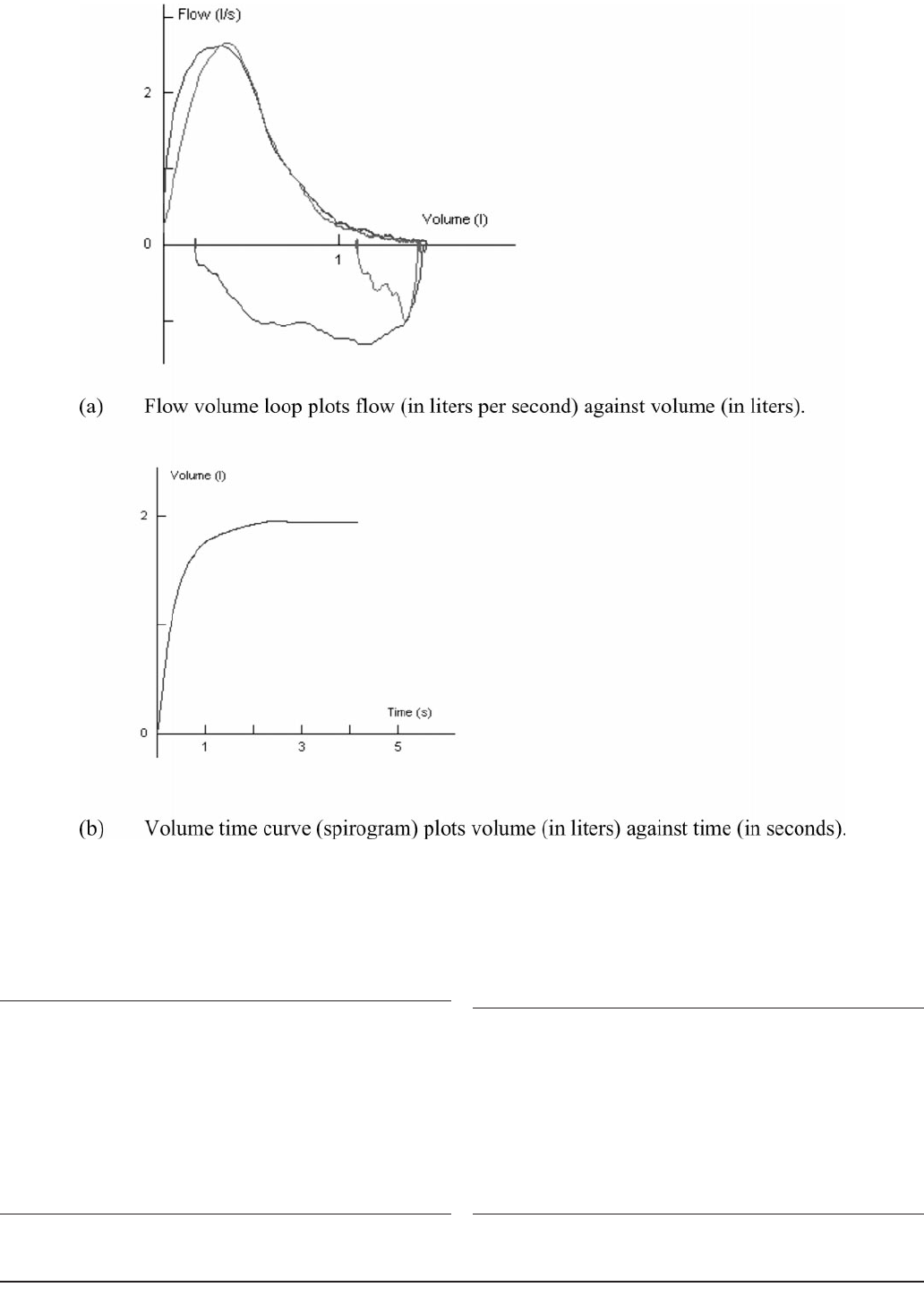
Journal of Athletic Training 233
Figure 3. Flow volume loop and volume time curve.
Table 2. Peak Flow Zones for Asthma Management*
Green zone
● PEF values are between 80% and 100% of personal best
● No asthma management changes are necessary at this time
Yellow zone
● PEF values are between 50% and 80% of personal best
● Caution is warranted; use of medications is required
Red zone
● PEF values are less than 50% of personal best
● Danger: emergency action is needed, including medications or
hospital visit
*Adapted with permission from Li JTC.
140
PEF indicates peak expiratory
flow.
Table 3. General Instructions for Using a Mechanical Peak Flow
Meter*
1. The movable indicator is placed at the beginning of the numbered
scale.
2. The patient stands or sits tall and straight.
3. The patient then inhales maximally.
4. The patient places his or her lips tightly around the mouthpiece.
5. The patient is told to ‘‘blast’’ the air out of his or her lungs and into
the device. The patient then forcefully exhales as fast and hard as
possible.
6. The value is recorded.
7. Steps 1–6 are repeated 2 more times.
8. The highest number is marked as the value for that time period.
*For example, a Mini-Wright peak flow meter (Clement Clarke Interna-
tional Ltd, Essex, UK). Adapted with permission from Li JTC.
140

234 Volume 40
•
Number 3
•
September 2005
Table 4. National Asthma Education and Prevention Program II: Usual Dosages for Long-Term Control Medications*
1
Medication Dosage Form Adult Dose Child Dose
Inhaled corticosteroids
Systemic corticosteroids
Methylprednisolone
Prednisolone
Prednisone
2, 4, 8, 16, 32 mg tablets
5 mg tablets
5 mg/5 cc
15 mg/5 cc
1, 2.5, 5, 10, 20, 50 mg tablets:
5 mg/cc, 5 mg/5cc
● 7.5–60 mg daily in a single dose
in
AM
or qod as needed for con-
trol
● Short-course ‘‘burst’’ to achieve
control; 40–60 mg/d as single
or 2 divided doses for 3–10 d
● 0.25–2 mg/kg daily in single dose
in
AM
or qod as needed for con-
trol
● Short-course ‘‘burst’’: 1–2 mg/kg/
d, maximum 60 mg/day for 3–
10 days
Long-acting inhaled beta
2
-agonists (Should not be used for symptom relief or for exacerbations. Use with inhaled corticosteroids.)
Salmeterol
Formoterol
MDI 21 mcg/puff
DPI 50 mcg/blister
DPI 12 mcg/single-use capsule
2 puffs q12h
1 blister q12h
1 capsule q12 h
1–2 puffs q12h
1 blister q12h
1 capsule q12h
Combined medication
Fluticasone/salmeterol DPI 100, 250, or 500 mcg/50 mcg 1 inhalation bid; dose depends on
severity of asthma
1 inhalation bid; dose depends on
severity of asthma
Cromolyn and nedocromil
Cromolyn
Nedocromil
MDI 1 mg/puff
Nebulizer 20 mg/ampule
MDI 1.75 mg/puff
2–4 puffs tid–qid
1 ampule tid–qid
2–4 puffs bid–qid
1–2 puffs tid–qid
1 ampule tid–qid
1–2 puffs bid-qid
Leukotriene modifiers
Montelukast 4 or 5 mg chewable tablet
10 mg tablet
10 mg qhs 4 mg qhs (2–5 yrs)
5 mg qhs (6–14 yrs)
10 mg qhs (.14 yrs)
Zafirlukast
Zileuton
10 or 20 mg tablet
300 or 600 mg tablet
40 mg daily (20 mg tablet bid)
2,400 mg daily (give tablets qid)
20 mg daily (7–11 yrs) (10 mg tablet
bid)
Methylxanthines (Serum monitoring is important [serum concentration of 5–15 mcg/mL at steady state])
Theophylline Liquids, sustained-release tablets,
and capsules
Starting dose 10 mg/kg/d up to 300
mg max; usual max 800 mg/d
Starting dose 10 mg/kg/d; usual
max:
● ,1 year of age: 0.2 (age in wk)
15 5 mg/kg/d
● $1 year of age: 16 mg/kg/d
*qod indicates every other day; bid, twice a day; tid, 3 times a day; qid, 4 times a day; qhs, at bedtime; MDI, metered dose inhaler; DPI, dry
powder inhaler.
the reversibility test with a b
2
-agonist is equivocal, then a
challenge test (eg, with methacholine) may be performed to
test for bronchial hyperresponsiveness.
19
During these tests,
progressively increasing concentrations of the aerosolized drug
are administered during a multistage procedure.
19
After each
stage, spirometry testing is performed to determine whether a
20% reduction in FEV
1
from baseline is obtained. If the re-
duction is less than 20% after all stages have been performed,
then the test is considered negative, and the patient is deter-
mined not to have bronchial hyperresponsiveness. It is impor-
tant to note, however, that these tests alone are not diagnostic
of asthma. A positive test must be interpreted in the context
of other information to make the definitive diagnosis of asth-
ma.
Exercise challenge and other surrogate challenges (such as
eucapnic hyperventilation) are described in the EIA section of
this statement.
19,21
Pharmacotherapy for Asthma
It is important to ascertain the correct diagnosis before med-
ications are prescribed and for the health care professional to
know the types of medications that are prescribed.
1,2,24–30,166,167
Only a small percentage of asthmatic patients take their med-
ications precisely as prescribed by their physician; the most
common cause of treatment failure is failure to use the pre-
scribed treatment.
167
Regardless, asthma can be managed
through various medications to prevent or control symptoms;
for an updated medication list, refer to the NAEPPII and
GINA guidelines.
1,2
The medications used to treat asthma are
classified as either controller or rescue (reliever) medications.
Controller Medications. Controller medications are daily,
long-term interventions used prophylactically to manage the
symptoms of mild, moderate, and severe asthma and gener-
ally should not be used to manage acute asthmatic symp-
toms.
1,2,25–28
Examples include inhaled corticosteroids, sys-
temic corticosteroids, cromones (sodium cromolyn and
nedocromil sodium), long-acting inhaled b
2
-agonists, theoph-
ylline, and leukotriene modifiers (Table 4 provides sample
agents).
Inhaled corticosteroids are effective controller medications
for treating persistent mild, moderate, and severe asthma.
1,2
They act by decreasing airway inflammation, mucus produc-
tion, and bronchial hyperresponsiveness.
168–173
Proper use of
inhaled corticosteroids can lead to a decrease in the number
and severity of asthma exacerbations, improve lung function,
lessen bronchial hyperresponsiveness, and reduce the need for
symptom relief with short-acting b
2
-agonists.
174–180
Inhaled
corticosteroids should not be used to treat acute asthmatic at-
tacks.
25
Their adverse effects include hoarseness, coughing,

Journal of Athletic Training 235
and occasionally thrush or oral candidiasis.
177,181
However,
rinsing the mouth after inhalation and using a spacer generally
help to prevent oral candidiasis.
Systemic corticosteroids are administered orally or paren-
terally for individuals who have severe persistent asthma that
remains poorly responsive to inhaler therapy.
182
Systemic ther-
apy has the same mechanisms of action as inhaled corticoste-
roids. However, long-term use of systemic agents can cause
more significant systemic adverse events than inhaled corti-
costeroids, including osteoporosis, glucose intolerance, glau-
coma, weight gain, skin thinning, bruising, fluid and electro-
lyte abnormalities, growth suppression, and muscle
weakness.
1,2,183
To minimize these adverse events, oral corti-
costeroids are often taken daily in the morning or every other
day, and patients should be monitored closely by a physi-
cian.
183
Oral corticosteroids are used much less frequently to-
day than in the past since the advent of high-potency inhaled
corticosteroids such as fluticasone and budesonide.
1,2
The cromones, cromolyn sodium and nedocromil sodium,
are inhaled asthma medications used to control mild persistent
asthma and are considered less effective anti-inflammatory
agents than inhaled corticosteroids.
25,26,184
Although the exact
mechanisms of action are poorly understood, they are thought
to inhibit IgE-mediated mediator release from mast cells and,
thus, to inhibit acute airflow limitations induced by exercise,
cold air, and allergens. The cromones are generally used to
treat mild persistent asthma and to prevent EIA.
