
Toxicological Profile for
Vinyl Chloride
January 2024
VINYL CHLORIDE ii
DISCLAIMER
Use of trade names is for identification only and does not imply endorsement by the Agency for Toxic
Substances and Disease Registry, the Public Health Service, or the U.S. Department of Health and Human
Services.

VINYL CHLORIDE iii
FOREWORD
This toxicological profile is prepared in accordance with guidelines* developed by the Agency for Toxic
Substances and Disease Registry (ATSDR) and the Environmental Protection Agency (EPA). The
original guidelines were published in the Federal Register on April 17, 1987. Each profile will be revised
and republished as necessary.
The ATSDR toxicological profile succinctly characterizes the toxicologic and adverse health effects
information for these toxic substances described therein. Each peer-reviewed profile identifies and
reviews the key literature that describes a substance's toxicologic properties. Other pertinent literature is
also presented, but is described in less detail than the key studies. The profile is not intended to be an
exhaustive document; however, more comprehensive sources of specialty information are referenced.
The focus of the profiles is on health and toxicologic information; therefore, each toxicological profile
begins with a relevance to public health discussion which would allow a public health professional to
make a real-time determination of whether the presence of a particular substance in the environment
poses a potential threat to human health. The adequacy of information to determine a substance's health
effects is described in a health effects summary. Data needs that are of significance to the protection of
public health are identified by ATSDR.
Each profile includes the following:
(A) The examination, summary, and interpretation of available toxicologic information and
epidemiologic evaluations on a toxic substance to ascertain the levels of significant
human exposure for the substance due to associated acute-, intermediate-, and chronic-
duration exposures;
(B) A determination of whether adequate information on the health effects of each substance
is available or in the process of development to determine levels of exposure that present
a significant risk to human health of acute, intermediate, and chronic health effects; and
(C) Where appropriate, identification of toxicologic testing needed to identify the types or
levels of exposure that may present significant risk of adverse health effects in humans.
The principal audiences for the toxicological profiles are health professionals at the Federal, State, and
local levels; interested private sector organizations and groups; and members of the public.
This profile reflects ATSDR’s assessment of all relevant toxicologic testing and information that has been
peer-reviewed. Staffs of the Centers for Disease Control and Prevention and other Federal scientists have
also reviewed the profile. In addition, this profile has been peer-reviewed by a nongovernmental panel
and was made available for public review. Final responsibility for the contents and views expressed in
this toxicological profile resides with ATSDR.
Christopher M. Reh, Ph.D.
Associate Director
Agency for Toxic Substances and Disease Registry
Centers for Disease Control and Prevention