1,2
Cromones
should be used as second-line drug therapy alternatives for
treating mild persistent asthma, perhaps combined with an in-
haled b
2
-agonist; however, several doses each day are usually
needed to control asthma.
1,2
Only minimal adverse events are
seen with these agents, including occasional coughing and an
unpleasant taste, particularly with nedocromil sodium.
Long-acting inhaled b
2
-agonists (eg, salmeterol and for-
moterol) have the same mechanisms of action as short-acting
b
2
-agonists but a 12-hour duration of action, compared with
4 to 6 hours for the short-acting agents.
185–190
A single dose
of formoterol or salmeterol before exercise can protect the ath-
lete from asthma symptoms associated with exercise for up to
12 hours.
191–193
Formoterol has a shorter onset of action than
salmeterol, approximately 5 minutes as compared with 15 to
30 minutes.
192,194
All b
2
-agonists, including formoterol and
salmeterol, are restricted asthma medications according to the
International Olympic Committee, World Anti-Doping Agen-
cy, and United States Anti-Doping Agency; elite athletes tak-
ing these medications, their physicians, and their athletic train-
ers should review the guidelines posted by these agencies.
Patients using long-acting b
2
-agonists regularly may display
a decrease in the duration of action.
195–197
In one study, for-
moterol had a shortened duration of action by day 14 of reg-
ular daily use.
198
Thus, patients should not expect these drugs
to remain effective over the 12-hour dosing interval after reg-
ular, daily, extended use. Studies also show that the use of
long-acting b
2
-agonists does not affect persistent airway in-
flammation.
199
These agents should only be used in combi-
nation with inhaled corticosteroids, which may be more ben-
eficial than ingesting each drug separately.
200–202
Combination
therapy (an inhaled, long-acting b
2
-agonist and an inhaled cor-
ticosteroid) has been shown to decrease the need for short-
acting b
2
-agonists, decrease nocturnal asthma, improve lung
function, decrease asthma exacerbations, and prevent
EIA.
200,202–205
Leukotriene modifiers, taken orally, block leukotriene syn-
thesis or block leukotriene receptors.
206–208
Leukotriene mod-
ifiers can be used to control allergen-, aspirin-, and exercise-
induced bronchoconstriction; improve lung function; and de-
crease asthma exacerbations.
209–215
Used primarily as second-
line therapy, leukotriene modifiers can reduce the dose of an
inhaled corticosteroid required to treat mild persistent asthma
and may improve asthma control.
214–217
Adverse events are
usually minimal, with reports of headaches and gastrointestinal
discomfort. However, zileuton (Zyflo, Abbott Laboratories,
Abbott Park, IL) may be associated with liver toxicity; there-
fore, liver function should be monitored regularly when using
this medication.
218
Unlike b
2
-agonists, the duration of action
of the leukotriene modifiers does not diminish over time.
215
In the past, theophylline has been used alone as a controller
agent, but now it is usually used in combination with another
agent, such as an inhaled corticosteroid.
219
Finally, some patients who have allergic asthma may benefit
over the long term from the administration of various forms
of allergy immunotherapy.
220–222
The decision to initiate such
therapy must be made by the patient and physician after a
careful evaluation. Even in the most successful cases, addi-
tional medical therapy is often required in conjunction with
immunotherapy.
Rescue (Reliever) Medications. Rescue medications act
rapidly to treat acute bronchoconstriction and associated symp-
toms of coughing, wheezing, shortness of breath (dyspnea),
and chest tightness.
1,2,25–28
Several classes of drugs act in this
manner, including rapid-acting inhaled b
2
-agonists, inhaled an-
ticholinergics, and short-acting theophylline.
Rapid-acting inhaled b
2
-agonists are the most commonly
used reliever therapy for chronic asthma. These b
2
-agonists
act quickly to cause bronchodilation by relaxing airway
smooth muscle, decreasing vascular permeability, and modi-
fying mediator release from mast cells.
185
Rapid-acting in-
haled b
2
-agonists are also the most frequently used agents to
prevent EIA and to treat its symptoms.
223
These medications
can be used as ‘‘rescue therapy’’ at times of an acute attack.
However, because of their relatively short duration of action
(2–4 hours), repeat treatments may be necessary for EIA.
Some authors
224,225
suggest that repeated use of short-acting
b
2
-agonists can lead to tolerance and less effectiveness over
time. Furthermore, the chronic use of long-acting inhaled b
2
-
agonists can decrease the effectiveness of the short-acting in-
haled b
2
-agonists.
189,225
Inhaled anticholinergic agents (eg, ipratropium) may be
used as bronchodilators.
226
These agents block acetylcholine
release from cholinergic innervation in airway smooth muscle
but have no anti-inflammatory action.
Short-acting theophylline is a bronchodilator that has been
used for many years to relieve asthma exacerbations.
29,227
Theophylline’s onset of action is delayed when compared with
that of b
2
-agonists, and so it is not currently used as first-line
rescue therapy.
1,2
Theophylline is a controversial drug because
its benefits might be outweighed by potential adverse events
such as seizures.
228
Adverse events can be serious and severe
if dosing is not closely monitored. Short-acting theophylline
should not be administered to patients who are already receiv-
ing chronic therapy with sustained-release theophylline thera-
py.
Short-acting oral b
2
-agonists, although rarely used, function
primarily by relaxing airway smooth muscle within a few min-
utes after administration and for a period of up to 4 hours;
however, they have no anti-inflammatory actions.
24,29
Adverse

236 Volume 40
•
Number 3
•
September 2005
Table 5. Sample Nonsteroidal Anti-Inflammatory Drugs
Generic
Name
Brand
Name(s) Manufacturer
Diclofenac, miso-
prostol
Arthrotec GD Searle LLC, Chicago, IL
Celecoxib Celebrex GD Searle LLC
Diclofenac Voltaren Novartis Pharmaceuticals Corp,
East Hanover, NJ
Diclofenac Cataflam Novartis Pharmaceuticals Corp
Diflunisal Dolobid Merck & Co, Inc, West Point, PA
Etodolac Lodine Wyeth-Ayerst International Inc,
Madison, NJ
Flurbiprofen Ansaid Pharmacia & Upjohn Co, Kalama-
zoo, MI
Ibuprofen Motrin McNeil-PPC, Fort Washington, PA
Advil Wyeth, Madison, NJ
Indomethacin Indocin Merck & Co, Inc
Ketoprofen Orudis Rhoˆne-Poulenc Rorer New Zealand
Ltd, Auckland, New Zealand
Ketorolac Toradol Roche Pharmaceuticals, Nutley, NJ
Nabumetone Relafen GlaxoSmithKline, Research Trian-
gle Park, NC
Naproxen Naprosyn Roche Pharmaceuticals
Aleve Bayer, Morristown, NJ
Oxaprozin Daypro GD Searle LLC
Piroxicam Feldene Pfizer Inc, New York, NY
Rofecoxib Vioxx Merck & Co, Inc
Salsalate Disalcid 3M Pharmaceuticals, Northridge,
CA
Sulindac Clinoril Merck & Co, Inc
events include tachycardia, hypertension, and decreased ap-
petite. If used chronically, increasing doses of short-acting oral
b
2
-agonists might indicate loss of control of asthma.
1,2
All
athletes using short-acting oral b
2
-agonists should be advised
that many sporting organizations restrict or prohibit the use of
these agents.
Finally, systemic glucocorticosteriods are administered oral-
ly or parenterally for individuals who have acute asthma ex-
acerbations. The mechanisms of action are similar to those for
corticosteroids used to treat chronic severe asthma.
1,2
Asthma Medication Delivery Techniques
Many of the newer asthma medications are delivered to the
lungs by inhalation devices.
23
The most common types of in-
halers are MDIs and dry powder inhaler devices. The MDIs
release a specific amount of a drug from a pressurized canister
to propel medication into the lungs when the patient takes a
breath. When using MDIs, patients must exhale first, then
place the inhaler at or slightly in front of the lips, and slowly
inhale at the same time that they are activating the inhaler to
release the drug. Patients hold their breath for a few seconds
(approximately 10) before exhaling. Patients who have diffi-
culty coordinating MDI activation with breathing generally
benefit from the use of a spacer.
229–231
A spacer is attached to
the MDI device to reduce side effects of inhaled corticoste-
roids in the mouth and for patients who have difficulty coor-
dinating the activation of an MDI and breathing. Dry powder
inhalers are often easier to use than MDIs, and they do not
permit use of a spacer.
Aspirin, Nonsteroidal Anti-Inflammatory Drugs, and
Asthma
Aspirin-sensitive athletes may manifest nasal congestion;
itchy, watery, or swollen eyes; coughing; difficulty breathing;
wheezing; urticaria; and possible shock when they ingest as-
pirin or other NSAIDs.
232–236
This is not a true allergy because
it is not caused by IgE, but it is treated in the same manner
as an allergic reaction. Athletic trainers should also be aware
of triad syndrome: athletes with asthma, nasal polyps, and as-
pirin sensitivity may have a severe asthma attack when they
take an NSAID.
237
Although only a small percentage of the population has as-
pirin-sensitive asthma,
238–240
this condition is particularly con-
cerning in an athletic population because many athletes who
have asthma use anti-inflammatory drugs to treat injuries.
Therefore, athletic trainers must understand that some patients
who have asthma could suffer fatal consequences if they take
aspirin or NSAIDs.
Aspirin-sensitive athletes should also avoid COX-2 inhibi-
tors, but acetaminophen in moderate doses can usually be tak-
en without difficulty.
241
Salsalate, choline magnesium trisali-
cylate, and dextropropoxyphene may be used as substitute
medication in patients with aspirin sensitivity if tolerat-
ed.
240,242
Athletic trainers should be familiar with the many
prescription and over-the-counter products that contain aspirin
and other NSAIDs, including ibuprofen (eg, Motrin, McNeil-
PPC, Fort Washington, PA; Advil, Wyeth, Madison, NJ) and
naproxen (eg, Aleve, Bayer, Morristown, NJ). Health care pro-
fessionals should supply as much information to the patient as
possible, including a list of products to avoid (Table 5).
Nonpharmacologic Treatment for Asthma
Athletes with asthma need to keep their asthma under op-
timal control to prevent exercise-induced breathing symp-
toms.
31,32,243,244
Masks and nose breathing help to warm and
moisturize inhaled air before it reaches the smaller airways.
This may decrease the inflammatory reaction in the airways
and thus decrease the frequency and intensity of EIA. These
maneuvers are effective for some but not all athletes.
224
Nose
breathing is not effective at high ventilation rates. Limiting
environmental exposures (eg, to cold air and pollen) may de-
crease symptoms in susceptible athletes; however, this may not
be practical in some sports.
Theoretically, exercise training might decrease symptoms by
conditioning the body to exercise, but research has not sup-
ported this theory.
245,246
Nevertheless, an asthmatic individual
should participate in exercise programs tailored to his or her
capacity to perform.
55
A refractory period can occur after exercise, when the air-
way response to exercise is inhibited for up to 2 to 3 hours.
Some athletes have taken advantage of this phenomenon to
help control EIA.
31,32,34,247–251
However, there are no specific
guidelines to follow, and each athlete must experiment to de-
termine the best individual protocol.
Because hyperosmolarity plays a role in mediating EIA,
limiting sodium in the diet has received some attention. Re-
stricting dietary salt may cause a relative decrease in airway
obstruction.
252,253
Both sodium and chloride appear to play
roles, but this remains an area of active investigation, and no
specific guidelines are available.
254
A diet supplemented with
n-3 polyunsaturated fatty acid in fish oil has shown favorable
results in elite athletes with EIA.
255

Journal of Athletic Training 237
Exercise-Induced Asthma or Bronchospasm
Most asthmatic individuals have a flare of their asthma after
exercise.
256,257
Some individuals only have asthma signs and
symptoms associated with exercise.
13
By definition, a tempo-
rary narrowing of the airways (bronchospasm) induced by
strenuous exercise in which the patient has no symptoms is
known as EIB.
13
When symptoms are present, EIB is de-
scribed as EIA.
13
This section reviews the incidence of EIA
and EIB in the athletic population and considers special di-
agnostic or therapeutic measures that should be taken in an
athletic population.
Exercise-induced asthma is commonly seen in athletes in all
levels of athletic competition.
5,9,10,31,243,244,258–269
In most pa-
tients who have chronic asthma (at least 80%), exercise is a
trigger for bronchoconstriction.
13,243
Exercise-induced asthma
can also occur in patients who do not otherwise have asthma,
such as in about 40% of patients who suffer from allergic
rhinitis in season.
243,270,271
The incidence of EIA in the gen-
eral population has been estimated to be between 12% and
15%.
13,271
Rates as high as 23% have been reported in school-
age children, and the incidence in athletes may also be this
high.
258,262,263,265
Exercise-induced asthma may be more com-
mon in urban environments than in rural areas.
261
Other fac-
tors, such as high ozone levels, might also account for in-
creased EIA rates.
272
Exercise-induced asthma can be a significant disability for
the athlete, especially in endurance sports.
262,263,273
For ex-
ample, EIA is relatively common in cross-country skiers, and
some studies suggest that the cold air athletes breathe while
cross-country skiing may provoke inflammation.
274,275
Simi-
larly, athletes who participate in swimming and long-distance
running have a high incidence of asthma.
262
Among Olympic
athletes, asthma appears to be more common in those who
participate in winter sports than in those who participate in
summer sports.
262,263
At least 1 in 5 United States athletes who
participated in the 1998 Winter Olympics had the condition,
compared with 1 in 6 at the 1996 Summer Olympic
Games.
262,263
Wilber et al
265
found a 23% overall incidence
of EIA among athletes in the 7 winter sports tested. In addi-
tion, more females than males participating in the Winter
Games reported an asthma condition or medication
use.
262,263,265
Of the winter sports athletes tested, females had
an incidence of 26%, compared with 18% in males.
265
Although EIA impairs performance, it can be overcome.
Amy Van Dyken, an athlete who suffered from relatively se-
vere asthma, won 4 gold medals in swimming in the 1996
Olympic Games.
273
Other well-known elite athletes have also
been able to excel when their asthma was under good con-
trol.
273
Pathophysiology of Exercise-Induced Asthma
Two major theories exist to explain EIA: the cooling/warm-
ing hypothesis and the drying hypothesis.
31,256,257,260,276–287
As ventilation increases, airways progressively cool, which re-
sults in bronchoconstriction. This theory is supported by the
higher incidence of EIA in athletes participating in cold en-
vironments.
263
In addition to cooling, the increased ventilation
can lead to airway dehydration as inhaled air is humidified.
The main effect of inhaling cold air is actually attributable to
the fact that cold air carries less moisture. As with chronic
asthma, inflammatory cells and mediators may increase in the
lung in response to exercise in patients with EIA.
248,279,288–290
Environmental allergens may enhance the likelihood of bron-
choconstriction, and irritants such as sulfur dioxide, nitrogen
dioxide, ozone, and chlorine have been implicated as causing
patients to have exercise-induced symptoms.
31,243,291
Exercise-Induced Asthma Diagnosis
Two requirements are needed to diagnose EIA: symptoms
and obstructed airways, both associated with exercise.
13,269
First, the patient has any of a constellation of symptoms
associated with exercise, including shortness of breath (dys-
pnea), coughing, chest tightness (or chest pain in children),
wheezing, and decreased exercise tolerance.
13,262,263
Symp-
toms generally occur 5 to 8 minutes after sufficiently intense
exercise starts. The EIA may be associated with specific sports
as well as specific environments.
262–267
Where allergens are
present, outdoor activities and cold air exposure may be more
likely to foster the appearance of EIA, which would not occur
in other environments.
Second, the patient should have objective evidence of air-
way obstruction associated with exercise.
224
Generally, a drop
from baseline of at least 10% to 15% in FEV
1
after a challenge
test supports the diagnosis of EIA.
19,292
Pulmonary function
should be monitored 5, 10, 15, and 30 minutes after the chal-
lenge.
19
The exercise needs to last for 6 to 8 minutes at an
intensity level high enough to raise the athlete’s heart rate to
at least 80% of maximum
19
and ventilation to approximately
40% to 60% of maximum.
19
Exercise challenges can be per-
formed in a laboratory (using a treadmill, a cycle ergometer,
a rowing machine, or a free running asthma screening test
[FRAST]).
293
Alternatively, an exercise challenge test in the
laboratory can attempt to mimic the conditions and intensity
of the sport.
20,21
Indeed, 78% of cross-country skiers reported
a false-negative test during standard laboratory exercise chal-
lenges, suggesting that the standard tests are not as sensitive
as sport-specific exercise challenge tests for endurance ath-
letes.
20,21
Cold, dry air and near-maximal exercise intensity
(greater than 90% peak heart rate) are required to provoke a
positive result, especially in the cold-weather athlete. Time of
day can be important: in a group of asthmatics, a greater drop
in pulmonary function (FEV
1
) to exercise challenge was mea-
sured in the evening than in the morning.
294
Additional challenge tests include eucapnic voluntary hy-
perventilation or inhalation of hypertonic saline.
21,269,295
The
former test requires the athlete to hyperventilate dry air con-
taining 5% carbon dioxide, 21% oxygen, and the balance of
nitrogen at 30 times FEV
1
for 6 minutes.
21
It is important to evaluate athletes with atypical EIA symp-
toms because upper airway conditions such as vocal cord dys-
function or abnormal movement of the arytenoids region may
be the cause.
15,53
The signs and symptoms of vocal cord dys-
function can be similar to asthma and can be confused with
EIA.
54,296,297
This laryngeal disorder involves the unintention-
al paradoxic adduction of the vocal cords with breathing and
can be triggered by exercise.
296,297
The patient is often female
and may also have gastroesophageal reflux disease or a psy-
chiatric illness. Vocal cord dysfunction often occurs with asth-
ma, making control of EIA difficult. Diagnosis of vocal cord
dysfunction involves the direct visualization of the paradoxic
vocal cord motion, but the condition is often suspected when
voice changes and inspiratory stridor occur during an attack,
as well as when the inspiratory (bottom) portion of the flow
volume loop is truncated.

238 Volume 40
•
Number 3
•
September 2005
Table 6. National Asthma Education and Prevention Program II:
Treatment of Exercise-Induced Asthma*
1
One goal of management is to enable patients to participate in any
activity they choose without experiencing asthma symptoms. EIB should
not limit either participation or success in vigorous activities.
Recommended treatments include:
C Beta
2
-agonists will prevent EIB in more than 80 percent of patients.
● Short-acting inhaled beta
2
-agonists used shortly before exercise
(or as close to exercise as possible) may be helpful for 2 to 3
hours.
● Salmeterol has been shown to prevent EIB for 10 to 12 hours
(Kemp et al 1994
301
).
C Cromolyn and nedocromil, taken shortly before exercise, are also
acceptable for preventing EIB.
C A lengthy warmup period before exercise may benefit patients who
can tolerate continuous exercise with minimal symptoms. The
warmup may preclude a need for repeated medications.
C Long-term–control therapy, if appropriate.
● There is evidence that appropriate long-term control of asthma
with anti-inflammatory medication will reduce airway responsive-
ness, and this is associated with a reduction in the frequency and
severity of EIB (Vathenen et al, 1991
302
).
Teachers and coaches need to be notified that a child has EIB, should
be able to participate in activities, and may need inhaled medication
before activity. Individuals involved in competitive athletics need to be
aware that their medication use should be disclosed and should adhere
to standards set by the U.S. Olympic Committee (Nastasi et al, 1995
303
).
The U.S. Olympic Committee’s Drug Control Hotline is 1-800-233-0393.
EIB indicates exercise-induced bronchospasm.
Table 7. United States Anti-Doping Agency Regulated Asthma
Medications
Drug class: b
2
-agonists:
Advair* (GlaxoSmithKline, Research Triangle Park, NC)
albuterol*
bambuterol
bitolterol
Brethaire* (Riker Laboratories, Inc, Northridge, CA)
Combivent* (Boehringer Ingelheim Pharmaceuticals Inc, Ridgefield
(CT)
fenoterol
Foradil* (Novartis Pharmaceuticals Corp, East Hanover, NJ)
formoterol*
metaproterenol
orciprenaline
pirbuterol
Proventil* (Schering-Plough Corp, Kenilworth, NJ)
reproterol
salbutamol*
salmeterol*
Serevent* (GlaxoSmithKline)
terbutaline*
Ventolin* (GlaxoSmithKline)
Xopenex* (Sepracor, Marlborough, MA)
Available at: http://www.usantidoping.org. Accessed June 6, 2005.
*Allowed by inhaler or nebulizer only to prevent or treat asthma or ex-
ercise-induced asthma. Abbreviated Therapeutic Use Exemption (TUE)
must be on file with United States Anti-Doping Agency or international
federations, as appropriate. A salbutamol (albuterol) level greater than
1000 ng/mL is prohibited even with abbreviated TUE.
Exercise-Induced Asthma Treatment
Most of the drugs described for the treatment of chronic
asthma are used to prevent EIA attacks.
1,2,269,298–300
Table 6
contains recommendations from the NAEPPII for the treat-
ment of EIA.
1
The key feature is that a b
2
-agonist can be used
both to prevent attacks and to treat them when they occur.
Once an asthmatic individual meets the requirements for stage
1 through 4 asthma, the NAEPPII treatment guidelines should
be followed.
Asthma Education
Throughout this position statement, information has been
presented to inform and educate the athletic trainer and allied
health personnel about asthma and asthma management. Of
particular importance is a properly prepared asthma manage-
ment plan. Educating athletes about asthma and having a writ-
ten management plan will help control their disease.
1,2,304
Sev-
eral groups
35–44,48–51
have shown that an effectively written
management plan can reduce medication errors, asthma ex-
acerbations, and hospital visits. Without a written asthma ac-
tion plan, many patients have a difficult time controlling their
asthma symptoms.
45–47,305
It is also imperative that an acces-
sible line of communication between the patient and health
care professional be identified.
An effective management plan should include a written doc-
ument that addresses the following: (1) goals of the patient,
(2) proper use and frequency of PEFR monitoring, (3) guide-
lines for altering medications based upon readings from PFMs
or asthma symptoms, (4) contact numbers for all health care
professionals, including emergency numbers, and (5) environ-
mental factors to avoid or monitor. The health care profes-
sionals developing the asthma management plan should dis-
cuss all goals or expectations with the athlete. This education
empowers the athlete and promotes better compliance.
Athletic trainers working with elite or Olympic athletes
must be familiar with International Olympic Committee,
World Anti-Doping Agency, and United States Anti-Doping
Agency medication guidelines (Table 7). Certain asthma med-
ications may be banned, restricted, or permitted, depending on
the organization and the medication. A banned medication is
one the athlete cannot take. In some cases, a prohibited sub-
stance is prohibited at all times or only prohibited in compe-
tition, meaning the athlete must allow sufficient time for the
substance to clear the system before competition. Restricted
medications must have prior physician approval and comple-
tion of forms (such as the Therapeutic Use Exemption, or
TUE) before the athlete can compete. For example, the United
States Anti-Doping Agency lists salbutamol/albuterol, salme-
terol, terbutaline, and formoterol as restricted b
2
-agonists that
require a TUE before competition.
Additional information about the diagnosis and management
of asthma can be obtained at the National Asthma Education
and Prevention Program Web site (http://www.nhlbi.nih.gov/
health/prof/lung/index.htm or http://www.nhlbi.nih.gov/about/
naepp/) and the GINA Web site (www.ginasthma.com).