VINYL CHLORIDE iv
VERSION HISTORY
Date
Description
January 2024
Final toxicological profile released
January 2023
Draft for public comment toxicological profile released
July 2006
Final toxicological profile released
September 1997
Final toxicological profile released
April 1993
Final toxicological profile released
August 1989
Final toxicological profile released
VINYL CHLORIDE v
CONTRIBUTORS & REVIEWERS
CHEMICAL MANAGER TEAM
Rae T. Benedict, Ph.D. (Lead)
Brittany Szafran, D.V.M., Ph.D.
Julie Melia, Ph.D., D.A.B.T.
Deborah Herber, Ph.D.
Jenny S. Crisman, B.S.
Parker Honey, B.S.
Sabah Tariq, M.S.P.H.
Christina Coley, M.S.
Mario Citra, Ph.D.
Kambria Haire, M.P.H., Ph.D.
ATSDR, Office of Innovation and Analytics,
Toxicology Section, Atlanta, GA
SRC, Inc., North Syracuse, NY
REVIEWERS
Interagency Minimal Risk Level Workgroup:
Includes ATSDR; National Center for Environmental Health (NCEH); National Institute for
Occupational Safety and Health (NIOSH); U.S. Environmental Protection Agency (EPA); National
Toxicology Program (NTP).
Additional reviews for science and/or policy:
ATSDR, Office of Community Health Hazard Assessment; ATSDR,
Office of Capacity Development
and Applied Prevention Science; ATSDR, Office of Science; NCEH, Division of Laboratory Sciences;
NCEH, Division of Environmental Health Science and Practice; EPA, Office of Research and
Development; EPA, Office of Water.
PEER REVIEWERS
1. Ivan Rusyn, MD, Ph.D.; University Professor; KC Donnelly Professor of Veterinary Integrative
Biosciences; Chair, Interdisciplinary Faculty of Toxicology; Director, Superfund Research
Center; Texas A&M University; College Station, Texas
2. Juliane Beier, Ph.D.; Assistant Professor; Department of Medicine; Division of Gastroenterology,
Hepatology and Nutrition; Pittsburgh Liver Research Center; Department of Environmental and
Occupational Health; University of Pittsburgh; Pittsburgh, Pennsylvania
3. Sa Liu, Ph.D., M.P.H., C.I.H.; Assistant Professor; School of Health Sciences; Purdue University;
Fellow, Robert Wood Johnson Foundation; Interdisciplinary Research Leaders Cohort 5;
Hampton Hall 1271; West Lafayette, Indiana
These experts collectively have knowledge of toxicology, chemistry, and/or health effects. All reviewers
were selected in conformity with Section 104(I)(13) of the Comprehensive Environmental Response,
Compensation, and Liability Act, as amended.
ATSDR scientists review peer reviewers’ comments and determine whether changes will be made to the
profile based on comments. The peer reviewers’ comments and responses to these comments are part of
the administrative record for this compound.
The listing of peer reviewers should not be understood to imply their approval of the profile's final
content. The responsibility for the content of this profile lies with ATSDR.
VINYL CHLORIDE vi
CONTENTS
D
ISCLAIMER .............................................................................................................................................. ii
FOREWORD ............................................................................................................................................... iii
VERSION HISTORY .................................................................................................................................. iv
CONTRIBUTORS & REVIEWERS ............................................................................................................ v
CONTENTS ................................................................................................................................................. vi
LIST OF FIGURES ................................................................................................................................... viii
LIST OF TABLES ....................................................................................................................................... ix
CHAPTER 1. RELEVANCE TO PUBLIC HEALTH ................................................................................. 1
1.1 OVERVIEW AND U.S. EXPOSURES ......................................................................................... 1
1.2 SUMMARY OF HEALTH EFFECTS ........................................................................................... 2
1.3 MINIMAL RISK LEVELS (MRLs) .............................................................................................. 8
CHAPTER 2. HEALTH EFFECTS ............................................................................................................ 12
2.1 INTRODUCTION ........................................................................................................................ 12
2.2 DEATH ........................................................................................................................................ 43
2.3 BODY WEIGHT .......................................................................................................................... 44
2.4 RESPIRATORY........................................................................................................................... 45
2.5 CARDIOVASCULAR ................................................................................................................. 47
2.6 GASTROINTESTINAL ............................................................................................................... 49
2.7 HEMATOLOGICAL ................................................................................................................... 49
2.8 MUSCULOSKELETAL .............................................................................................................. 50
2.9 HEPATIC ..................................................................................................................................... 51
2.10 RENAL ........................................................................................................................................ 61
2.11 DERMAL ..................................................................................................................................... 62
2.12 OCULAR ..................................................................................................................................... 63
2.13 ENDOCRINE ............................................................................................................................... 63
2.14 IMMUNOLOGICAL ................................................................................................................... 64
2.15 NEUROLOGICAL ....................................................................................................................... 68
2.
16 REPRODUCTIVE ....................................................................................................................... 72
2.17 DEVELOPMENTAL ................................................................................................................... 73
2.18 OTHER NONCANCER ............................................................................................................... 79
2.19 CANCER ...................................................................................................................................... 81
2.20 GENOTOXICITY ........................................................................................................................ 93
CHAPTER 3. TOXICOKINETICS, SUSCEPTIBLE POPULATIONS, BIOMARKERS,
CHEMICAL INTERACTIONS ......................................................................................... 108
3.1 TOXICOKINETICS ................................................................................................................... 108
3.1.1 Absorption ........................................................................................................................... 108
3.1.2 Distribution ......................................................................................................................... 110
3.1.3 Metabolism .......................................................................................................................... 112
3.1.4 Excretion ............................................................................................................................. 115
3.1.5 Physiologically Based Pharmacokinetic (PBPK)/Pharmacodynamic (PD) Models ........... 118
3.1.5.1 EPA (1987) Animal Models .......................................................................................................... 118
3.1.5.2 U.S. Air Force (1990) Rat, Mouse, and Hamster Models .............................................................. 119
3.1.5.3 Clewell et al. (1995) Human Models ............................................................................................. 121
3.1.5.4 Reitz et al. (1996) Rat, Mouse, and Human Models ...................................................................... 122
VINYL CHLORIDE vii
3.1.5.5 Other Models ................................................................................................................................. 122
3.1.6 Animal-to-Human Extrapolations ....................................................................................... 123
3.2 CHILDREN AND OTHER POPULATIONS THAT ARE UNUSUALLY
SUSCEPTIBLE .......................................................................................................................... 124
3.3 BIOMARKERS OF EXPOSURE AND EFFECT ..................................................................... 128
3.3.1 Biomarkers of Exposure ...................................................................................................... 129
3.3.2 Biomarkers of Effect ........................................................................................................... 130
3.4 INTERACTIONS WITH OTHER CHEMICALS ..................................................................... 132
CHAPTER 4. CHEMICAL AND PHYSICAL INFORMATION ........................................................... 135
4.1 CHEMICAL IDENTITY ........................................................................................................... 135
4.2 PHYSICAL AND CHEMICAL PROPERTIES ........................................................................ 135
CHAPTER 5. POTENTIAL FOR HUMAN EXPOSURE ....................................................................... 137
5.1 OVERVIEW .............................................................................................................................. 137
5.2 PRODUCTION, IMPORT/EXPORT, USE, AND DISPOSAL ................................................ 138
5.2.1 Production ........................................................................................................................... 138
5.2.2 Import/Export ...................................................................................................................... 141
5.2.3 Use ...................................................................................................................................... 141
5.2.4 Disposal ............................................................................................................................... 141
5.3 RELEASES TO THE ENVIRONMENT ................................................................................... 142
5.3.1 Air ....................................................................................................................................... 142
5.3.2 Water ................................................................................................................................... 144
5.3.3 Soil ...................................................................................................................................... 145
5.4 ENVIRONMENTAL FATE ...................................................................................................... 145
5.4.1 Transport and Partitioning ................................................................................................... 145
5.4.2 Transformation and Degradation ........................................................................................ 147
5.5 LEVELS IN THE ENVIRONMENT ......................................................................................... 150
5.5.1 Air ....................................................................................................................................... 152
5.5.2 Water ................................................................................................................................... 154
5.5
.3 Sediment and Soil ............................................................................................................... 157
5.5.4 Other Media ........................................................................................................................ 157
5.6 GENERAL POPULATION EXPOSURE .................................................................................. 159
5.7 POPULATIONS WITH POTENTIALLY HIGH EXPOSURES .............................................. 161
CHAPTER 6. ADEQUACY OF THE DATABASE ................................................................................ 163
6.1 EXISTING INFORMATION ON HEALTH EFFECTS ........................................................... 163
6.2 IDENTIFICATION OF DATA NEEDS .................................................................................... 163
6.3 ONGOING STUDIES ................................................................................................................ 170
CHAPTER 7. REGULATIONS AND GUIDELINES ............................................................................. 172
CHAPTER 8. REFERENCES .................................................................................................................. 175
APPENDICES
APPENDIX A. ATSDR MINIMAL RISK LEVEL WORKSHEETS .................................................... A-1
APPENDIX B. LITERATURE SEARCH FRAMEWORK FOR VINYL CHLORIDE ......................... B-1
APPENDIX C. FRAMEWORK FOR ATSDR’S SYSTEMATIC REVIEW OF HEALTH EFFECTS
DATA FOR VINYL CHLORIDE .................................................................................. C-1
APPENDIX D. USER’S GUIDE ............................................................................................................. D-1
APPENDIX E. QUICK REFERENCE FOR HEALTH CARE PROVIDERS ....................................... E-1
APPENDIX F. GLOSSARY .................................................................................................................... F-1
APPENDIX G. ACRONYMS, ABBREVIATIONS, AND SYMBOLS ................................................. G-1
VINYL CHLORIDE viii
LIST OF FIGURES
1-
1. Health Effects Found in Humans and Animals Following Inhalation Exposure to Vinyl Chloride ..... 3
1-2. Health Effects Found in Animals Following Oral Exposure to Vinyl Chloride ................................... 4
1-3. Summary of Sensitive Targets of Vinyl Chloride – Inhalation ............................................................ 9
1-4. Summary of Sensitive Targets of Vinyl Chloride – Oral ................................................................... 10
2-1. Overview of the Number of Studies Examining Vinyl Chloride Health Effects ................................ 15
2-2. Levels of Significant Exposure to Vinyl Chloride – Inhalation ......................................................... 31
2-3. Levels of Significant Exposure to Vinyl Chloride – Oral .................................................................. 41
2-4. Key Characteristics of Hepatotoxicity Associated with Vinyl Chloride ............................................ 60
3-1. Proposed Metabolic Pathways for Vinyl Chloride ........................................................................... 113
5-1. Number of NPL Sites with Vinyl Chloride Contamination .............................................................. 137
6-1. Summary of Existing Health Effects Studies on Vinyl Chloride by Route and Endpoint ................ 164
VINYL CHLORIDE ix
LIST OF TABLES
1-
1. Minimal Risk Levels (MRLs) for Vinyl Chloride .............................................................................. 11
2-1. Levels of Significant Exposure to Vinyl Chloride– Inhalation .......................................................... 16
2-2. Levels of Significant Exposure to Vinyl Chloride– Oral ................................................................... 39
2-3. Results of Epidemiological Studies Evaluating Exposure to Vinyl Chloride and Liver Effects
(Noncancer) ........................................................................................................................................ 52
2-4. Results of Epidemiological Studies Evaluating Exposure to Vinyl Chloride and
Immunological Effects ....................................................................................................................... 64
2-5. Results of Epidemiological Studies Evaluating Exposure to Vinyl Chloride and Neurological
Effects ................................................................................................................................................. 68
2-6. Results of Epidemiological Studies Evaluating Exposure to Vinyl Chloride and
Developmental Effects ....................................................................................................................... 74
2-7. Results of Epidemiological Studies Evaluating Exposure to Vinyl Chloride and Insulin
Resistance ........................................................................................................................................... 80
2-8. Summary of Epidemiological Studies Evaluating Possible Associations between Vinyl
Chloride Exposure and Risk of Selected Cancer Types ..................................................................... 83
2-9. Genotoxicity of Vinyl Chloride In Vitro ............................................................................................ 94
2-10. Genotoxicity of Vinyl Chloride In Vivo ........................................................................................... 95
3-1. Vinyl Chloride Partition Coefficients ............................................................................................... 110
3-2. Physiological Parameters Used to Estimate Parameters from Vinyl Chloride Gas Uptake
Experiments ...................................................................................................................................... 119
3-3. Estimates of Metabolic Parameters Obtained from Gas Uptake Experiments ................................. 120
4-1. Chemical Identity of Vinyl Chloride ................................................................................................ 135
4-2. Physical and Chemical Properties of Vinyl Chloride ....................................................................... 136
5-1. Facilities that Produce, Process, or Use Vinyl Chloride ................................................................... 139
5-2. U.S. Production Capacity of Vinyl Chloride .................................................................................... 140
5-3. Releases to the Environment from Facilities that Produce, Process, or Use Vinyl Chloride ........... 142
5-4. Vinyl Chloride Detected in Samples Collected Throughout the United States from 2011 to
2021 .................................................................................................................................................. 151
5-5. Vinyl Chloride Detected in Samples Collected Throughout the United States in 2022 and
2023 .................................................................................................................................................. 151
VINYL CHLORIDE x
5-6. Vinyl Chloride Levels in Water, Soil, and Air of National Priorities List (NPL) Sites ................... 152
5-7. Summary of Annual Concentrations of Vinyl Chloride in ppbv Measured in Ambient Air at
Locations Across the United States .................................................................................................. 153
5-8. Safe Drinking Water Act (SDWA) 6-Year Reviews (1998–2005 and 2006–2011) ......................... 156
5-9. Reasonable Maximum Exposure Daily Inhalation Dose in µg/kg/day and Administered
Dermal Dose of Chloroethane for the Target Person ....................................................................... 160
6-1. Ongoing Studies on Vinyl Chloride ................................................................................................. 170
7-1. Regulations and Guidelines Applicable to Vinyl Chloride .............................................................. 172
VINYL CHLORIDE 1
CHAPTER 1. RELEVANCE TO PUBLIC HEALTH
1.1 OVERVIEW AND U.S. EXPOSURES
Vinyl chloride is a volatile compound used almost exclusively by the plastics industry to produce
polyvinyl chloride (PVC) and several copolymers in the United States. The majority of the vinyl chloride
produced at manufacturing facilities is converted to PVC and vinyl chloride derived copolymers on-site.
Nearly all vinyl chloride shipped to facilities off-site is also converted to PVC or PVC copolymers. In
many cases, vinyl chloride is transported by pipeline directly to the plant producing the polymer. The
physical form of vinyl chloride is a gas or neat liquid (99.9% minimum purity) stored or transported
under pressure.
Anthropogenic sources are responsible for all of the vinyl chloride found in the environment. Most of the
vinyl chloride released to the environment eventually partitions to the atmosphere. Vinyl chloride has
been detected at low levels in the ambient air in the vicinity of vinyl chloride and PVC manufacturing
plants, hazardous waste sites, and hydro fracking flowback pits. The compound has leached into
groundwater from spills, landfills, and industrial sources; it can also enter groundwater after being
produced as a byproduct during the bacterial degradation of trichloroethylene, tetrachloroethylene, and
1,1,1-trichloroethane.
When released to the atmosphere, vinyl chloride is expected to be removed by reaction with
photochemically generated hydroxyl radicals (half-life of 1–2 days). When released to water,
volatilization is expected to be the primary environmental fate process. In waters containing
photosensitizers, such as humic materials, sensitized photodegradation may also be important. Vinyl
chloride released to soil either volatilizes rapidly from soil surfaces or leaches readily through soil,
ultimately entering groundwater.
Segments of the general population living in the vicinity of emission sources (e.g., hazardous waste sites,
plastic manufacturing facilities) may be exposed to vinyl chloride by inhalation of contaminated air.
Community members living on or near hazardous waste sites may experience long-term exposure to low
levels of vinyl chloride as it has been found in multiple National Priority List (NPL) sites identified by the
U.S. Environmental Protection Agency (EPA). The majority of the general population is not expected to
be exposed to vinyl chloride through ingestion of drinking water, due to its volatility and restrictions on
its release to potable water as an indirect drinking water additive. Workers, particularly employees at
VINYL CHLORIDE 2
1. RELEVANCE TO PUBLIC HEALTH
vinyl chloride and PVC manufacturing facilities, are exposed to vinyl chloride mainly by inhalation,
although minor absorption through the skin is possible. Workers involved in the handling and processing
of PVC resins are exposed to lower levels of vinyl chloride than employees at vinyl chloride and PVC
manufacturing facilities since fabricated products contain only trace quantities of vinyl chloride present as
residual monomer. Since the early 1970s, improvements in manufacturing facilities, engineering controls,
and workplace practices have substantially reduced or nearly eliminated workplace exposures in the
United States and most other industrialized countries that manufacture vinyl chloride and produce or
fabricate PVC products. The 1974 ban on use of vinyl chloride in U.S. consumer products resulted in a
reduction in possible exposures in the general population (IARC 2012).
1.2 SUMMARY OF HEALTH EFFECTS
Information on the toxicity of vinyl chloride comes primarily from a large database of occupational
worker studies and inhalation studies in animals, with similar effects being exhibited in all species tested.
Chronic-duration oral studies of vinyl chloride in animals focus primarily on carcinogenicity; however,
two studies reported noncancer effects in the liver.
As shown in Figures 1-1 and 1-2, the most sensitive effects appear to be liver damage and
carcinogenicity, exacerbated immune response, and delayed fetal ossification. Neurological effects are
also commonly reported in humans and animals, although they generally occur at higher inhalation
concentrations. A systematic review of the noncancer endpoints resulted in the following hazard
identification conclusions:
• Hepatic effects are a presumed health effect for humans.
• Neurological effects are a presumed health effect for humans.
• Immunological effects are a suspected health effect for humans.
• Developmental effects are a suspected health effect for humans.
A systematic review was also performed for insulin resistance. The hazard identification conclusion was
that insulin resistance was not classifiable due to an insufficient level of evidence in both human and
animal studies.

VINYL CHLORIDE 3
1. RELEVANCE TO PUBLIC HEALTH
Figure 1-1. Health Effects Found in Humans and Animals Following Inhalation
Exposure to Vinyl Chloride
VINYL CHLORIDE 4
1. RELEVANCE TO PUBLIC HEALTH
Figure 1-2. Health Effects Found in Animals Following Oral Exposure to Vinyl
Chloride

VINYL CHLORIDE 5
1. RELEVANCE TO PUBLIC HEALTH
Hepatic Effects. Results from numerous inhalation and oral animal studies support the identification of
the liver as a presumed target in humans. Occupational studies have identified a consistent group of liver
effects resulting from vinyl chloride exposure, including hypertrophy, hyperplasia of hepatocytes and
sinusoidal cells, sinusoidal dilation, focal cellular degeneration, steatohepatitis, portal fibrosis, and
cirrhosis (Berk et al. 1975; Cave et al. 2010; Du and Wang 1998; Falk et al. 1974; Fedeli et al. 2019a;
Gedigke et al. 1975; Ho et al. 1991; Hsiao et al. 2004; Hsieh et al. 2007; Jones and Smith 1982; Lilis et al.
1975; Liss et al. 1985; Maroni et al. 2003; Marsteller et al. 1975; Mastrangelo et al. 2004; Mundt et al.
2017; NIOSH 1977; Popper and Thomas 1975; Suciu et al. 1975; Tamburro et al. 1984; Vihko et al.
1984; Ward et al. 2001; Zhu et al. 2005a). Plasma metabolomics analysis in vinyl chloride workers
showed alterations in lipid and amino acid metabolites, which may contribute to the observed liver
toxicity (Guardiola et al. 2016). Animal inhalation studies demonstrate that the severity of hepatic effects
increased with increasing vinyl chloride concentration, ranging from cellular hypertrophy and sinusoidal
compression, to vacuolization, hepatic hyperplasia, fibrosis, and necrosis (Jia et al. 2022; Lester et al.
1963; Sokal et al. 1980; Thornton et al. 2002; Torkelson et al. 1961; Wisniewska-Knypl et al. 1980).
Centrilobular hypertrophy, steatosis (fatty liver) and steatohepatitis (inflammation) resulted from
intermediate-duration (15–364 days) inhalation exposures of 10, 50, and 100 ppm, respectively (Sokal et
al. 1980; Thornton et al. 2002; Wisniewska-Knypl et al. 1980). Mice fed a high-fat diet (not included in
Levels of Significant Exposure, LSE Tables) and exposed to vinyl chloride experienced liver damage,
neutrophil infiltration, apoptosis, and oxidative and endoplasmic reticulum stress in the liver compared to
mice fed a normal or low-fat diet (Chen et al. 2019; Fujiwara 2018; Jia et al. 2022; Lang et al. 2018,
2020; Liang et al. 2018; Liu et al. 2023; Wahlang et al. 2020). Chronic-duration oral exposure of rats to
1.7 mg/kg/day resulted in liver cell polymorphisms and development of hepatic cysts (Til et al. 1983,
1991). In addition to noncancer effects, the liver was sensitive to tumor development. For intermediate-
and chronic-duration (>365 days) inhalation and chronic-duration oral exposures, the development of
liver angiosarcoma resulted from exposures as low as 50 ppm and 0.3 mg/kg/day, respectively (Drew et
al. 1983; Holmberg et al. 1976; Hong et al. 1981; Maltoni et al. 1981).
Immune Effects. Workers exposed to high concentrations of vinyl chloride in air experienced Raynaud's
phenomenon (a condition in which the fingers blanch and become numb with discomfort upon exposure
to the cold), acroosteolysis (resorption of the distal bony phalanges), joint and muscle pain, enhanced
collagen deposition, stiffness of the hands, and scleroderma-like skin changes and these effects may have
an immunologic basis. The immunologic findings in workers with these conditions include an increase in
circulating immune complexes, cryoglobulinemia (precipitation of abnormal proteins in the blood)
(Bogdanikowa and Zawilska 1984; Grainger et al. 1980; Saad et al. 2017), increased incidence of B-cell
VINYL CHLORIDE 6
1. RELEVANCE TO PUBLIC HEALTH
proliferation (Ward 1976), hyperimmunoglobulinemia (Ward 1976), and complement activation
(Grainger et al. 1980; Saad et al. 2017; Ward 1976). Serum immunoglobulins (IgA, IgG, and IgM) and
other inflammatory markers (i.e., ceruloplasmin, orosomucoid) were elevated in highly exposed male
vinyl chloride workers (Bencko et al. 1988; Bogdanikowa and Zawilska 1984; Wagnerova et al. 1988),
and proinflammatory cytokine levels (tumor necrosis factor-α, interleukin-1β, interleukin-6, and
interleukin-8) were increased in the serum of vinyl chloride-exposed workers with steatohepatitis (liver
inflammation with fat accumulation) (Cave et al. 2010). There is evidence of a structurally altered IgG
and it has been proposed that vinyl chloride (or a metabolite) binds to IgG (Grainger et al. 1980).
Immunological effects are not well studied in animals; however, reported findings included increased
spleen weight in rats (Sokal et al. 1980), increased thymus weight in immunized rabbits (Sharma et al.
1980), and an increase in spontaneous and/or mitogen-stimulated lymphocyte proliferation in mice and
immunized rabbits (Sharma and Gehring 1979; Sharma et al. 1980).
Neurological Effects. Inhalation-related neurological effects in humans include dizziness, drowsiness
and fatigue, headache, euphoria, irritability, nervousness, sleep disturbances, nausea, visual and hearing
disturbances and loss of consciousness (Ho et al. 1991; Langauer-Lewowicka et al. 1983; Lilis et al.
1975; Marsteller et al. 1975; NIOSH 1977; Spirtas et al. 1975; Suciu et al. 1975; Veltman et al. 1975;
Walker 1976). Signs of pyramidal and cerebellar disturbances have also been observed (not specified;
Langauer-Lewowicka et al. 1983). Dizziness has been reported by volunteers acutely exposed to
12,000 ppm, while nausea and subsequent headache resulted from exposures of 20,000 to 25,000 ppm
(Lester et al. 1963; Patty et al. 1930). Peripheral neurological effects have been reported, including
paresthesia, tingling or warmth in the extremities, numbness or pain in the fingers, and depressed reflexes
(Lilis et al. 1975; NIOSH 1977; Perticoni et al. 1986; Sakabe 1975; Spirtas et al. 1975; Suciu et al. 1975;
Veltman et al. 1975; Walker 1976). Effects in animals from acute-duration (≤14 days) inhalation
exposures include ataxia, decreased coordination, decreased reflexes, twitching, tremors, and
unconsciousness (Hehir et al. 1981; Jaeger et al. 1974; Lester et al. 1963; Mastromatteo et al. 1960; Patty
et al. 1930).
Developmental Effects. Early studies examining parental employment and/or residential proximity to
vinyl chloride facilities and birth defects reported links to fetal loss and birth defects of the central
nervous system (Infante et al. 1976a, 1976b; NIOSH 1977); however, most studies failed to demonstrate a
correlation between the developmental toxicity and either parental occupation or proximity to the facility
(Bao et al. 1988; Edmonds et al. 1975, 1978; Rosenman et al. 1989; Theriault et al. 1983). Case-control
studies evaluating exposure to multiple compounds in air and drinking water during pregnancy did not
VINYL CHLORIDE 7
1. RELEVANCE TO PUBLIC HEALTH
demonstrate an association between vinyl chloride concentration and risk of neural tube defects including
spina bifida (Ruckart et al. 2013; Swartz et al. 2015), oral clefts (Ruckart et al. 2013), or autism spectrum
disorder (Talbott et al. 2015). Developmental effects were observed in animal studies using the inhalation
route. Gestational exposures of 2,500 ppm resulted in ureter dilatation in rat offspring, while delayed
ossification was observed following 500 ppm exposures in mice (John et al. 1977, 1981). No adverse
effects were noted in an inhalation embryo-fetal developmental study in rats exposed to vinyl chloride at
concentrations up to 1,100 ppm (Thornton et al. 2002).
Cancer. The development of cancer in humans as a result of vinyl chloride exposure has been
demonstrated in a number of studies of workers in the vinyl chloride production industry. The strongest
evidence comes from the greater-than-expected incidences of liver angiosarcoma (details in Section 2.19),
which is considered to be very rare in humans (25–30 cases/year in the United States) (Heath et al. 1975).
The latency period for the development of hepatic angiosarcoma was 24–56 years in workers exposed
prior to 1974 (Collins et al. 2014). Other liver tumors, including hepatocellular carcinoma and
cholangiocellular carcinoma, have also been associated with occupational exposure to vinyl chloride
(details in Section 2.19). The latency period for the development of hepatocellular carcinoma has been
estimated to range from 32 to 67 years (Mundt et al. 2017).
Studies in several animal species support the conclusion that vinyl chloride is carcinogenic. In rats,
chronic-duration exposure to 5–5,000 ppm vinyl chloride vapors resulted in significant incidence of
mammary gland carcinomas, Zymbal’s gland carcinomas, nephroblastoma, and liver angiosarcoma
(Maltoni et al. 1981). Intermediate- (15–364 days) and chronic-duration (≥365 days) exposures of 50–
2,500 ppm vinyl chloride resulted in significant incidence of liver angiosarcoma, carcinoma, and
angioma, lung adenoma, mammary gland carcinoma, adipose tissue hemangiosarcoma, and
hemangiosarcoma of the subcutis and peritoneum in mice (details in Section 2.19). With the exception of
liver angiosarcomas, which have been observed in all species (including humans), there is little
consistency in tumor types across species.
Chronic-duration oral administration of 1.7–5 mg/kg/day of vinyl chloride resulted in the development of
neoplastic liver nodules, hepatocellular carcinoma, and lung and liver angiosarcoma in rats (Feron et al.
1981; Til et al. 1983, 1991). Studies in rats, mice, and hamsters provide evidence that exposure early in
life increases the risk of hemangiosarcoma in liver, skin, and spleen, stomach angiosarcoma, and
mammary gland carcinoma, as compared to the risk associated with exposure after 12 months of age
(Drew et al. 1983; Maltoni and Cotti 1988; Maltoni et al. 1981). Due to the latency period for vinyl
VINYL CHLORIDE 8
1. RELEVANCE TO PUBLIC HEALTH
chloride-induced cancer, exposure of animals early in life may have increased the likelihood of
developing tumors and affected the type of tumor that developed.
The Department of Health and Human Services has determined vinyl chloride to be a known human
carcinogen (NTP 2021). The International Agency for Research on Cancer (IARC) has concluded that
sufficient evidence for carcinogenicity in humans and animals exists and has placed vinyl chloride in
carcinogenicity category 1 (i.e., carcinogenic to humans) (IARC 2012). Similarly, EPA concluded that
vinyl chloride is a known human carcinogen by the inhalation route of exposure, based on human
epidemiological data (EPA 2000). By analogy, vinyl chloride is classified as carcinogenic by the oral
route because of positive animal bioassay data as well as pharmacokinetic data allowing dose
extrapolation across routes. By inference, EPA considers vinyl chloride highly likely to be carcinogenic
by the dermal route because it acts systemically.
1.3 MINIMAL RISK LEVELS (MRLs)
The inhalation database was considered adequate for deriving acute- and intermediate-duration MRLs but
inadequate for derivation of a chronic-duration MRL. As presented in Figure 1-3, the available inhalation
data for vinyl chloride suggest that the liver, immune system, and the developing fetus are the most
sensitive target of toxicity in laboratory animals.
The oral database was considered adequate for deriving a chronic-duration MRL. The oral database was
inadequate for derivation of acute- or intermediate-duration MRLs. As presented in Figure 1-4, the
available oral data for vinyl chloride suggest that the liver is the most sensitive target of toxicity in
laboratory animals.
The MRL values for vinyl chloride are summarized in Table 1-1 and discussed in greater detail in
Appendix A.

VINYL CHLORIDE 9
1. RELEVANCE TO PUBLIC HEALTH
Figure 1-3. Summary of Sensitive Targets of Vinyl Chloride – Inhalation
Available data indicate that the liver and immune system are the most sensitive targets of vinyl
chloride inhalation exposure.
Numbers in circles are the lowest LOAELs for all health effects in animals; numbers in triangles are the
lowest LOAELs for all health effects in humans.

VINYL CHLORIDE 10
1. RELEVANCE TO PUBLIC HEALTH
Figure 1-4. Summary of Sensitive Targets of Vinyl Chloride – Oral
Available data indicate that the liver is the most sensitive target of vinyl chloride oral exposure.
Numbers in circles are the lowest LOAELs for all health effects in animals.
No reliable dose response data were available for humans.