CONCLUSIONS
Asthma can affect individuals regardless of age, sex, or so-
cioeconomic status. This National Athletic Trainers’ Associa-
tion asthma position statement is designed to present guide-
lines for the recognition, prophylaxis, and management of
asthma. The information should lead to improvements in the
quality of care certified athletic trainers and other heath care
providers can offer to patients with asthma, particularly ath-
letes and especially those with EIA. In the end, these guide-

Journal of Athletic Training 239
lines should reduce the incidence of asthma complications and
improve the quality of life for patients with asthma, especially
those in whom exercise is an important trigger.
DISCLAIMER
The NATA publishes its position statements as a service to
promote the awareness of certain issues to its members. The
information contained in the position statement is neither ex-
haustive not exclusive to all circumstances or individuals. Var-
iables such as institutional human resource guidelines, state or
federal statutes, rules, or regulations, as well as regional en-
vironmental conditions, may impact the relevance and imple-
mentation of these recommendations. The NATA advises its
members and others to carefully and independently consider
each of the recommendations (including the applicability of
same to any particular circumstance or individual). The posi-
tion statement should not be relied upon as an independent
basis for care but rather as a resource available to NATA mem-
bers or others. Moreover, no opinion is expressed herein re-
garding the quality of care that adheres to or differs from
NATA’s position statements. The NATA reserves the right to
rescind or modify its position statements at any time.
ACKNOWLEDGMENTS
We gratefully acknowledge the efforts of Michael C. Koester, MD,
ATC; James L. Moeller, MD, FACSM; Kenneth W. Rundell, PhD;
Chad Starkey, PhD, ATC; Randall L. Wilber, PhD; and the Pro-
nouncements Committee in the preparation of this document.
REFERENCES
1. National Asthma Education and Prevention Program. Expert Panel Re-
port II: Guidelines for the Diagnosis and Management of Asthma. Be-
thesda, MD: National Institutes of Health; 1997. Publication No. 97-
4051:12–18.
2. Global Strategy for Asthma Management and Prevention. Bethesda,
MD: National Institutes of Health, National Heart, Lung, and Blood
Institute; 2002. NIH publication No. 02-3659.
3. Nystad W. Asthma. Int J Sports Med. 2000;21(suppl):S-98–S-102.
4. Balatbat JH. Asthma: an overview from prevalence to plan. J Contin
Educ Top Issues. 2002;2:80–92.
5. Rundell KW, Im J, Mayers LB, Wilber RL, Szmedra L, Schmitz HR.
Self-reported symptoms and exercise-induced asthma in the elite athlete.
Med Sci Sports Exerc. 2001;33:208–213.
6. Tiddens H, Silverman M, Bush A. The role of inflammation in airway
disease: remodeling. Am J Respir Crit Care Med. 2000;162(2, pt 2):S-
7–S-10.
7. Bousquet J, Jeffery PK, Busse WW, Johnson M, Vignola AM. Asthma:
from bronchoconstriction to airways inflammation and remodeling. Am
J Respir Crit Care Med. 2000;161:1720–1745.
8. Wenzel S. Severe asthma in adults. Am J Respir Crit Care Med. 2005;
172:149–160.
9. Rupp NT, Guill NF, Brudno DS. Unrecognized exercise-induced bron-
chospasm in adolescent athletes. Am J Dis Child. 1992;146:941–944.
10. Rupp NT, Brudno DS, Guill MF. The value of screening for risk of
exercise-induced asthma in high school athletes. Ann Allergy. 1993;70:
339–342.
11. Hammerman SI, Becker JM, Rogers J, Quedenfeld TC, D’Alonzo GE
Jr. Asthma screening of high school athletes: identifying the undiagnosed
and poorly controlled. Ann Allergy Asthma Immunol. 2002;88:380–384.
12. Hallstrand TS, Curtis JR, Koepsell TD, et al. Effectiveness of screening
examinations to detect unrecognized exercise-induced bronchoconstric-
tion. J Pediatr. 2002;141:343–348.
13. Weiler JM. Exercise-induced asthma: a practical guide to definitions,
diagnosis, prevalence, and treatment. Allergy Asthma Proc. 1996;17:
315–325.
14. Lacroix VJ. Exercise-induced asthma. Physician Sportsmed. 1999;
27(12):75–91.
15. Abu-Hasan M, Tannous B, Weinberger M. Exercise-induced dyspnea in
children and adolescents: if not asthma then what? Ann Allergy Asthma
Immunol. 2005;94:366–371.
16. West JB. Respiratory Physiology: The Essentials. Philadelphia, PA: Lip-
pincott Williams & Wilkins; 2000.
17. Kanner RE, Morris AH, eds. Clinical Pulmonary Function Testing: A
Manual of Uniform Laboratory Procedures. Salt Lake City, UT: Inter-
mountain Thoracic Society; 1975.
18. Petty TL. Simple office spirometry. Clin Chest Med. 2001;22:845–859.
19. Crapo RO, Casaburi R, Coates AL, et al. Guidelines for methacholine
and exercise challenge testing—1999. Am J Respir Crit Care Med. 2000;
161:309–329.
20. Rundell KW, Wilber RL, Szmedra L, Jenkinson DM, Mayers LB, Im J.
Exercise-induced asthma screening of elite athletes: field versus labo-
ratory exercise challenge. Med Sci Sports Exerc. 2000;32:309–316.
21. Rundell KW, Anderson SD, Spiering BA, Judelson DA. Field exercise
vs laboratory eucapnic voluntary hyperventilation to identify airway hy-
perresponsiveness in elite cold weather athletes. Chest. 2004;125:909–
915.
22. Peak flow meters and spirometers in general practice. Drug Ther Bull.
1997;35:52–55.
23. Meadows-Oliver M, Banasiak NC. Asthma medication delivery devices.
J Pediatr Health Care. 2005;19:121–123.
24. Im J. Asthma. RxConsultant. 2003;12:1–8.
25. Baker VO, Friedman J, Schmitt R. Asthma management, part II: phar-
macologic management. J Sch Nurs. 2002;18:257–269.
26. Self TH, Kelly HW. Asthma. In: Young LY, Koda-Kimble MA, eds.
Applied Therapeutics: The Clinical Use of Drugs. 6th ed. Vancouver,
WA: Applied Therapeutics, Inc; 1995:19-1–19-31.
27. Drugs for asthma. Med Lett Drugs Ther. 1999;41:5–10.
28. Szilagyi PG, Kemper KJ. Management of chronic childhood asthma in
the primary care office. Pediatr Ann. 1999;28:43–52.
29. Sherman J, Hendeles L. Practical pharmacology for pediatric asthma.
Pediatr Ann. 2000;29:768–773.
30. Houglum JE. Asthma medications: basic pharmacology and use in the
athlete. J Athl Train. 2000;35:179–187.
31. Hough DO, Dec KL. Exercise-induced asthma and anaphylaxis. Sports
Med. 1994;18:162–172.
32. Gong H Jr. Breathing easy: exercise despite asthma. Physician Sports-
med. 1992;20(3):158–167.
33. McDonald E, Cook D, Newman T, Griffith L, Cox G, Guyatt G. Effect
of air filtration systems on asthma: a systematic review of randomized
trials. Chest. 2002;122:535–1542.
34. Reiff DB, Choudry NB, Pride NB, Ind PW. The effect of prolonged
submaximal warm-up exercise on exercise-induced asthma. Am Rev Res-
pir Dis. 1989;139:479–484.
35. Turner MO, Taylor D, Bennett R, Fitzgerald JM. A randomized trial
comparing peak expiratory flow and symptom self-management plans
for patients with asthma attending a primary care clinic. Am J Respir
Crit Care Med. 1998;157:540–546.
36. Kolbe J, Vamos M, James F, Elkind G, Garrett J. Assessment of practical
knowledge of self-management of acute asthma. Chest. 1996;109:86–
90.
37. Lieu TA, Quesenberry CP Jr., Capra AM, Sorel ME, Martin KE, Men-
doza GR. Outpatient management practices associated with reduced risk
of pediatric asthma hospitalization and emergency department visits. Pe-
diatrics. 1997;100(3, pt 1):334–341.
38. Gibson PG, Wlodarczyk J, Hensley MJ, Murree-Allen K, Olson LG,
Saltos N. Using quality-control analysis of peak expiratory flow record-
ings to guide therapy for asthma. Ann Intern Med. 1995;123:488–492.
39. Boggs PB, Hayati F, Washburne WF, Wheeler DA. Using statistical pro-
cess control charts for the continual improvement of asthma care. Jt
Comm J Qual Improv. 1999;25:163–181.
40. Allen RM, Jones MP, Oldenburg B. Randomised trial of an asthma self-
management programme for adults. Thorax. 1995;50:731–738.

240 Volume 40
•
Number 3
•
September 2005
41. Bailey WC, Richards JM Jr, Brooks CM, Soong SJ, Windsor RA, Man-
zella BA. A randomized trial to improve self-management practices of
adults with asthma. Arch Intern Med. 1990;150:1664–1668.
42. Hilton S, Sibbald B, Anderson HR, Freeling P. Controlled evaluation of
the effects of patient education on asthma morbidity in general practice.
Lancet. 1986;1:26–29.
43. Yoon R, McKenzie DK, Bauman A, Miles DA. Controlled trial evalu-
ation of an asthma education programme for adults. Thorax. 1993;48:
1110–1116.
44. Wilson SR, Scamagas P, German DF, et al. A controlled trial of two
forms of self-management education for adults with asthma. Am J Med.
1993;94:564–576.
45. Beilby JJ, Wakefield MA, Ruffin RE. Reported use of asthma manage-
ment plans in South Australia. Med J Aust. 1997;166:298–301.
46. Dales RE, Kerr PE, Schweitzer I, Reesor K, Gougeon L, Dickinson G.
Asthma management preceding an emergency department visit. Arch
Intern Med. 1992;152:2041–2044.
47. Scarfone RJ, Zorc JJ, Capraro GA. Patient self-management of acute
asthma: adherence to national guidelines a decade later. Pediatrics.
2001;108:1332–1338.
48. Adams RJ, Boath K, Homan S, Campbell DA, Ruffin RE. A randomized
trial of peak-flow and symptom-based action plans in adults with mod-
erate-to-severe asthma. Respirology. 2001;6:297–304.
49. Myers TR. Improving patient outcomes with tools for asthma self-mon-
itoring: a review of the literature. Dis Manag Health Outcomes. 2002;
10:631–642.
50. Clark NM, Partridge MR. Strengthening asthma education to enhance
disease control. Chest. 2002;121:1661–1669.
51. Lawrence G. Asthma self-management programs can reduce the need
for hospital-based asthma care. Respir Care. 1995;40:39–43.
52. Partridge MR. Delivering optimal care to the person with asthma: what
are the key components and what do we mean by patient education?
Eur Respir J. 1995;8:298–305.
53. Bittleman DB, Smith RJH, Weiler JM. Abnormal movment of the ary-
tenoid region during exercise presenting as exercise-induced asthma in
an adolescent athlete. Chest. 1994;106:615–616.
54. McFadden ER Jr, Zawadski DK. Vocal cord dysfunction masquerading
as exercise-induced asthma: a physiologic cause for ‘‘choking’’ during
athletic activities. Am J Respir Crit Care Med. 1996;153:942–947.
55. Clark CJ, Cochrane LM. Physical activity and asthma. Curr Opin Pulm
Med. 1999;5:68–75.
56. Guthrie CM, Tingen MS. Asthma: a case study, review of pathophysi-
ology, and management strategies. J Am Acad Nurse Pract. 2002;14:
457–461.
57. Wiggs BR, Bosken C, Pare´ PD, James A, Hogg JC. A model of airway
narrowing in asthma and in chronic obstructive pulmonary disease. Am
Rev Respir Dis. 1992;145:1251–1258.
58. Aikawa T, Shimura S, Sasaki H, Ebina M, Takishima T. Marked goblet
cell hyperplasia with mucus accumulation in the airways of patients who
died of severe acute asthma attack. Chest. 1992;101:916–921.