VINYL CHLORIDE 11
1. RE
LEVANCE TO PUBLIC HEALTH
Table 1-1. Minimal Risk Levels (MRLs) for Vinyl Chloride
a
Exposure
route
Exposure
duration
Provisional
MRL
Critical effect
POD type
POD value
Uncertainty/
modifying
factor
Reference
Inhalation
Acute
0.5 ppm
(1.3 mg/m
3
)
Delayed ossification
NOAEL
HEC
15 ppm
UF: 30
John et al.
1977, 1981
Intermediate
0.02 ppm
(0.05 mg/m
3
)
Increased incidence of
centrilobular hypertrophy
BMCL
HEC
0.5 ppm
UF: 30
Thornton et
al. 2002
Chronic
None
–
–
–
–
–
Oral
Acute
None
–
–
–
–
–
Intermediate
None
–
–
–
–
–
Chronic
0.003 mg/kg/day
Liver cell polymorphism
NOAEL
HED
0.09 mg/kg/day
UF: 30
Til et al.
1983, 1991
a
See Appendix A for additional information.
BMCL = benchmark concentration lower confidence limit; HEC = human equivalent concentration; HED = human equivalent dose; NOAEL = no-observed-
adverse-effect level; POD = point of departure; UF = uncertainty factor
VINYL CHLORIDE
CHAPTER 2. HEALTH EFFECTS
2.1 INT
RODUCTION
The pr
imary purpose of this chapter is to provide public health officials, physicians, toxicologists, and
other interested individuals and groups with an overall perspective on the toxicology of vinyl chloride. It
contains descriptions and evaluations of toxicological studies and epidemiological investigations and
provides conclusions, where possible, on the relevance of toxicity and toxicokinetic data to public health.
When available, mechanisms of action are discussed along with the health effects data; toxicokinetic
mechanistic data are discussed in Section 3.1.
A glossary and list of acronyms, abbreviations, and symbols can be found at the end of this profile.
To hel
p public health professionals and others address the needs of persons living or working near hazardous
waste sites, the information in this section is organized by health effect. These data are discussed in terms of
route of exposure (inhalation, oral, and dermal) and three exposure periods: acute (≤14 days), intermediate
(15–364 days), and chronic (≥365 days).
As discu
ssed in Appendix B, a literature search was conducted to identify relevant studies examining health
effect endpoints. Figure 2-1 provides an overview of the database of studies in humans or experimental
animals included in this chapter of the profile. These studies evaluate the potential health effects associated
with inhalation, oral, or dermal exposure to vinyl chloride, but may not be inclusive of the entire body of
literature. A systematic review of the scientific evidence of the health effects associated with exposure to
vinyl chloride was also conducted; the results of this review are presented in Appendix C.
Huma
n controlled exposure inhalation studies and animal inhalation studies are presented in Table 2-1
and Figure 2-2, and animal oral studies are presented in Table 2-2 and Figure 2-3; no dermal data were
identified for vinyl chloride. Summaries of human observational studies are also provided by health
effect in Tables 2-3 through 2-8.
Level
s of significant exposure (LSEs) for each route and duration are presented in tables and illustrated in
figures. The points in the figures showing no-observed-adverse-effect levels (NOAELs) or lowest-
observed-adverse-effect levels (LOAELs) reflect the actual doses (levels of exposure) used in the studies.
Effects have been classified into “less serious LOAELs” or “serious LOAELs (SLOAELs).” “Serious”
12
VINYL CHLORIDE 13
2. HEALTH EFFECTS
effects (SLOAELs) are those that evoke failure in a biological system and can lead to morbidity or
mortality (e.g., acute respiratory distress or death). "Less serious" effects are those that are not expected
to cause significant dysfunction or death, or those whose significance to the organism is not entirely clear.
ATSDR acknowledges that a considerable amount of judgment may be required in establishing whether
an endpoint should be classified as a NOAEL, "less serious" LOAEL, or "serious" LOAEL, and that in
some cases, there will be insufficient data to decide whether the effect is indicative of significant
dysfunction. However, the Agency has established guidelines and policies that are used to classify these
endpoints. ATSDR believes that there is sufficient merit in this approach to warrant an attempt at
distinguishing between "less serious" and "serious" effects. The distinction between "less serious" effects
and "serious" effects is considered to be important because it helps the users of the profiles to identify
levels of exposure at which major health effects start to appear. LOAELs or NOAELs should also help in
determining whether or not the effects vary with dose and/or duration, and place into perspective the
possible significance of these effects to human health. Levels of exposure associated with cancer (Cancer
Effect Levels, CELs) of vinyl chloride are indicated in Table 2-2 and Figure 2-3.
A User's Guide has been provided at the end of this profile (see Appendix D). This guide should aid in
the interpretation of the tables and figures for LSEs and MRLs.
The health effects of vinyl chloride have been evaluated in epidemiological and laboratory animal studies.
As illustrated in Figure 2-1, most of the health effects data come from inhalation exposure studies in
humans and animals. Human and animal data are available for each health effect category and exposure
duration category. The most examined endpoints were cancer (approximately 50%), hepatic
(approximately 40%), and neurological (10%). Only five animal studies evaluated toxicity following oral
exposure and these studies examined a limited number of endpoints (death, body weight, hematological,
hepatic, and cancer). The oral animal data are derived from chronic-duration studies only. Many of the
available human studies for vinyl chloride are characterized as case reports/series or occupational health
studies of vinyl chloride workers. These studies are often limited by the absence of exposure data or a
comparison group; however, they were conducted during a time period where workers were highly
exposed to vinyl chloride and provide important information on vinyl chloride hazards. The human
database also contains many cohort, cross-sectional, and case-control studies of vinyl chloride health
effects, especially for hepatic and cancer outcomes.
VINYL CHLORIDE 14
2. HEALTH EFFECTS
The human and animal studies suggest several sensitive targets of vinyl chloride toxicity.
• Hepatic endpoints: Hepatic effects are a presumed health effect for humans based on evidence
of fibrosis, cirrhosis, and steatohepatitis in vinyl chloride workers following chronic-duration
inhalation exposure. Moderate evidence of hepatic effects in animals includes increased liver
weight and histopathological liver lesions in rats and mice following intermediate- and chronic-
duration inhalation and chronic-duration oral exposure.
• Immune endpoints: Immunological effects are a suspected health effect based on an increase in
circulating immune complexes, immunoglobulins, complement factors, and levels of
inflammatory cytokines in occupational worker studies. Limited evidence in animal studies
includes increases in spleen weight and spontaneous and mitogen-stimulated lymphocyte
proliferation.
• Neurological endpoints: Neurological effects are a presumed health effect for humans based on
limited information including neurological symptom reporting and a single report of peripheral
neuropathy in humans. There is a moderate level of evidence in animal studies based on clinical
signs in multiple acute-duration inhalation studies.
• Developmental endpoints. Developmental effects are a suspected health effect for humans
based on strong evidence from acute-duration inhalation exposures in mice and rabbits. The most
sensitive developmental endpoint was delayed ossification in mice following prenatal inhalation
exposure. Human data were limited to a small number of ecological and case-control studies that
did not report developmental effects.
• Other noncancer endpoints. Limited evidence of increased insulin resistance in humans was
based on two epidemiology studies with altered serum biomarkers of this effect. Insulin
resistance was not observed in several intermediate-duration inhalation studies in mice; however,
these studies used only a single low concentration of vinyl chloride (0.85 ppm) and did not
evaluate effects at higher concentrations.
• Cancer endpoints. The development of cancer in humans as a result of vinyl chloride exposure
has been demonstrated in a number of studies of workers in the vinyl chloride production
industry. The strongest evidence is for liver angiosarcoma; however, other liver tumors,
including hepatocellular carcinoma and cholangiocellular carcinoma, have also been associated
with occupational exposure to vinyl chloride. Data from studies in rats, mice, and hamsters
support the conclusion that vinyl chloride is carcinogenic. Several tumor types were observed in
animal studies, including hemangiosarcoma in liver, skin, and spleen, stomach angiosarcoma,
mammary gland carcinoma, Zymbal’s gland carcinoma, and nephroblastoma.

VINYL CHLORIDE 15
2. HEALTH EFFECTS
Figure 2-1. Overview of the Number of Studies Examining Vinyl Chloride Health Effects*
Most studies examined the potential for cancer and hepatic and neurological effects of vinyl chloride
Fewer studies evaluated health effects in animals than humans (counts represent studies examining endpoint)
*Includes studies discussed in Chapter 2. A total of 224 studies (including those finding no effect) have examined toxicity; most studies examined multiple
endpoints.

VINYL CHLORIDE 16
2. HEALTH EFFECTS
Table 2-1. Levels of Significant Exposure to Vinyl Chloride– Inhalation
(ppm)
Figure
key
a
Species
(strain)
No./group
Exposure
parameters
Doses
Parameters
monitored
Endpoint
NOAEL
Less
serious
LOAEL
Serious
LOAEL
Effects
ACUTE EXPOSURE
Lester et al. 1963
1
Human
3 M, 3 F
3 days
2 times/day
5 minutes
0, 4,000,
8,000,
12,000,
16,000,
20,000
CS
Neuro
8,000
12,000
Dizziness
Patty et al. 1930
2
Human
2 NS
3 minutes
25,000
CS
Neuro
25,000
Dizziness, disorientation
Hehir et al. 1981
3
Rat
(Fischer-
344) 85–
92M, 79–
100 F
1 hour
(WB)
0, 50, 500,
5,000,
50,000
CS, BW,
GN, HP
Bd wt
50,000
Neuro
50,000
Hehir et al. 1981
4
Rat
(Fischer-
344) 50–
90 M, 50–
90 F
2 weeks
5 days/week
1 hour/day
(WB)
0, 500
CS, BW, HP
Bd wt
500
Neuro
500
Jaeger et al. 1974
5
Rat
(Sprague-
Dawley) 2–
5 M
1, 5 days
6 hours/day
0, 5,000,
50,000,
100,000
CS, BC, HP
Hepatic
50,000
100,000
Hepatocellular vacuolization,
increased alanine-α-ketoglutarate
transaminase and SDH
Neuro
50,000
100,000
Anesthesia
John et al. 1977, 1981
6
Rat
(Sprague-
Dawley)
16–31 F
GDs 6–15
10 days
7 hours/day
(WB)
0, 500, 2,500
LE, BW, FI,
OW, DX
Hepatic
500
2,500
9 or 10% increase in absolute and
relative liver weight, respectively
Develop
500
2,500
Ureter dilation

VINYL CHLORIDE 17
2. HEALTH EFFECTS
Table 2-1. Levels of Significant Exposure to Vinyl Chloride– Inhalation
(ppm)
Figure
key
a
Species
(strain)
No./group
Exposure
parameters
Doses
Parameters
monitored
Endpoint
NOAEL
Less
serious
LOAEL
Serious
LOAEL
Effects
Lester et al. 1963
7
Rat
(Sherman)
2 NS
2 hours
50,000,
60,000,
70,000,
100,000,
150,000
LE, CS, GN,
HP
Death
150,000
½ died
Resp
150,000
Edema and congestion in lungs
Neuro
50,000
70,000
LOAEL: moderate intoxication
SLOAEL: loss of righting reflex
Mastromatteo et al. 1960
8
Rat (NS)
5 NS
30 minutes
0, 100,000,
200,000,
300,000
LE, CS, GN,
HP
Death
300,000
5/5 died
Resp
100,000
Lung hyperemia
Hepatic
100,000
200,000
Fatty infiltration changes
Renal
200,000
300,000
Renal congestion
Neuro
100,000
Narcosis
Prodan et al. 1975
9
Rat (NS)
10–30 NS
2 hours
1 time
146,625–
205,275
LE, CS
Death
146,625
7/30 died
Reynolds et al. 1975a
10
Rat
(Holtzman)
M
1, 5 days
6 hours/day
50,000
GN, HP
Hepatic
50,000
Reynolds et al. 1975b
11
Rat (NS) M
1 day
6 hours/day
50,000
BC, HP
Hepatic
50,000
Thornton et al. 2002
12
Rat
(Sprague-
Dawley)
25 F
GDs 6–19
6 hours/day
(WB)
0, 10, 100,
1,100
LE, CS, BW,
FI, GN, OW,
DX
Bd wt
1,100
Hepatic
1,100
Renal
10
100
20% increase in relative kidney
weight
Develop
1,100

VINYL CHLORIDE 18
2. HEALTH EFFECTS
Table 2-1. Levels of Significant Exposure to Vinyl Chloride– Inhalation
(ppm)
Figure
key
a
Species
(strain)
No./group
Exposure
parameters
Doses
Parameters
monitored
Endpoint
NOAEL
Less
serious
LOAEL
Serious
LOAEL
Effects
Hehir et al. 1981
13
Mouse
(ICR) 82 or
90 M, 88 or
90 F
1 hour
(WB)
0, 50, 500,
5,000,
50,000
CS, BW,
GN, HP
Bd wt
50,000
Resp
50
500
50,000
LOAEL: pneumonitis
SLOAEL: hyperventilation,
respiratory difficulties
Cardio
50,000
Gastro
50,000
Musc/skel
50,000
Hepatic
50,000
Renal
50,000
Ocular
50,000
Immuno
50,000
Neuro
5,000
50,000
50% of males with twitching,
ataxia; 25% of females with
hyperactivity, ataxia
Cancer
5,000
CEL: 24/143 bronchioalveolar
adenoma
John et al. 1977, 1981
14
Mouse (CF-
1) 19–26 F
GDs 6–15
10 days
7 hours/day
(WB)
0, 50, 500
LE, BW, FI,
OW, DX
Death
500
5/29 died
Hepatic
500
Develop
50
b
500
Delayed ossification of skull and
sternebrae; unfused sternebrae
Mastromatteo et al. 1960
15
Mouse (NS)
5 NS
30 minutes
0, 100,000,
200,000,
300,000
LE, CS, GN,
HP
Death
200,000
1/5 died
Resp
100,000
Lung hyperemia
Hepatic
200,000
300,000
Liver congestion
Renal
100,000
Degenerative tubular epithelium
Neuro
100,000
Narcosis

VINYL CHLORIDE 19
2. HEALTH EFFECTS
Table 2-1. Levels of Significant Exposure to Vinyl Chloride– Inhalation
(ppm)
Figure
key
a
Species
(strain)
No./group
Exposure
parameters
Doses
Parameters
monitored
Endpoint
NOAEL
Less
serious
LOAEL
Serious
LOAEL
Effects
Prodan et al. 1975
16
Mouse (NS)
20–90 NS
2 hours
1 time
87,975–
195,500
LE, CS
Death
107,525
15/61 died
John et al. 1977, 1981
17
Rabbit
(New
Zealand) 5–
20 F
GDs 6–18
13 days
7 hours/day
0, 500, 2,500
LE, BW, FI,
OW, DX
Hepatic
2,500
Develop
500
38% of fetuses with delayed
ossification of sternebrae; 16% of
fetuses with delayed ossification at
2,500 ppm
Prodan et al. 1975
18
Rabbit (NS)
4 NS
2 hours
1 time
195,500 to
273,700
LE, CS, GN
Death
224,825
¼ died
Mastromatteo et al. 1960
19
Guinea pig
(NS) 5 NS
30 minutes
0, 100,000,
200,000,
300,000,
400,000
LE, CS, GN,
HP
Death
300,000
1/5 died
Resp
100,000
Slight pulmonary hyperemia
Cardio
400,000
Hepatic
200,000
300,000
Fatty degeneration
Ocular
400,000
Endocr
400,000
Immuno
400,000
Neuro
100,000
Tremor, loss of consciousness
Patty et al. 1930
20
Guinea pig
(NS) 3–6,
18 NS
Up to 8 hours
0, 5,000,
10,000,
25,000,
50,000,
100,000,
150,000–
250,000,
400,000
LE, CS, GN
Death
100,000
Death (incidence not reported)
Neuro
10,000
25,000
Narcosis
VINYL CHLORIDE 20
2. HEALTH EFFECTS
Table 2-1. Levels of Significant Exposure to Vinyl Chloride– Inhalation
(ppm)
Figure
key
a
Species
(strain)
No./group
Exposure
parameters
Doses
Parameters
monitored
Endpoint
NOAEL
Less
serious
LOAEL
Serious
LOAEL
Effects
Prodan et al. 1975
21
Guinea pig
(NS) 4–12
NS
2 hours
1 time
195,500–
273,700
LE, CS
Death
224,825
1/6 died
INTERMEDIATE EXPOSURE
Bi et al. 1985
22
Rat (Wistar)
38 M
3, 6 months
6 days/week
6 hours/day
0, 11.1,
105.6, 2,918
BW, GN,
OW, HP
Bd wt
11.1
105.6
15–17% decreased bodyweight at
3 and 6 months
Cardio
2,918
Hepatic
11.1
Dose response with 14-68%
increased relative liver weights at
6 months
Renal
2,918
12% increased relative kidney
weight at 3 months
Immuno
2,918
Repro
105.6
8–11% decreased relative testes
weight with testicular necrosis at
6 months
Drew et al. 1983
23
Rat
(Fischer-
344) 112–
224 F
6 months
5 days/week
6 hours/day
0, 100
LE, HP
Cancer
100
CEL: hepatic hemangiosarcoma,
hepatocellular carcinoma,
neoplastic nodules; mammary
fibroadenoma
Froment et al. 1994
24
Rat
(Sprague-
Dawley)
22 M, 22 F
33 days
6 days/week
8 hours/day
0, 500
LE, CS, GN,
HP
Cancer
500 M
CEL: hepatocellular carcinoma,
angiosarcoma of the liver, benign
cholangioma, nephroblastoma,
angiomyoma, leukemia, Zymbal
gland carcinoma, pituitary
adenoma, mammary carcinoma
and fibroma

VINYL CHLORIDE 21
2. HEALTH EFFECTS
Table 2-1. Levels of Significant Exposure to Vinyl Chloride– Inhalation
(ppm)
Figure
key
a
Species
(strain)
No./group
Exposure
parameters
Doses
Parameters
monitored
Endpoint
NOAEL
Less
serious
LOAEL
Serious
LOAEL
Effects
Hehir et al. 1981
25
Rat
(Fischer-
344) 50–
90 M, 50–
90 F
20 weeks
5 days/week
1 hour/day
(WB)
0, 50
CS, BW
Bd wt
50
Neuro
50
Hong et al. 1981
26
Rat (CD) 4-
16 M, 4–
16 F
1–10 months
5 days/week
6 hours/day
0, 50, 250,
1,000
LE, BW, FI,
HP
Death
50
17/26 died
Cancer
250
CEL: liver hemangiosarcoma,
neoplastic nodules
Sokal et al. 1980
27
Rat (Wistar)
85 M
10 months
5 days/week
5 hours/day
0, 50, 500,
20,000
CS, BW, BC,
BI, UR, GN,
OW, HP
Bd wt
20,000
23% decrease in body weight
Cardio
20,000
10% decrease in relative heart
weight
Musc/skel
20,000
Hepatic
50
Fatty change at 50 ppm; increased
incidence of hepatocyte
polymorphisms (53%) and
proliferative reticuloendothelial
cells (38%) at 500 ppm
Renal
50
500
13% increase in relative kidney
weight; 19% increase at
20,000 ppm
Immuno
50
17% increase in relative spleen
weight; 36% and 31% increase at
500 and 20,000 ppm, respectively
Repro
50
500
Spermatogenic epithelial necrosis
VINYL CHLORIDE 22
2. HEALTH EFFECTS
Table 2-1. Levels of Significant Exposure to Vinyl Chloride– Inhalation
(ppm)
Figure
key
a
Species
(strain)
No./group
Exposure
parameters
Doses
Parameters
monitored
Endpoint
NOAEL
Less
serious
LOAEL
Serious
LOAEL
Effects
Thornton et al. 2002
28
Rat
(Sprague-
Dawley)
30 M, 30 F
2 generations
13–16 weeks
(M)
16–19 weeks
(F)
6 hours/day
(WB)
0, 10, 100,
1,100
LE, CS, BW,
FI, GN, OW,
HP, RX, DX
Bd wt
1,100
Hepatic
10 F
c
Centrilobular hypertrophy in
6/30 F1 female rats (BMCL
10
=
2.05 ppm)
10 M
Increase in absolute (13–17%) and
relative (7–
15%) liver weights in F0
males; at 100 ppm: centrilobular
hypertrophy in 15/30 F0 males and
19/30 F1 males, increase in
absolute (18–20%) and relative
(11–13%) liver weight in F1 males
Immuno
1,100
Repro
1,100
Torkelson et al. 1961
29
Rat (NS)
20–24 M,
24 F
6 months
5 days/week
0.5–
7 hours/day
0, 100, 200
LE, CS, BW,
BC, UR, GN,
OW, HP
Bd wt
200
Hemato
200
Hepatic
100
Increased relative liver weight
Renal
200
Wisniewska-Knypl et al. 1980
30
Rat (Wistar)
7–10 M
10 months
5 days/week
5 hours/day
0, 50, 500,
20,000
BI, OW, HP
Hepatic
50
Fatty changes
Adkins et al. 1986
31
Mouse (A/J)
70–72 M,
30–70 F
6 months
5 days/week
6 hours/day
0, 50, 200,
500
LE, GN, HP
Death
500 F
23/70 died
500 M
37/70 died
Cancer
50
CEL: 74–88% of animals with
pulmonary adenoma; 100% with
pulmonary adenoma at 500 ppm
with same result in repeat study

VINYL CHLORIDE 23
2. HEALTH EFFECTS
Table 2-1. Levels of Significant Exposure to Vinyl Chloride– Inhalation
(ppm)
Figure
key
a
Species
(strain)
No./group
Exposure
parameters
Doses
Parameters
monitored
Endpoint
NOAEL
Less
serious
LOAEL
Serious
LOAEL
Effects
Chen et al. 2019
32
Mouse
(C57BL/6J)
8–10 M
12 weeks
5 days/week
6 hours/day
(low fat diet)
0, 0.85
BW, FI, BC,
BI, HP
Bd wt
0.85
Hepatic
0.85
Other
noncancer
0.85
Drew et al. 1983
33
Mouse
(Swiss CD-
1) 71–162 F
6 months
5 days/week
6 hours/day
0, 50
LE, GN, HP
Death
50
Mean survival time significantly
less than controls (340 days versus
474 days)
Cancer
50
CEL: hemangiosarcoma of skin,
peritoneum; mammary gland
carcinoma; lung carcinoma
Drew et al. 1983
34
Mouse
(B6C3F1)
69–162 F
6 months
5 days/week
6 hours/day
0, 50
LE, GN, HP
Death
50
Mean survival time significantly
less than controls (316 days versus
780 days)
Cancer
50
CEL: hemangiosarcoma of
subcutis, peritoneum; mammary
gland carcinoma
Hong et al. 1981
35
Mouse (CD-
1) 8–28 M,
8–28 F
1,3,6 months
5 days/week
6 hours/day
0, 50, 250,
1,000
LE, CS, HP
Death
50
15/16 died
Cancer
50 F
CEL: mammary gland
adenocarcinoma/carcinoma
Jia et al. 2022
36
Mouse
(C57BL/6J)
8 M
13 weeks
5 days/week
2 hours/day
(WB, normal
diet)
0, 63, 313
BW, BC, BI,
OW, HP
Bd wt
313
Hepatic
63
313
Decreased absolute liver weight
and hepatic steatosis