59. Shimura S, Andoh Y, Haraguchi M, Shirato K. Continuity of airway
goblet cells and intraluminal mucus in the airways of patients with bron-
chial asthma. Eur Respir J. 1996;9:1395–1401.
60. Huber HL, Koessler KL. The pathology of bronchial asthma. Arch Intern
Med. 1922;30:689–760.
61. Dunnill MS. The pathology of asthma, with special reference to changes
in the bronchial mucosa. J Clin Pathol. 1960;13:27–33.
62. Wenzel SE, Westcott JY, Smith HR, Larsen GL. Spectrum of prostanoid
release after bronchoalveolar allergen challenge in atopic asthmatics and
in control groups: an alteration in the ratio of bronchoconstrictive to
bronchoprotective mediators. Am Rev Respir Dis. 1989;139:450–457.
63. Persson CG. Role of plasma exudation in asthmatic airways. Lancet.
1986;2:1126–1129.
64. Djukanovic´ R, Wilson JW, Britten KM, et al. Quantitation of mast cells
and eosinophils in the bronchial mucosa of symptomatic atopic asth-
matics and healthy control subjects using immunohistochemistry. Am
Rev Respir Dis. 1990;142:863–871.
65. Koshino T, Arai Y, Miyamoto Y, et al. Mast cell and basophil number
in the airway correlate with the bronchial responsiveness of asthmatics.
Int Arch Allergy Immunol. 1995;107:378–379.
66. O’Byrne PM. Airway inflammation and asthma. Aliment Pharmacol
Ther. 1996;10(suppl 2):18–24.
67. Hays SR, Fahy JV. The role of mucus in fatal asthma. Am J Med. 2003;
115:68–69.
68. Kessler GF, Austin JH, Graf PD, Gamsu G, Gold WM. Airway constric-
tion in experimental asthma in dogs: tantalum bronchographic studies.
J Appl Physiol. 1973;35:703–708.
69. Carroll N, Elliot J, Morton A, James A. The structure of large and small
airways in nonfatal and fatal asthma. Am Rev Respir Dis. 1993;147:
405–410.
70. Awadh N, Mu¨ller NL, Park CS, Abboud RT, FitzGerald JM. Airway
wall thickness in patients with near fatal asthma and control groups:
assessment with high resolution computed tomographic scanning. Tho-
rax. 1998;53:248–253.
71. McFadden ER Jr. Development, structure, and physiology in the normal
lung and in asthma. In: Middleton E, Ellis EF, Adkinson NF Jr, Yungin-
ger JW, Reed CH, Busse WW, eds. Allergy Principles and Practice. St.
Louis, MO: Mosby; 1998:508–519.
72. McFadden ER Jr, Kiser R, DeGroot WJ. Acute bronchial asthma: rela-
tions between clinical and physiologic manifestations. N Engl J Med.
1973;288:221–225.
73. National Center for Health Statistics. NCHS Data on Asthma. Available
at: http://www.cdc.gov/nchs/data/factsheets/asthma.pdf. Accessed June
6, 2005.
74. D’Amato G, Liccardi G, Cazzola M. Environment and development of
respiratory allergy, I: outdoors. Monaldi Arch Chest Dis. 1994;49:406–
411.
75. D’Amato G, Liccardi G, D’Amato M. Environment and development of
respiratory allergy, II: indoors. Monaldi Arch Chest Dis. 1994;49:412–
420.
76. Steerenberg PA, Van Amsterdam JGC, Vandebriel BJ, Vos JG, Van Bree
L, Van Loveren H. Environmental and lifestyle factors may act in con-
cert to increase the prevalence of respiratory allergy including asthma.
Clin Exp Allergy. 1999;29:1303–1308.
77. Sunyer J, Anto` JM, Kogevinas M, et al. Risk factors for asthma in young
adults: Spanish Group of the European Community Respiratory Health
Survey. Eur Respir J. 1997;10:2490–2494.
78. Grant EN, Wagner R, Weiss KB. Observations on emerging patterns of
asthma in our society. J Allergy Clin Immunol. 1999;104(2, pt 2):S-1–
S-9.
79. Koenig JQ. Air pollution and asthma. J Allergy Clin Immunol. 1999;
104(4, pt 1):717–722.
80. Custovic A, Simpson A, Woodcock A. Importance of indoor allergens
in the induction of allergy and elicitation of allergic disease. Allergy.
1998;53(48 suppl):115–20.
81. Jones AP. Asthma and the home environment. J Asthma. 2000;37:103–
124.
82. Platts-Mills TAE. Allergen avoidance. J Allergy Clin Immunol. 2004;
113:388–391.
83. Platts-Mills TAE, Ward GW Jr, Sporik R, Gelber LE, Chapman MD,
Heymann PW. Epidemiology of the relationship between exposure to
indoor allergens and asthma. Int Arch Allergy Appl Immunol. 1991;94:
339–345.
84. Gruchalla RS, Pongracic J, Plaut M, et al. Inner City Asthma Study:
relationships among sensitivity, allergen exposure, and asthma morbid-
ity. J Allergy Clin Immunol. 2005;115:478–485.
85. Platts-Mills TAE, Vervloet D, Thomas WR, Aalberse RC, Chapman
MD. Indoor allergens and asthma: report of the Third International
Workshop. J Allergy Clin Immunol. 1997;100:S-1–S-24.
86. Hirsch T, Hering M, Bu¨rkner K, et al. House-dust-mite allergen concen-
trations (Der f 1) and mold spores in apartment bedrooms before and
after installation of insulated windows and central heating systems. Al-
lergy. 2000;55:79–83.
87. Schoenwetter WF. Building a healthy house. Ann Allergy Asthma Im-
munol. 1997;79:1–4.
88. Woodfolk JA, Luczynska CH, de Blay F, Chapman MD, Platts-Mills
TAE. Cat allergy. Ann Allergy. 1992;69:273–275.

Journal of Athletic Training 241
89. Sarpong SB, Karrison T. Season of birth and cockroach allergen sensi-
tization in children with asthma. J Allergy Clin Immunol. 1998;101(4,
pt 1):566–568.
90. Kuster PA. Reducing risk of house dust mite and cockroach allergen
exposure in inner-city children with asthma. Pediatr Nurs. 1996;22:297–
303.
91. Kang BC, Wu CW, Johnson J. Characteristics and diagnosis of cock-
roach-sensitive bronchial asthma. Ann Allergy. 1992;68:237–244.
92. Liccardi G, Cazzola M, D’Amato M, D’Amato G. Pets and cockroaches:
two increasing causes of respiratory allergy in indoor environments.
Characteristics of airways sensitization and prevention strategies. Respir
Med. 2000;94:1109–1118.
93. Platt SD, Martin CJ, Hunt SM, Lewis CW. Damp housing, mould
growth, and symptomatic health state. BMJ. 1989;298:1673–1678.
94. Potter PC, Juritz J, Little F, McCaldin M, Dowdle EB. Clustering of
fungal allergen-specific IgE antibody responses in allergic subjects. Ann
Allergy. 1991;66:149–153.
95. Henderson FW, Henry MM, Ivins SS, et al. Correlates of recurrent
wheezing in school-age children: The Physicians of Raleigh Pediatric
Associates. Am J Respir Crit Care Med. 1995;151:1786–1793.
96. Gergen PJ, Turkeltaub PC. The association of individual allergen reac-
tivity with respiratory disease in a national sample: data from the second
National Health and Nutrition Examination Survey, 1976–80 (NHANES
II). J Allergy Clin Immunol. 1992;90(4, pt 1):579–588.
97. Duffy DL, Mitchell CA, Martin NG. Genetic and environmental risk
factors for asthma: a cotwin-control study. Am J Respir Crit Care Med.
1998;157(3, pt 1):840–845.
98. Omenaas E, Bakke P, Eide GE, Elsayed S, Gulsvik A. Serum house-
dust-mite antibodies and reduced FEV
1
in adults of a Norwegian com-
munity. Am J Respir Crit Care Med. 1995;152(4, pt 1):1158–1163.
99. Omenaas E, Bakke P, Eide GE, Elsayed S, Gulsvik A. Serum house dust
mite antibodies: predictor of increased bronchial responsiveness in
adults of a community. Eur Respir J. 1996;9:919–925.
100. Turner KJ, Stewart GA, Woolcock AJ, Green W, Alpers MP. Relation-
ship between mite densities and the prevalence of asthma: comparative
studies in two populations in the Eastern Highlands of Papua New Guin-
ea. Clin Allergy. 1988;18:331–340.
101. Vervloet D, Charpin D, Haddi E, et al. Medication requirements and
house dust mite exposure in mite-sensitive asthmatics. Allergy. 1991;46:
554–558.
102. Tariq SM, Matthews SM, Hakim EA, Stevens M, Arshad SH, Hide DW.
The prevalence of and risk factors for atopy in early childhood: a whole
population birth cohort study. J Allergy Clin Immunol. 1998;101:587–
593.
103. Call RS, Smith TF, Morris E, Chapman MD, Platts-Mills TAE. Risk
factors for asthma in inner city children. J Pediatr. 1992;121:862–866.
104. Platts-Mills TAE, Woodfolk JA, Chapman MD, Heymann PW. Changing
concepts of allergic disease: the attempt to keep up with real changes
in lifestyles. J Allergy Clin Immunol. 1996;98(6, pt 3):S-297–S-306.
105. Forastie´re F, Corbo GM, Michelozzi P, et al. Effects of environment and
passive smoking on the respiratory health in children. Int J Epidemiol.
1992;21:66–73.
106. Gortmaker SL, Walker DK, Jacobs FH, Ruch-Ross H. Parental smoking
and the risk of childhood asthma. Am J Public Health. 1982;72:574–
579.
107. Koenig JQ, Larson TV, Hanley QS, et al. Pulmonary function changes
in children associated with fine particulate matter. Environ Res. 1993;
63:26–38.
108. Murray B, Morrison BJ. The effect of cigarette smoke from the mother
on bronchial responsiveness and severity of symptoms in children with
asthma. J Allergy Clin Immunol. 1986;77:575–581.
109. Halken S, Host A, Nilsson L, Taudorf E. Passive smoking as a risk
factor for development of obstructive respiratory disease and allergic
sensitization. Allergy. 1995;50:97–105.
110. Siroux V, Pin I, Oryszczyn MP, Le Moual N, Kauffmann F. Relationships
of active smoking to asthma and asthma severity in the EGEA study:
Epidemiological Study on the Genetics and Environment of Asthma. Eur
Respir J. 2000;15:470–477.
111. Bar-Or O, Inbar O. Swimming and asthma: benefits and deleterious ef-
fects. Sports Med. 1992;14:397–405.
112. Massin N, Bohadana AB, Wild P, Hery M, Toamain JP, Hubert G. Re-
spiratory symptoms and bronchial responsiveness in lifeguards exposed
to nitrogen trichloride in indoor swimming pools. Occup Environ Med.
1998;55:258–263.
113. Bernard A, Carbonnelle S, Michel O, et al. Lung hyperpermeability and
asthma prevalence in schoolchildren: unexpected associations with the
attendance at indoor chlorinated swimming pools. Occup Environ Med.
2003;60:385–394.
114. Thickett KM, McCoach JS, Gerber JM, Sadhra S, Burge PS. Occupa-
tional asthma caused by chloramines in indoor swimming-pool air. Eur
Respir J. 2002:19:827–832.
115. Nemery B, Hoet PHM, Nowak D. Indoor swimming pools, water chlo-
rination and respiratory health. Eur Respir J. 2002;19:790–793.
116. Zwemer RJ, Karibo J. Use of laminar control device as adjunct to stan-
dard environmental control measures in symptomatic asthmatic children.
Ann Allergy. 1973;31:284–290.
117. Villaveces JW, Rosengren H, Evans J. Use of a laminar air flow portable
filter in asthmatic children. Ann Allergy. 1977;38:400–404.
118. Kooistra JB, Pasch R, Reed CE. The effects of air cleaners on hay fever
symptoms in air-conditioned homes. J Allergy Clin Immunol. 1978;61:
315–319.