VINYL CHLORIDE 24
2. HEALTH EFFECTS
Table 2-1. Levels of Significant Exposure to Vinyl Chloride– Inhalation
(ppm)
Figure
key
a
Species
(strain)
No./group
Exposure
parameters
Doses
Parameters
monitored
Endpoint
NOAEL
Less
serious
LOAEL
Serious
LOAEL
Effects
Lang et al. 2018
37
Mouse
(C57BL/6J)
4–12 M
12 weeks
5 days/week
6 hours/day
(low fat diet)
0, 0.85
BW, FI, BC,
BI, HP
Bd wt
0.85
Hepatic
0.85
Other
noncancer
0.85
Lang et al. 2020
38
Mouse
(C56B1/6J)
8–10 NS
12 weeks
5 days/week
6 hours/day
0, 0.85
BW, FI, BC,
BI, HP, OW
Bd wt
0.85
Hepatic
0.85
Liang et al. 2018
39
Mouse
(C57BL/6J)
5–13M
12 weeks
5 days/week
6 hours/day
0, 0.85
BW, BC, BI,
HP
Bd wt
0.85
Cardio
0.85
Liu et al. 2023
40
Mouse
(C57BL/6J)
5 M
12 weeks
5 days/week
6 hours/day
(WB, control
diet)
0, 0.85
BW, FI, BC,
OW, HP
Bd wt
0.85
Hepatic
0.85
Maltoni et al. 1981
41
Mouse
(Swiss) 30–
75 M, 30–
75 F
30 weeks
5 days/week
4 hours/day
0, 50, 250,
2,500, 6,000,
10,000
BW, GN, HP
Cancer
50
CEL: liver angiosarcoma and
angioma

VINYL CHLORIDE 25
2. HEALTH EFFECTS
Table 2-1. Levels of Significant Exposure to Vinyl Chloride– Inhalation
(ppm)
Figure
key
a
Species
(strain)
No./group
Exposure
parameters
Doses
Parameters
monitored
Endpoint
NOAEL
Less
serious
LOAEL
Serious
LOAEL
Effects
Schaffner 1978
42
Mouse (NS)
3–14 M
1–6 months
5 days/week
5 hours/day
0, 2,500,
6,000
HP
Hepatic
2,500
Hyperplasia of hepatocytes and
activated sinusoidal cells
Sharma and Gehring 1979
43
Mouse (CD-
1) 12 M
2–8 weeks
5 days/week
6 hours/day
0, 10, 101,
983
CS, BW, BC,
OW
Bd wt
983
Hemato
983
Hepatic
983
Decreased relative liver weight
Renal
983
Immuno
10
Increased spontaneous
lymphocyte proliferation
Suzuki 1978, 1981
44
Mouse (CD-
1) 1–7 M
5–6 months
5 days/week
5 hours/day
0, 2500,
6,000
GN, HP
Resp
2,500
Proliferation and hypertrophy of
bronchial epithelium;
hypersecretion of mucin;
hyperplasia of alveolar epithelium
Suzuki 1983
45
Mouse (CD-
1) 30–60M
4 weeks
5 days/week
6 hours/day
0, 1, 10, 100,
300, 600
HP
Cancer
100
CEL: lung alveoli tumors
Wahlang et al. 2020
46
Mouse
(C57BL/6N)
3–6 M, 3–
6 F
12 weeks
5 days/week
6 hours/day
0, 0.85
BW, FI, WI,
BC, BI, HP,
OW
Bd wt
0.85
Hepatic
0.85
Repro
0.85
Other
noncancer
0.85

VINYL CHLORIDE 26
2. HEALTH EFFECTS
Table 2-1. Levels of Significant Exposure to Vinyl Chloride– Inhalation
(ppm)
Figure
key
a
Species
(strain)
No./group
Exposure
parameters
Doses
Parameters
monitored
Endpoint
NOAEL
Less
serious
LOAEL
Serious
LOAEL
Effects
Wang et al. 2019a
47
Mouse
(C57BL/6N)
10 M
16 weeks
5 days/week
2 hours/day
0, 57.3,
286.7,
1,433.6
BW, BC, BI,
HP, OW
Bd wt
1,433.6
Hepatic
57.3
286.7
Fat droplets, eosinophilic changes,
nuclear condensation; at
1,433.6 ppm: steatosis, large lipid
droplets, hepatic edema,
cytoplasmic loosening, and
hepatocyte nuclear fragmentation
Zelko et al. 2022
48
Mouse
(C57BL/6)
25 M
12 weeks
5 days/week
6 hours/day
0, 0.8
BW, BC, HE,
IX
Bd wt
0.8
Hemato
0.8
Immuno
0.8
Increased pulmonary interstitial
macrophages
Other
noncancer
0.8
Impaired glucose tolerance
Drew et al. 1983
49
Hamster
(Golden
Syrian)
143–224 F
6 months
5 days/week
6 hours/day
0, 200
LE, GN, HP
Death
200
Mean survival time significantly
decreased in 2-month-old
hamsters (390 days versus
463 days)
Cancer
200
CEL: liver hemangiosarcoma; skin
hemangiosarcoma, spleen
hemangiosarcoma; mammary
gland carcinoma
Maltoni et al. 1981
50
Hamster
(Golden
Syrian) 30–
62 M
30 weeks
5 days/week
4 hours/day
0, 50, 250,
500, 2500,
6,000,
10,000
BW, GN, HP
Cancer
500
CEL: liver angiosarcoma

VINYL CHLORIDE 27
2. HEALTH EFFECTS
Table 2-1. Levels of Significant Exposure to Vinyl Chloride– Inhalation
(ppm)
Figure
key
a
Species
(strain)
No./group
Exposure
parameters
Doses
Parameters
monitored
Endpoint
NOAEL
Less
serious
LOAEL
Serious
LOAEL
Effects
Sharma et al. 1980
51
Rabbit
(New
Zealand)
5 M
8 weeks
5 days/week
6 hours/day
(WB)
10, 101, 983
BW, OW, IX
Bd wt
983
Cardio
983
Hepatic
983
Renal
983
Endocr
983
Immuno
10
Increased spontaneous splenic
lymphocyte proliferation
Neuro
983
Torkelson et al. 1961
52
Rabbit (NS)
3 M, 3 F
6 months
5 days/week
7 hours/day
0, 100, 200
LE, BW, BC,
UR, GN,
OW, HP
Bd wt
200
Hepatic
100
200
Centrilobular degeneration and
necrosis
Renal
200
CHRONIC EXPOSURE
Bi et al. 1985
53
Rat (Wistar)
35–36 M
12 months
6 days/week
6 hours/day
(WB)
0, 11.1,
105.6, 2,918
BW, GN,
OW, HP
Bd wt
11.1
105.6
2,918
Dose response with 10–35%
decreased body weight at 9, 12,
and 18 months for 105.6 and
2,918 ppm; 26–35% decreased
body weight at 12 and 18 months
at 2,918 ppm
Hepatic
2,918
20% increase in relative liver
weight
Renal
2,918
17% increase in relative kidney
weight

VINYL CHLORIDE 28
2. HEALTH EFFECTS
Table 2-1. Levels of Significant Exposure to Vinyl Chloride– Inhalation
(ppm)
Figure
key
a
Species
(strain)
No./group
Exposure
parameters
Doses
Parameters
monitored
Endpoint
NOAEL
Less
serious
LOAEL
Serious
LOAEL
Effects
Repro
11.1
105.6
27/74 with degenerative
seminiferous tubule changes;
incidence for testes damage 18.9,
29.7, 36.5, and 56%, respectively
Cancer
105.6
CEL: 7/19 liver angiosarcoma and
2/19 lung angiosarcoma; at
2,918 ppm 17/19 liver
angiosarcoma and 9/19 lung
angiosarcoma
Drew et al. 1983
54
Rat
(Fischer-
344) 112–
280 F
12, 18, or
24 months
5 days/week
6 hours/day
0, 100
LE, GN, HP
Death
100
Mean survival time significantly
less than controls (≤634 days
versus 703 days)
Cancer
100
CEL: hepatic hemangiosarcoma,
hepatocellular carcinoma,
neoplastic nodules; mammary
gland fibroadenoma and
adenocarcinoma
Holmberg et al. 1976
55
Rat (albino)
12 M, 12 F
26 or
52 weeks
5 days/week
6 hours/day
0, 50, 500
CS, BW,
GN, OW, HP
Cancer
50
CEL: lung, kidney, abdominal
hemangiosarcoma
Lee et al. 1978
56
Rat (CD)
36 M, 36 F
1–12 months
5 days/week
6 hours/day
0, 50, 250,
1,000
BW, FI, HE,
GN, HP
Cancer
250 F
CEL: hepatic hemangiosarcoma

VINYL CHLORIDE 29
2. HEALTH EFFECTS
Table 2-1. Levels of Significant Exposure to Vinyl Chloride– Inhalation
(ppm)
Figure
key
a
Species
(strain)
No./group
Exposure
parameters
Doses
Parameters
monitored
Endpoint
NOAEL
Less
serious
LOAEL
Serious
LOAEL
Effects
Maltoni et al. 1981
57
Rat
(Sprague-
Dawley)
30–300 B
52 weeks
5 days/week
4 hours/day
0, 1, 5, 10,
25, 50, 100,
150, 200,
250, 500,
2,500, 6,000,
10,000,
30,000
BW, GN, HP
Cancer
5 F
CEL: mammary gland carcinoma
Drew et al. 1983
58
Mouse
(Swiss CD-
1) 71–216 F
12 or
18 months
5 days/week
6 hours/day
0, 50
LE, GN, HP
Death
50
Mean survival time significantly
less than controls (≤347 days
versus 474 days)
Cancer
50
CEL: lung; hemangiosarcoma of
peritoneum, subcutis; mammary
gland carcinoma
Drew et al. 1983
59
Mouse
(B6C3F1)
69–216 F
12 months
5 days/week
6 hours/day
0, 50
LE, GN, HP
Death
50
Mean survival time significantly
less than controls (301 days versus
780 days)
Cancer
50
CEL: hemangiosarcoma of
peritoneum, subcutis; mammary
gland carcinoma
Lee et al. 1977a, 1978
60
Mouse (CD-
1) 36 M,
36 F
1–12 months
5 days/week
6 hours/day
0, 50, 250,
1,000
GN, HP
Cancer
50
CEL: hepatic hemangiosarcoma;
bronchiolo-alveolar adenoma;
malignant lymphoma
50 F
CEL: mammary gland adenoma
and adenocarcinoma