119. Wood RA, Mudd KE, Egglestone PA. The distribution of cat and dust
mite allergens on wall surfaces. J Allergy Clin Immunol. 1992;89(1, pt
1):126–130.
120. Shirai T, Matsui T, Suzuki K, Chida K. Effect of pet removal on pet
allergic asthma. Chest. 2005;127:1565–1571.
121. Folinsbee LJ. Does nitrogen dioxide exposure increase airways respon-
siveness? Toxicol Ind Health. 1992;8:273–283.
122. Pope CA III, Dockery DW, Spengler JD, Raizenne ME. Respiratory
health and PM
10
pollution: a daily time series analysis. Am Rev Respir
Dis. 1991;144(3, pt 1):668–674.
123. Delfino RJ, Coate BD, Zeiger RS, Seltzer JM, Street DH, Koutrakis P.
Daily asthma severity in relation to personal ozone exposure and outdoor
fungal spores. Am J Respir Crit Care Med. 1996;154(3, pt 1):633–641.
124. Delfino RJ, Zeiger RS, Seltzer JM, Street DH. Symptoms in pediatric
asthmatics and air pollution: differences in effects by symptoms severity,
anti-inflammatory medication use and particulate averaging time. Envi-
ron Health Perspect. 1998;106:751–761.
125. Koenig JQ, Pierson WE, Horike M, Frank R. Effects of SO
2
plus NaCl
aerosol combined with moderate exercise on pulmonary function in asth-
matic adolescents. Environ Res. 1981;25:340–348.
126. Suphioglu C, Singh MB, Taylor P, et al. Mechanism of grass-pollen-
induced asthma. Lancet. 1992;339:569–572.
127. Helenius I, Haahtela T. Allergy and asthma in elite summer sport ath-
letes. J Allergy Clin Immunol. 2000;106:444–452.
128. Solomon WR. Airborne pollen prevalence in the United States. In:
Grammer LC, Greenberger PA, eds. Patterson’s Allergic Diseases. Phil-
adelphia, PA: Lippincott Williams & Wilkins; 2002:131–144.
129. D’Amato G, Spieksma FT, Liccardi G, et al. Pollen-related allergy in
Europe. Allergy. 1998;53:567–578.
130. Rundell KW, Spiering BA, Evans TM, Baumann JM. Baseline lung
function, exercise-induced bronchoconstriction, and asthma-like symp-
toms in elite women ice hockey players. Med Sci Sports Exerc. 2004;
36:405–410.
131. Lung function testing: selection of reference values and interpretative
strategies. American Thoracic Society. Am Rev Respir Dis. 1991;144:
1202–1218.
132. Ferguson GT, Enright PL, Buist AS, Higgins MW. Office spirometry for
lung health assessment in adults: a consensus statement from the Na-
tional Lung Health Education Program. Respir Care. 2000;45:513–530.
133. Guidelines for the measurement of respiratory function: recommenda-
tions of the British Thoracic Society and the Association of Respiratory
Technicians and Physiologists. Respir Med. 1994;88:165–194.
134. Crapo RO, Morris AH, Gardner RM. Reference spirometric values using
techniques and equipment that meet ATS recommendations. Am Rev
Respir Dis. 1981;123:659–664.

242 Volume 40
•
Number 3
•
September 2005
135. Polgar G, Promadhat V. Pulmonary Function Testing in Children. Phil-
adelphia, PA: WB Saunders; 1971.
136. Standardization of spirometry: 1994 update. American Thoracic Society.
Am Respir Crit Care Med. 1995;152:1107–1136.
137. Koyama H, Nishimura K, Ikeda A, Tsukino M, Izumi T. Comparison of
four types of portable peak flow meters (Mini-Wright, Assess, Pulmo-
graph and Wright Pocket meters). Respir Med. 1998;92:505–511.
138. Kelly CA, Gibson GJ. Relation between FEV
1
and peak expiratory flow
in patients with chronic airflow obstruction. Thorax. 1988;43:335–336.
139. Ladebauche P. Peak flow meters: child’s play. Office Nurse. 1996:22–
28.
140. Li JTC. Do peak flow meters lead to better asthma control? J Respir
Dis. 1995;16:381–398.
141. Ignacio-Garcia JM, Gonzales-Santos P. Asthma self-management edu-
cation program by home monitoring of peak expiratory flow. Am J Res-
pir Crit Care Med. 1995;151:353–359.
142. Measuring peak flow at home. Drug Ther Bull. 1991;29:87–88.
143. Lahdensuo A, Haahtela T, Herrala J, et al. Randomised comparison of
cost effectiveness of guided self management and traditional treatment
of asthma in Finland. BMJ. 1998;316:1138–1139.
144. Lahdensuo A, Haahtela T, Herrala J, et al. Randomised comparison of
guided self management and traditional treatment of asthma over one
year. BMJ. 1996;312:748–752.
145. Wagner MH, Jacobs J. Improving asthma management with peak flow
meters. Contemp Pediatr. 1997;14:111–119.
146. Bheekie A, Syce JA, Weinberg EG. Peak expiratory flow rate and symp-
tom self monitoring of asthma initiated from community pharmacies. J
Clin Pharm Ther. 2001;26:287–296.
147. Cowie RL, Revitt SG, Underwood MF, Field SK. The effect of a peak
flow-based action plan in the prevention of exacerbations of asthma.
Chest. 1997;112:1534–1538.
148. Rachelefsky G, Fitzgerald S, Page D, Santamaria B. An update on the
diagnosis and management of pediatric asthma: based on the National
Heart, Lung, and Blood Institute expert panel report. Nurse Pract. 1993;
18:51–62.
149. Killian KJ, Watson R, Otis J, St Amand TA, O’Bryne PM. Symptom
perception during acute bronchoconstriction. Am J Respir Crit Care
Med. 2000;162(2, pt 1):490–496.
150. Kendrick AH, Higgs CMB, Whitfield MJ, Laszlo G. Accuracy of per-
ception of severity of asthma: patients treated in general practice. BMJ.
1993;307:422–424.
151. Klements EM. Monitoring peak flow rates as a health-promoting behav-
ior in managing and improving asthma. Clin Excell Nurse Pract. 2001;
5:147–151.
152. Malo JL, L’Archeveˆque J, Trudeau C, d’Aquino C, Cartier A. Should
we monitor peak expiratory flow rates or record symptoms with a simple
diary in the management of asthma? J Allergy Clin Immunol. 1993;91:
702–709.
153. Verschelden P, Cartier A, L’Archeveˆque J, Trudeau C, Malo JL. Com-
pliance with and accuracy of daily self-assessment of peak expiratory
flows (PEF) in asthmatic subjects over a three month period. Eur Respir
J. 1996;9:880–885.
154. Sawyer G, Miles J, Lewis S, Fitzharris P, Pearce N, Beasley R. Classi-
fication of asthma severity: should the international guidelines be
changed? Clin Exp Allergy. 1998;28:1565–1570.
155. Eid N, Yandell B, Howell L, Eddy M, Sheikh S. Can peak expiratory
flow predict airflow obstruction in children with asthma? Pediatrics.
2000;105:354–358.
156. Brand PL, Duiverman EJ, Waalkens HJ, van Essen-Zandvliet EE, Ker-
rebijn KF. Peak flow variation in childhood asthma: correlation with
symptoms, airways obstruction, and hyperresponsiveness during long
term treatment with inhaled corticosteroids: Dutch CNSLD Study. Tho-
rax. 1999;54:103–107.
157. Folgering H, vd Brink W, v Heeswijk O, v Herwaarden C. Eleven peak
flow meters: a clinical evaluation. Eur Respir J. 1998;11:188–193.
158. Jain P, Kavuru MS. Peak expiratory flow vs spirometry in a patient with
asthma. Respir Care. 2000;45:969–970.
159. Nolan S, Tolley E, Leeper K, Strayhorn Smith V, Self T. Peak expiratory
flow associated with change in positioning of the instrument: comparison
of five peak flow meters. J Asthma. 1999;36:291–294.
160. Connolly CK, Murthy NK, Tighe E, Alcock S, Harding T. Mini and
Wright peak flow meters. Thorax. 1991;46:289P.
161. Dickinson SA, Hitchins DJ, Miller MR. Accuracy of measurement of
peak flow using different peak flow meters. Thorax. 1991;46:289P.
162. Jain P, Kavuru MS. Peak expiratory flow vs spirometry in a patient with
asthma. Respir Care. 2000;45:969–970.
163. Gardner RM, Crapo RO, Jackson BR, Jensen RL. Evaluation of accu-
racy and reproducibility of peak flow meters at 1,400 m. Chest. 1992;
101:948–952.
164. Chafin CC, Tolley E, George C, et al. Are there gender differences in
the use of peak flow meters? J Asthma. 2001;38:541–543.
165. den Otter JJ, Knitel M, Akkermans RPM, Van Schayck CP, Folgering
HTM, van Weel C. Spirometry in general practice: the performance of
practice assistants scored by lung function technicians. Br J Gen Pract.
1997;47:41–42.
166. Flood-Page P, Barnes NC. What are the alternatives to increasing inhaled
corticosteroids for the long term control of asthma? BioDrugs. 2001;15:
185–198.
167. Spector SL, Kinsman R, Mawhinney H, et al. Compliance of patients
with asthma with an experimental aerosolized medication: implications
for controlled clinical trials. J Allergy Clin Immunol. 1986;77(1, pt 1):
65–70.
168. Jeffery PK, Godfrey RW, A
¨
delroth E, Nelson F, Rogers A, Johansson
SA. Effects of treatment on airway inflammation and thickening of base-
ment membrane reticular collagen in asthma: a quantitative light and
electron microscopic study. Am Rev Respir Dis. 1992;145(4, pt 1):890–
899.
169. Djukanovic´ R, Wilson JW, Britten KM, et al. Effect of an inhaled cor-
ticosteroid on airway inflammation and symptoms in asthma. Am Rev
Respir Dis. 1992;145:669–674.
170. Laitinen LA, Laitinen A, Haahtela T. A comparative study of the effects
of an inhaled corticosteroid, budesonide, and a b
2
-agonist, terbutaline,
on airway inflammation in newly diagnosed asthma: a randomized, dou-
ble-blind, parallel-group controlled trial. J Allergy Clin Immunol. 1992;
90:32–42.
171. van Essen-Zandvliet EE, Hughes MD, Waalkens HJ, Duiverman EJ, Po-
cock SJ, Kerrebijn KF. Effects of 22 months of treatment with inhaled
corticosteroids and/or beta2-agonists on lung function, airway respon-
siveness, and symptoms in children with asthma: the Dutch Chronic
Non-Specific Lung Disease Study Group. Am Rev Respir Dis. 1992;146:
547–554.
172. Munck A, Mendel DB, Smith LI, Orti E. Glucocorticoid receptors and
actions. Am Rev Respir Dis. 1990;141(2, pt 2):S-2–S-10.
173. Schleimer RP. Effects of glucocorticosteroids on inflammatory cells rel-
evant to their therapeutic applications in asthma. Am Rev Respir Dis.
1990;141(2, pt 2):S-59–S-69.
174. Shelhamer JH, Levine SJ, Wu T, Jacoby DB, Kaliner MA, Rennard SL.
NIH conference: airway inflammation. Ann Intern Med. 1995;123:288–
304.
175. Haahtela T, Ja¨rvinen M, Kava T, et al. Comparison of a b
2
-agonist,
terbutaline, with an inhaled corticosteroid, budesonide, in newly detected
asthma. N Engl J Med. 1991;325:388–392.
176. Barnes PJ, Pedersen S, Busse WW. Efficacy and safety of inhaled cor-
ticosteroids. New developments. Am J Respir Crit Care Med. 1998;
157(3, pt 2):S-1–S-53.
177. Barnes PJ, Pedersen S. Efficacy and safety of inhaled corticosteroids in
asthma: report of a workshop held in Eze, France, October 1992. Am
Rev Respir Dis. 1993;148(4, pt 2):S-1–S-26.