VINYL CHLORIDE 30
2. HEALTH EFFECTS
Table 2-1. Levels of Significant Exposure to Vinyl Chloride– Inhalation
(ppm)
Figure
key
a
Species
(strain)
No./group
Exposure
parameters
Doses
Parameters
monitored
Endpoint
NOAEL
Less
serious
LOAEL
Serious
LOAEL
Effects
Drew et al. 1983
61
Hamster
(Golden
Syrian)
143–280 F
12, 18, or
24 months
5 days/week
6 hours/day
0, 200
LE, GN, HP
Death
200
Mean survival time significantly
less than controls (≤355 days
versus 463 days)
Cancer
200
CEL: liver hemangiosarcoma; skin
carcinoma, hemangiosarcoma;
spleen hemangiosarcoma;
mammary gland carcinoma;
stomach adenoma
a
The number corresponds to entries in Figure 2-2. The only human studies included in this table are controlled exposure studies. Other epidemiological studies
are described in text and tables in the health effect sections below.
b
Used to derive an acute-duration inhalation Minimal Risk Level (MRL) of 0.5 ppm. The NOAEL of 50 ppm was adjusted for continuous exposure and was
converted to a human equivalency concentration using the default animal:human blood gas partition coefficient ratio of 1 (50 ppm x 7 hours/24 hours = 15 ppm)
and divided by an uncertainty factor of 30 (3 for animal to human after dosimetric adjustment and 10 for human variability), resulting in an MRL of 0.5 ppm.
c
Used to derive an intermediate-duration inhalation MRL of 0.02 ppm based on the BMCL
10HEC
of 0.5 ppm and an uncertainty factor of 30 (3 for animal to human
after dosimetric adjustment and 10 for human variability).
BC = blood chemistry; Bd wt or BW = body weight; BI = biochemical changes; BMCL
10
= benchmark concentration lower confidence limit 10%;
Cardio = cardiovascular; CEL = cancer effect level; CS = clinical signs; Develop = developmental; DX = developmental toxicity; Endocr = endocrine;
F = female(s); FI = food intake; Gastro = gastrointestinal; GD = gestational day; GN = gross necropsy; Hemato = hematological; HP = histopathology;
Immuno = immunological; IX = immune function; LE = lethality; LOAEL = lowest-observed-adverse-effect level; M = male(s); Musc/skel =
muscular/skeletal; Neuro
= neurological; NOAEL = no-observed-adverse-effect level; NS = not specified; OW = organ weight; Repro = reproductive; Resp = respiratory; RX = reproductive
toxicity; SDH = sorbitol dehydrogenase; SLOAEL = serious lowest-observed-adverse-effect level; UR = urinalysis; (WB) = whole body; WI = water intake

VINYL CHLORIDE 31
2. HEALTH EFFECTS
Figure 2-2. Levels of Significant Exposure to Vinyl Chloride – Inhalation
Acute (≤14 days)

VINYL CHLORIDE 32
2. HEALTH EFFECTS
Figure 2-2. Levels of Significant Exposure to Vinyl Chloride – Inhalation
Acute (≤14 days)

VINYL CHLORIDE 33
2. HEALTH EFFECTS
Figure 2-2. Levels of Significant Exposure to Vinyl Chloride – Inhalation
Acute (≤14 days)
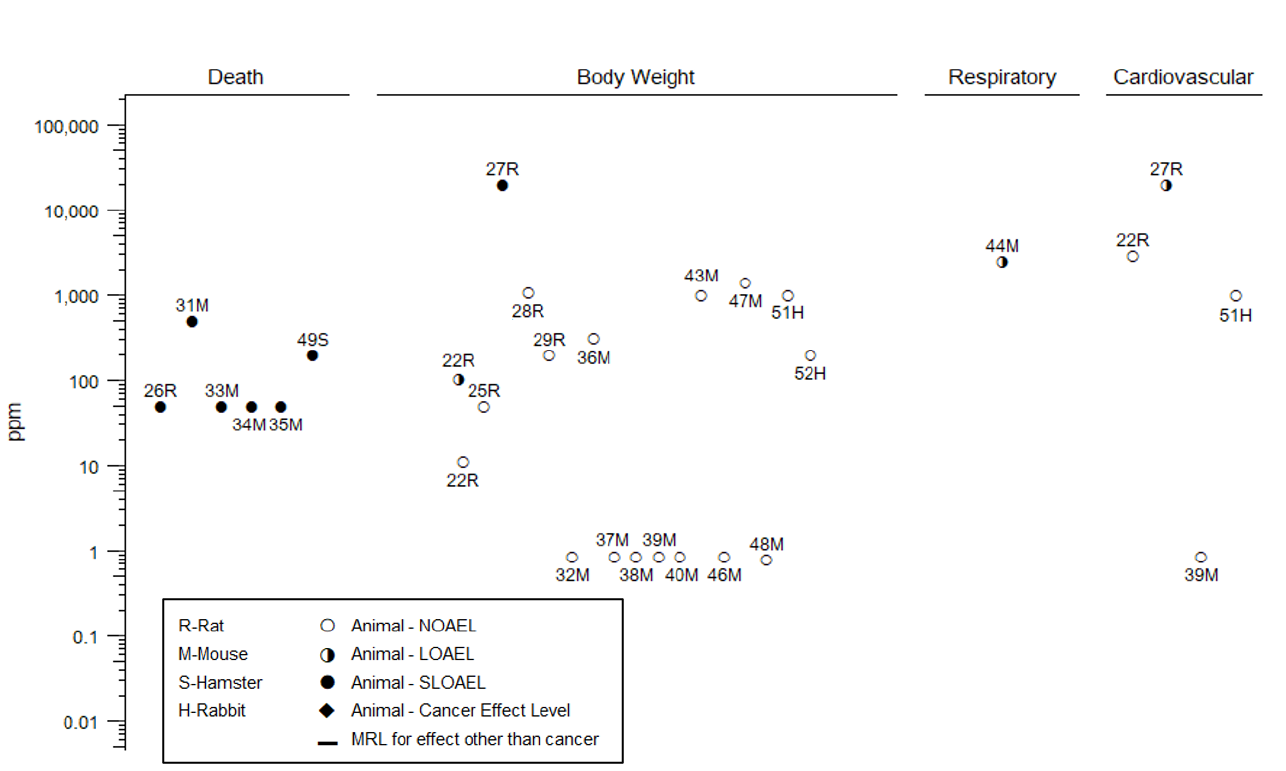
VINYL CHLORIDE 34
2. HEALTH EFFECTS
Figure 2-2. Levels of Significant Exposure to Vinyl Chloride – Inhalation
Intermediate (15–364 days)

VINYL CHLORIDE 35
2. HEALTH EFFECTS
Figure 2-2. Levels of Significant Exposure to Vinyl Chloride – Inhalation
Intermediate (15–364 days)

VINYL CHLORIDE 36
2. HEALTH EFFECTS
Figure 2-2. Levels of Significant Exposure to Vinyl Chloride – Inhalation
Intermediate (15–364 days)

VINYL CHLORIDE 37
2. HEALTH EFFECTS
Figure 2-2. Levels of Significant Exposure to Vinyl Chloride – Inhalation
Intermediate (15–364 days)

VINYL CHLORIDE 38
2. HEALTH EFFECTS
Figure 2-2. Levels of Significant Exposure to Vinyl Chloride – Inhalation
Chronic (≥365 days)

VINYL CHLORIDE 39
2. HEALTH EFFECTS
Table 2-2. Levels of Significant Exposure to Vinyl Chloride– Oral
(mg/kg/day)
Figure
key
a
Species
(strain)
No./group
Exposure
parameters
Doses
Parameters
monitored
Endpoint
NOAEL
Less
serious
LOAEL
Serious
LOAEL
Effects
CHRONIC EXPOSURE
Feron et al. 1981
1
Rat (Wistar)
60–80 M,
60–80 F
84 weeks–
2.7 years
5 days/week
4 hours/day
(F), (GO)
0, 1.7, 5,
14.1, 300
LE, CS, BW,
FI, BC, UR,
GN, HP
Death
5 F
7/60 dead at 80 weeks
14.1 M
8/60 dead at 80 weeks
Resp
5
Breathing difficulties at 18 months
Hemato
5
14.1
6–8% statistically significant
decrease in clotting time
Hepatic
5 F
Extensive necrosis
14.1 M
Extensive necrosis
1.7
Cellular alteration
Neuro
5
14.1
Humpback position, lethargy,
emaciation
Cancer
5
Female CEL: 19/59 with
hepatocellular carcinoma; 9/57 with
liver angiosarcoma at
14.1 mg/kg/day
Male CEL: 6/56 with liver
angiosarcoma, 4/56 with lung
angiosarcoma; 8/59 with
hepatocellular carcinoma at
14.1 mg/kg/day
Knight and Gibbons 1987
2
Rat (Wistar)
8–20 B NS
2 years
1 time/day
(GO)
0, 3, 30, 300
LE, BW, BI,
GN
Death
30
33% mortality
Hepatic
3
Mottled appearance and
hemorrhagic patches
Dermal
30
Increased skin thickness, collagen
Cancer
30
CEL: liver angiosarcoma
Maltoni et al. 1981
3
Rat
(Sprague-
Dawley)
40 M, 40 F
52 weeks
5 times/week
(GO)
0, 3.33,
16.65, 50
BW, GN, HP
Cancer
16.65 F
CEL: liver angiosarcoma