178. Long-term effects of budesonide or nedocromil in children with asthma:
The Childhood Asthma Management Program Research Group. N Engl
J Med. 2000;343:1054–1063.
179. Greenberger PA, Tatum AJ, D’Epiro P. Asthma: acute and long-term
concerns. Patient Care. 1999;15:154–174.
180. Bleecker ER, Welch MJ, Weinstein SF, et al. Low-dose inhaled flutica-
sone propionate versus oral zafirlukast in the treatment of persistent
asthma. J Allergy Clin Immunol. 2000;105:1123–1129.
181. Williamson IJ, Matusiewicz SP, Brown PH, Greening AP, Crompton GK.

Journal of Athletic Training 243
Frequency of voice problems and cough in patients using pressurized
aerosol inhaled steroid preparations. Eur Respir J. 1995;8:590–592.
182. Mash B, Bheekie A, Jones PW. Inhaled versus oral steroids for adults
with chronic asthma (Cochrane Review). In: The Cochrane Library. Is-
sue 4. Chichester, UK: John Wiley and Sons, Ltd: 2003.
183. Dunlap NE, Fulmer JD. Corticosteroid therapy in asthma. Clin Chest
Med. 1984;5:669–683.
184. Serafin WE. Drugs used in the treatment of asthma. In: Hardman JG,
Limbird LE, Molinoff PB, Ruddon RW, Gilman AG, eds. The Phar-
macological Basis of Therapeutics. 9th ed. New York, NY: McGraw-
Hill; 1996:659–682.
185. Nelson HS. b-adrenergic bronchodilators. N Engl J Med. 1995;333:499–
506.
186. Boulet LP. Long versus short-acting beta 2-agonists: implications for
drug therapy. Drugs. 1994;47:207–222.
187. Pearlman DS, Chervinsky P, LaForce C, et al. A comparison of salme-
terol with albuterol in the treatment of mild-to-moderate asthma. N Engl
J Med. 1992;327:1420–1425.
188. Kesten S, Chapman KR, Broder I, et al. A three-month comparison of
twice daily inhaled formoterol versus four times daily inhaled albuterol
in the management of stable asthma. Am Rev Respir Dis. 1991;144:622–
625.
189. Anderson SD, Brannan JD. Long-acting b
2
-adrenoceptor agonists and
exercise-induced asthma: lessons to guide us in the future. Paediatr
Drugs. 2004;6:161–175.
190. Wenzel SE, Lumry W, Manning M, et al. Efficacy, safety, and effects
on quality of life of salmeterol versus albuterol in patients with mild to
moderate persistent asthma. Ann Allergy Asthma Immunol. 1998;80:463–
470.
191. Selroos O. Formoterol used as needed: clinical effectiveness. Respir
Med. 2001;95:17–20.
192. Richter K, Jamicki S, Jorres RA, Magnussen H. Acute protection against
exercise-induced bronchoconstriction by formoterol, salmeterol, and ter-
butaline. Eur Respir J. 2002;19(suppl B):865–871.
193. Bronsky EA, Yegen U
¨
, Yeh CM, Larsen LV, Della Cioppa G. Formoterol
provides long-lasting protection against exercise-induced bronchospasm.
Ann Allergy Asthma Immunol. 2002;89:417–412.
194. Ferrari M, Segattini C, Zanon R, et al. Comparison of the protective
effect of formoterol and of salmerterol against exercise-induced bron-
chospasm when given immediately before a cycloergometric test. Res-
piration. 2002;69:509–512.
195. Hancox RJ, Subbarao P, Kamada D, Watson RM, Hargreave FE, Inman
MD. Beta2-agonist tolerance and exercise-induced bronchospasm. Am J
Respir Crit Care Med. 2002;165:1068–1070.
196. Ramage L, Lipworth BJ, Ingram CG, Cree IA, Dhillon DP. Reduced
protection against exercise induced bronchoconstriction after chronic
dosing with salmerterol. Respir Med. 1994;88:363–368.
197. Nelson JA, Strauss L, Skowronski M, Ciufo R, Novak R, McFadden ER
Jr. Effect of long-term salmeterol treatment on exercise-induced asthma.
N Engl J Med. 1998;339:141–146.
198. Garcia R, Guerra P, Feo F, et al. Tachyphylaxis following regular use of
formoterol in exercise-induced bronchospasm. J Investig Allergol Clin
Immunol. 2001;11:176–182.
199. Gardiner PV, Ward C, Booth H, Allison A, Hendrick DJ, Walters EH.
Effect of eight weeks of treatment with salmeterol on bronchoalveolar
lavage inflammatory indices in asthmatics. Am J Respir Crit Care Med.
1994;150:1006–1011.
200. Lemanske RF, Sorkness CA, Mauger EA, et al. Inhaled corticosteroid
reduction and elimination in patients with persistent asthma receiving
salmeterol: a randomized controlled trial. JAMA. 2001;285:2594–2603.
201. Lazarus SC, Boushey HA, Fahy JV, et al. Long-acting b
2
-agonist mono-
therapy vs continued therapy with inhaled corticosteroids in patients
with persistent asthma: a randomized controlled trial. JAMA. 2001;285:
2583–2593.
202. Weiler JM, Nathan RA, Rupp NT, Kalberg CJ, Emmett A, Dorinsky
PM. Effect of fluticasone/salmeterol administered via a single device on
exercise-induced bronchospasm in patients with persistent asthma. Ann
Allergy Asthma Immunol. 2005;94:65–72.
203. Shrewsbury S, Pyke S, Britton M. Meta-analysis of increased dose of
inhaled steroid or addition of salmeterol in symptomatic asthma (MI-
ASMA). BMJ. 2000;320:1368–1373.
204. Pauwels RA, Lo¨fdahl CG, Postma DS, et al. Effect of inhaled formoterol
and budesonide on exacerbations of asthma: Formoterol and Corticoste-
roids Establishing Therapy (FACET) International Study Group. N Engl
J Med. 1997;337:1405–1411.
205. Holimon TD, Chafin CC, Self TH. Nocturnal asthma uncontrolled by
inhaled corticosteroids: theophylline or long-acting beta2 agonists?
Drugs. 2001;61:391–418.
206. Montelukast for persistent asthma. Med Lett Drugs Ther. 1998;40:71–
73.
207. Stempel DA. Leukotriene modifiers in the treatment of asthma. Respir
Care. 1998;43:481–489.
208. O’Byrne PM, Israel E, Drazen JM. Antileukotreines in the treatment of
asthma. Ann Intern Med. 1997;127:472–480.
209. Lipworth BJ. Leukotriene-receptor antagonists. Lancet. 1999;353:57–62.
210. Drazen JM, Israel E, O’Byrne PM. Treatment of asthma with drugs
modifying the leukotriene pathway. N Engl J Med. 1999;340:197–206.
211. Drazen JM. Asthma therapy with agents preventing leukotriene synthesis
or action. Proc Assoc Am Physicians. 1999;111:547–559.
212. Dahle´n B, Margolskee DJ, Zetterstro¨m O, Dahle´n S. Effect of the leu-
kotriene receptor antagonist MK-0679 on baseline pulmonary function
in aspirin sensitive asthmatic subjects. Thorax. 1993;48:1205–1210.
213. Barnes NC, Miller CJ. Effect of leukotriene receptor antagonist therapy
on the risk of asthma exacerbations in patients with mild to moderate
asthma: an integrated analysis of zafirlukast trials. Thorax. 2000;55:478–
483.
214. Christian Virchow J, Prasse A, Naya I, Summerton L, Harris A. Zafir-
lukast improves asthma control in patients receiving high-dose inhaled
corticosteroids. Am J Respir Crit Care Med. 2000;162:578–585.
215. Leff JA, Busse WW, Pearlman D, et al. Montelukast, a leukotriene-
receptor antagonist, for the treatment of mild asthma and exercise-in-
duced bronchoconstriction. N Engl J Med. 1998;339:147–152.
216. Laviolette M, Malmstrom K, Lu S, et al. Montelukast added to inhaled
beclomethasone in treatment of asthma: Montelukast/Beclomethasone
Additivity Group. Am J Respir Crit Care Med. 1999;160:1862–1868.
217. Lo¨fdahl CG, Reiss TF, Leff JA, et al. Randomised, placebo controlled
trial of effect of a leukotriene receptor antagonist, montelukast, on ta-
pering inhaled corticosteroids in asthmatic patients. BMJ. 1999;319:87–
90.
218. Joshi EM, Heasley BH, Chordia MD, Macdonald TL. In vitro metabo-
lism of 2-acetylbenzothiophene: relevance to zileuton hepatotoxicity.
Chem Res Toxicol. 2004;17:137–143.
219. Ukena D, Harnest U, Sakalauskas R, et al. Comparison of addition of
theophylline to inhaled steroid with doubling of the dose of inhaled
steroid in asthma. Eur Respir J. 1997;10:2754–2760.
220. Norman PS. Immunotherapy: 1999–2004. J Allergy Clin Immunol. 2004;
113:1013–1023.
221. Stokes J, Casale TB. Rationale for new treatments aimed at IgE im-
munomodulation. Ann Allergy Asthma Immunol. 2004;93:212–217.
222. Creticos PS, Chen YH, Schroeder JT. New approaches in immunother-
apy: allergen vaccination with immunostimulatory DNA. Immunol Al-
lergy Clin North Am. 2004;24:569–581.
223. Godfrey S, Bar-Yishay E. Exercise-induced asthma revisited. Respir
Med. 1993;87:331–344.
224. Holzer K, Brukner P, Douglass J. Evidence-based management of ex-
ercise-induced asthma. Curr Sports Med Rep. 2002;1:86–92.
225. Sears MR, Lotvall J. Past, present and future–beta2-adrenoceptor ago-
nists in asthma management. Respir Med. 2005;99:152–170.
226. Kno¨pfli BH, Bar-Or O, Arau´go CG. Effect of ipratropium bromide on
EIB in children depends on vagal activity. Med Sci Sports Exerc. 2005;
37:354–359.
227. Weinberger M, Hendeles L. Theophylline in asthma. N Engl J Med.
1996;334:1380–1388.
228. Sessler CN. Theophylline toxicity: clinical features of 116 consecutive
cases. Am J Med. 1990;88:567–576.
229. Dozor AJ. Spacer crusader. Lancet. 2005;365:572.
230. Newman SP. Spacer devices for metered dose inhalers. Clin Pharma-
cokinet. 2004;43:349–60.

244 Volume 40
•
Number 3
•
September 2005
231. Newman KB, Milne S, Hamilton C, Hall K. A comparison of albuterol
administered by metered-dose inhaler and spacer with albuterol by neb-
ulizer in adults presenting to an urban emergency department with acute
asthma. Chest. 2002;121:1036–1041.
232. Stevenson DD. Aspirin and NSAID sensitivity. Immunol Allergy Clin
North Am. 2004;24:491–505.
233. Namazy JA, Simon RA. Sensitivity to nonsteroidal anti-inflammatory
drugs. Ann Allergy Asthma Immunol. 2002;89:542–550.
234. Jawien J. A new insight into aspirin-induced asthma. Eur J Clin Invest.
2002;32:134–138.
235. de Almeida MA, Gaspar AP, Carvalho FS, Nogueira JM, Pinto JE. Ad-
verse reactions to acetaminophen, ASA, and NSAIDs in children: what
alternatives? Allergy Asthma Proc. 1997;18:313–318.
236. Vaszar LT, Stevenson DD. Aspirin-induced asthma. Clin Rev Allergy
Immunol. 2001;21:71–87.
237. Rivas B. The management of ASA syndrome. J Invest Allergol Clin
Immunol. 1997;7:392–396.
238. Hedman J, Kaprio J, Poussa T, Nieminen MM. Prevalence of asthma,
aspirin intolerance, nasal polyposis and chronic obstructive pulmonary
disease in a population-based study. Int J Epidemiol. 1999;28:717–722.
239. Levy S, Volans G. The use of analgesics in patients with asthma. Drug
Safe. 2001;24:829–841.
240. Szczeklik A, Stevenson DD. Aspirin-induced asthma: advances in path-
ogenesis and management. J Allergy Clin Immunol. 1999;104:5–13.
241. Settipane RA, Stevenson DD. Cross sensitivity with acetaminophen in
aspirin-sensitive subjects with asthma. J Allergy Clin Immunol. 1989;
84:26–33.
242. Szczeklik A, Stevenson DD. Aspirin-induced asthma: advances in path-
ogenesis, diagnosis, and management. J Allergy Clin Immunol. 2003;
111:913–921.
243. Mellion MB, Kobayashi RH. Exercise-induced asthma. Am Fam Phy-
sician. 1992;45:2671–2677.
244. Rupp NT. Diagnosis and management of exercise-induced asthma. Phy-
sician Sportsmed. 1996;24(1):77–87.
245. Baker WH, Weiler JM. The role of exercise training and conditioning
in patients with asthma. In: Weiler JM, ed. Allergic and Respiratory
Disease in Sports Medicine. New York, NY: Marcel Dekker; 1997:179–
206.
246. Cochrane LM, Clark CJ. Benefits and problems of a physical training
programme for asthmatic patients. Thorax. 1990;45:345–351.
247. Ben Dov I, Bar-Yishay E, Godfrey S. Refractory period after exercise-
induced asthma unexplained by respiratory heat loss. Am Rev Respir
Dis. 1982;125:530–534.
248. Belcher NG, O’Hickey S, Arm JP, Lee TH. Pathogenetic mechanisms
of exercise-induced asthma and the refractory period. N Engl Reg Al-
lergy Proc. 1988;9:199–201.
249. McKenzie DC, McLuckie SL, Stirling DR. The protective effects of
continuous and interval exercise in athletes with exercise-induced asth-
ma. Med Sci Sports Exerc. 1994;26:951–956.
250. Morton AR, Fitch KD, Davis T. The effect of ‘‘warm-up’’ on exercise-
induced asthma. Ann Allergy. 1979;42:257–260.
251. Schnall RP, Landau LI. Protective effects of repeated short sprints in
exercise-induced asthma. Thorax. 1980;35:828–832.
252. Mickleborough TD, Gotshall RW, Cordain L, Lindley M. Dietary salt
alters pulmonary function during exercise in exercise-induced asthmat-
ics. J Sports Sci. 2001;19:865–873.
253. Gotshall RW, Mickleborough TD, Cordain L. Dietary salt restriction
improves pulmonary function in exercise-induced asthma. Med Sci
Sports Exerc. 2000;32:1815–1819.
254. Mickleborough TD, Gotshall RW, Kluka EW, Miller CW, Cordian L.
Dietary chloride as a possible determinant of the severity of exercise-
induced asthma. Eur J Appl Physiol. 2001;85:450–456.
255. Mickleborough TD, Murray RL, Ionescu AA, Lindley MR. Fish oil sup-
plementation reduces severity of exercise-induced bronchoconstriction
in elite athletes. Am J Respir Crit Care Med. 2003;168:1181–1189.
256. Weiler JM, ed. Allergic and Respiratory Disease in Sports Medicine.
New York, NY: Marcel Dekker; 1997.
257. McFadden ER Jr, ed. Exercise-Induced Asthma. New York, NY: Marcel
Dekker; 1999.
258. Homnick DN, Marks JH. Exercise and sports in the adolescent with
chronic pulmonary disease. Adolesc Med. 1998;9:467–481.
259. Milgrom H, Taussig LM. Keeping children with exercise-induced asth-
ma active. Pediatrics. 1999;104:38–42.
260. Langdeau JB, Boulet LP. Prevalence and mechanisms of development
of asthma and airway hyperresponsiveness in athletes. Sports Med.
2001;31:601–616.
261. Sudhir P, Prasad CE. Prevalence of exercise-induced bronchospasm in
schoolchildren: an urban-rural comparison. J Trop Pediatr. 2003;49:
104–108.
262. Weiler JM, Layton T, Hunt M. Asthma in United States Olympic athletes
who participated in the 1996 Summer Games. J Allergy Clin Immunol.
1998;102:722–726.
263. Weiler JM, Ryan EJ III. Asthma in United States Olympic athletes who
participated in the 1998 Olympic Winter Games. J Allergy Clin Immu-
nol. 2000;106:267–271.
264. Storms WW. Review of exercise-induced asthma. Med Sci Sports Exerc.
2003;35:1464–1470.
265. Wilber RL, Rundell KW, Szmedra L, Jenkinson DM, Im J, Drake SD.
Incidence of exercise-induced bronchospasm in Olympic winter sport
athletes. Med Sci Sports Exerc. 2000;32:732–737.
266. Rundell KW, Jenkinson DM. Exercise-induced bronchospasm in the elite
athlete. Sports Med. 2002;32:583–600.
267. Feinstein RA, LaRussa J, Wang-Dohlman A, Bartolucci AA. Screening
adolescent athletes for exercise-induced asthma. Clin J Sport Med. 1996;
6:119–123.
268. Tan RA, Spector SL. Exercise-induced asthma. Sports Med. 1998;25:1–6.
269. Spector SL. Update on exercise-induced asthma. Ann Allergy. 1993;71:
571–577.
270. Bierman EW, Kawabori I, Pierson WE. Incidence of exercise-induced
asthma in children. Pediatrics. 1975;56(5, pt 2 suppl.):847–850.
271. Kovan JR, Mackowiak TJ. Exercise-induced asthma. Athl Ther Today.
2001;6(5):22–25, 34–35,64.
272. McConnell R, Berhane K, Gilliland F, et al. Asthma in exercising chil-
dren exposed to ozone: a cohort study. Lancet. 2002;359:386–391.
273. Peter MM, Silvers WS, Thompson FL, Weiler JM. Case studies of asth-
ma in elite and world-class athletes: the roles of the athletic trainer and
physician. In: Weiler JM, ed. Allergic and Respiratory Disease in Sports
Medicine. New York, NY: Marcel Dekker; 1997:353–375.
274. Karjalainen EM, Laitinen A, Sue-Chu M, Altraja A, Bjermer L, Laitinen
LA. Evidence of airway inflammation and remodeling in ski athletes
with and without bronchial hyperresponsiveness to methacholine. Am J
Respir Crit Care Med. 2000;161:2086–91.
275. Larsson K, Ohlse´n P, Larsson L, Malmberg P, Rydstro¨m P-O, Ulriksen
H. High prevalence of asthma in cross country skiers. BMJ. 1993;307:
1326–1329.
276. Anderson SD, Daviskas E. Pathophysiology of exercise-induced asthma:
the role of respiratory water loss. In: Weiler JM, ed. Allergic and Re-
spiratory Disease in Sports Medicine. New York, NY: Marcel Dekker;
1997:87–114.
277. Anderson SD, Holzer K. Exercise-induced asthma: is it the right diag-
nosis in elite athletes? J Allergy Clin Immunol. 2000;106:419–428.
278. McFadden ER Jr, Nelson JA, Skowronski ME, Lenner KA. Thermally
induced asthma and airway drying. Am J Respir Crit Care Med. 1999;
160:221–226.
279. Makker HK, Holgate ST. Pathophysiology: role of mediators in exercise-
induced asthma. In: Weiler JM, ed. Allergic and Respiratory Disease in
Sports Medicine. New York, NY: Marcel Dekker; 1997:115–136.
280. Anderson SD, Daviskas E. The mechanism of exercise-induced asthma
is... J Allergy Clin Immunol. 2000;106:453–459.
281. Deal EC Jr., McFadden ER Jr, Ingram RH Jr, Strauss RH, Jaeger JJ.
Role of respiratory heat exchange in production of exercise-induced
asthma. J Appl Physiol. 1979;46:467–475.
282. Chen WY, Horton DJ. Heat and water loss from the airways and exer-
cise-induced asthma. Respiration. 1977;34:305–313.
283. Gilbert IA, McFadden ER Jr. Airway cooling and rewarming: the second
reaction sequence in exercise-induced asthma. J Clin Invest. 1992;90:
699–704.

Journal of Athletic Training 245
284. Anderson SD, Kippelen P. Exercise-induced bronchoconstriction: path-
ogenesis. Curr Allergy Asthma Rep. 2005;5:116–122.
285. McFadden ER Jr, Lenner KA, Strohl KP. Postexertional airway rewarm-
ing and thermally induced asthma: new insights into pathophysiology
and possible pathogenesis. J Clin Invest. 1986;78:18–25.
286. Smith CM, Anderson SD, Walsh S, McElrea MS. An investigation of
the effects of heat and water exchange in the recovery period after ex-
ercise in children with asthma. Am Rev Respir Dis. 1989;140:598–605.
287. McFadden ER Jr, Gilbert IA. Exercise-induced asthma. N Engl J Med.
1994;330:1362–1367.
288. Beck KC. Control of airway function during and after exercise in asth-
matics. Med Sci Sports Exerc. 1999;31:S-4–S-11.
289. Makker HK, Holgate ST. Mechanisms of exercise-induced asthma. Eur
J Clin Invest. 1994;24:571–585.
290. Furuichi S, Hashimoto S, Gon Y, Matsumoto K, Horie T. p38 mitogen-
activated protein kinase and C-Jun-NH2 terminal kinase regulate inter-
leukin-8 and RANTES production in hyperosmolarity stimulated human
bronchial epithelial cells. Respirology. 2002;7:193–200.
291. Rundell KW. Pulmonary function decay in women ice hockey players:
is there a relationship to ice rink air quality? Inhal Toxicol. 2004;16:
117–123.
292. Zainudin NM, Aziz BA, Haifa AL, Deng CT, Omar AH. Exercise-in-
duced bronchoconstriction among Malay schoolchildren. Respirology.
2001;6:151–155.
293. Jones A, Bowen M. Screening for childhood asthma using an exercise
test. Br J Gen Pract. 1994;44:127–131.
294. Vianne EO, Boaventura LC, Terra-Filho J, Nakama GY, Martinez JAB,
Martinez RJ. Morning-to-evening variation in exercise-induced bron-
chospasm. J Allergy Clin Immunol. 2002;110:236–240.
295. Anderson SD. Exercise-induced asthma and the use of hypertonic saline
aerosol as a bronchial challenge. Respirology. 1996;3:175–181.
296. Tilles SA. Vocal cord dysfunction in children and adolescents. Curr
Allergy Asthma Rep. 2003;3:467–472.
297. Rundell KW, Spiering BA. Inspiratory stridor in elite athletes. Chest.
2003;123:468–474.
298. Wilkerson LA. Exercise-induced asthma. J Am Osteopath Assoc. 1998;
98:211–215.
299. Smith BW, LaBotz M. Pharmacologic treatment of exercise-induced
asthma. Clin Sports Med. 1998;17:343–363.
300. Fowler C. Preventing and managing exercise-induced asthma. Nurse
Pract. 2001;26:25–33.
301. Kemp JP, Dockhorn RJ, Busse WW, Bleecker ER, Van As A. Prolonged
effect of inhaled salmeterol against exercise-induced bronchospasm. Am
J Respir Crit Care Med. 1994;150(6 Pt 1):1612–1615.
302. Vathenen AS, Knox AJ, Wisniewski A, Tattersfield AE. Time course of
change in bronchial reactivity with an inhaled corticosteroid in asthma.
Am Rev Respir Dis. 1991;143:1317–1321.
303. Nastasi KJ, Heinly TL, Blaiss MS, Exercise-induced asthma and the
athlete. J Asthma. 1995;32:249–257.
304. Finch L. Asthma education is key. J Respir Care Pract. 2000:85–116.
305. Dawson KP, Van Asperen P, Higgins C, Sharpe C, Davis A. An evalu-
ation of the action plans of children with asthma. J Paediatr Child
Health. 1995;31:21–23.