VINYL CHLORIDE 40
2. HEALTH EFFECTS
Table 2-2. Levels of Significant Exposure to Vinyl Chloride– Oral
(mg/kg/day)
Figure
key
a
Species
(strain)
No./group
Exposure
parameters
Doses
Parameters
monitored
Endpoint
NOAEL
Less
serious
LOAEL
Serious
LOAEL
Effects
Maltoni et al. 1981
4
Rat
(Sprague-
Dawley)
75 M,75 F
52 weeks
5 times/week
(GO)
0, 0.03, 0.3,
1
BW, GN, HP
Cancer
0.3
CEL: liver angiosarcoma,
hepatoma
Til et al. 1983, 1991
5
Rat (Wistar)
50–100 M,
50–100 F
149 weeks
4 hours/day
(F)
0, 0.018,
0.17, 1.7
LE, CS, BW,
FI, BC, HP
Death
1.7 F
14% mortality
Bd wt
1.7
Hemato
1.7
Hepatic
0.17
b
1.7
33–34% increase in the incidence
of liver cell polymorphism; cysts
(females only)
Cancer
1.7
CEL: 3/49 males and 3/49 females
with hepatocellular carcinoma;
1/49 males and 2/49 females with
liver angiosarcoma
a
The number corresponds to entries in Figure 2-3.
b
Used to derive a chronic-duration oral Minimal Risk Level (MRL) of 0.003 mg/kg/day based on the PBPK-modeled equivalent human NOAEL of 0.09 mg/kg/day
and an uncertainty factor of 30 (3 for species extrapolation with a dosimetric adjustment and 10 for human variability).
BC = blood chemistry; Bd wt or BW = body weight; BI = biochemical changes; CEL = cancer effect level; CS = clinical signs; (F) = feed; F = female(s); FI = food
intake; (GO) = gavage in oil; GN= gross necropsy; Hemato = hematological; HP = histopathology; LE = lethality; LOAEL = lowest-observed-adverse-effect level;
M = male(s); NOAEL = no-observed-adverse-effect level; Neuro = neurological; NS = not specified; PBPK = physiologically based pharmacokinetic;
Resp = respiratory; UR = urinalysis
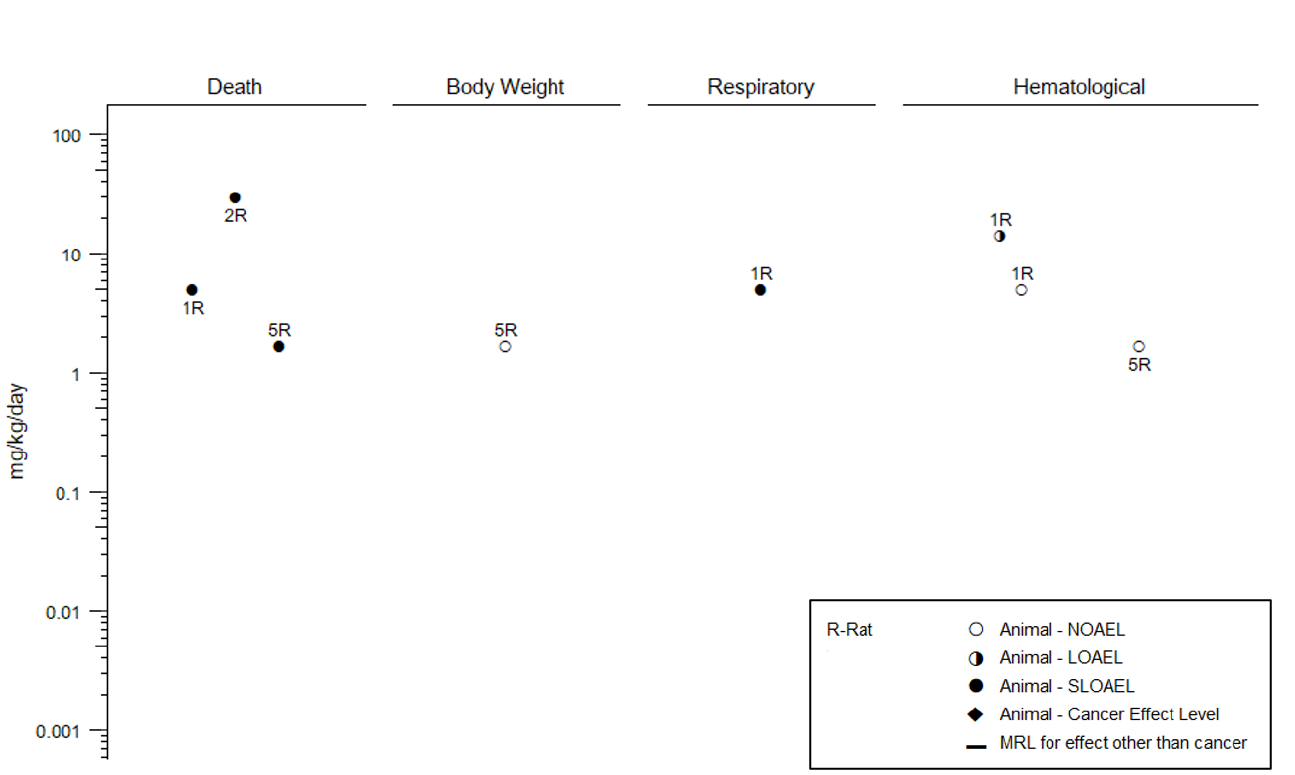
VINYL CHLORIDE 41
2. HEALTH EFFECTS
Figure 2-3. Levels of Significant Exposure to Vinyl Chloride – Oral
Chronic (≥365 days)
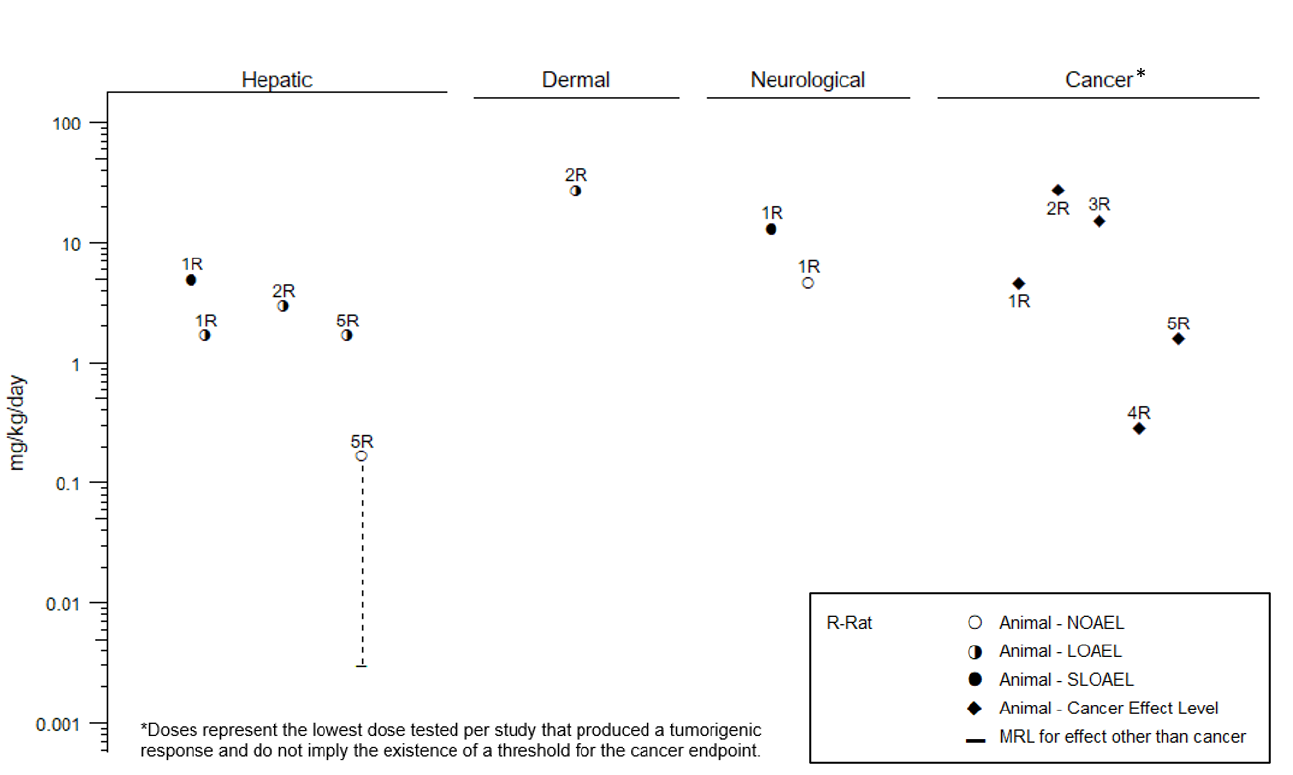
VINYL CHLORIDE 42
2. HEALTH EFFECTS
Figure 2-3. Levels of Significant Exposure to Vinyl Chloride – Oral
Chronic (≥365 days)
VINYL CHLORIDE 43
2. HEALTH EFFECTS
2.2 DEATH
Human Studies. A report by Danziger (1960) described the deaths of two vinyl chloride workers. In one
case, a worker exposed to high concentrations of vinyl chloride emitted from an open valve was found
dead. In another case, a worker responsible for cleaning a polymerization tank was found dead in the
tank. Autopsies performed on these men showed congestion of the internal organs, particularly the lungs
and kidneys, and failure of the blood to clot. Circumstances surrounding the deaths suggested that the
deaths were due to breathing very high levels of vinyl chloride. Retrospective mortality studies
associating exposure with cancer are described in Section 2.19. In general, epidemiology studies did not
report an increase in all-cause mortality for workers exposed to vinyl chloride (Belli et al. 1987; Buffler et
al. 1979; Carreón et al. 2014; Fedeli et al. 2019a; Hagmar et al. 1990; Hsieh et al. 2011; Laplanche et al.
1987, 1992; Mundt et al. 2000, 2017; Ott et al. 1975; Scarselli et al. 2022; Ward et al. 2001; Wong et al.
2002a).
Animal Studies. Brief exposures to concentrations of vinyl chloride ranging from 100,000 to
400,000 ppm have been shown to be fatal in rats (Lester et al. 1963; Mastromatteo et al. 1960; Prodan et
al. 1975), guinea pigs (Mastromatteo et al. 1960; Patty et al. 1930; Prodan et al. 1975), mice
(Mastromatteo et al. 1960; Prodan et al. 1975), and rabbits (Prodan et al. 1975). At these concentrations,
deaths occurred within 30–60 minutes. An increased mortality rate was also observed at much lower
concentrations in maternal mice in a developmental toxicity study (John et al. 1977, 1981). In this study,
mortality was observed following exposure to 500 ppm for 10 days during gestation.
Decreased survival occurred in intermediate- and chronic-duration inhalation studies (Adkins et al. 1986;
Drew et al. 1983; Feron et al. 1979a; Hong et al. 1981, Lee et al. 1977a, 1978). A treatment-related
increase in the mortality rate was observed in mice exposed to 500 ppm of vinyl chloride for 6 hours/day,
5 days/week, for 6 months (Adkins et al. 1986). In mice and rats maintained for 12 months following a
6-month, 6 hour/day, 5 day/week exposure regime, survival was decreased at concentrations as low as
50 ppm (Hong et al. 1981). Substantial increases in the mortality rate of mice and rats exposed to
250 ppm vinyl chloride for 12 months were observed by Lee et al. (1977a, 1978). In addition, small
increases in the mortality of mice and rats during the 12-month exposure period were observed at 50 ppm
in these reports.
Drew et al. (1983) examined the influence of age on survival of female mice, rats, and hamsters exposed
to 50, 100, or 200 ppm vinyl chloride, respectively. For a 12-month exposure duration (6 hours/day,
VINYL CHLORIDE 44
2. HEALTH EFFECTS
5 days/week), mortality was highest in younger animals where exposure began at 2 months of age
compared to animals that were first exposed at 8 or 14 months of age. All animals were maintained for
up to 24 months; therefore, the post-exposure period was considerably longer for the younger animals.
Tumor incidence was higher in younger animals, suggesting that mortality may be related to
carcinogenesis in this study (Section 2.19 Cancer). This study was limited in that only one dose of vinyl
chloride was tested in each species.
Decreased survival has been observed in rats as a result of chronic oral ingestion of vinyl chloride.
Significant increases in mortality were observed by Feron et al. (1981) when Wistar rats were allowed to
consume vinyl chloride doses as low as 5 mg/kg/day in the diet for 4 hours/day over a 2.7-year period or
when gavaged with 30 mg/kg/day for 2 years (Knight and Gibbons 1987) The effects of consumption of
vinyl chloride during a lifespan study in Wistar rats lasting almost 3 years (149 weeks) were examined by
Til et al. (1983, 1991). These authors found a decreased survival rate at a vinyl chloride dosage of
1.7 mg/kg/day. In both of these studies, vinyl chloride was administered by incorporating PVC resin that
was high in vinyl chloride content into the diet.
2.3 BODY WEIGHT
Human Studies. An occupational health study (i.e., vinyl chloride worker study with no exposure
measurements or comparison group) reported that workers exposed to high concentrations of vinyl
chloride experienced anorexia (Suciu et al. 1975). No additional information on body weight is available
from human studies of vinyl chloride exposure.
Animal Studies. No effects on body weight were noted in acute-duration studies of adult mice exposed to
inhalation concentrations up to 10,000 ppm vinyl chloride 4 hours/day for 5–6 days (Kudo et al. 1990) or
adult rats exposed to up to 50,000 ppm for 1 hour or 500 ppm 5 days/week, for 2 weeks (Hehir et al.
1981). Body weight decreases were observed in some, but not all, intermediate- and chronic-duration
inhalation studies. Significant body weight decreases were found in rats exposed to 100 ppm vinyl
chloride 6 hours/day, 6 days/week for 12 months (Bi et al. 1985), or 5,000 ppm vinyl chloride
7 hours/day, 5 days/week for 4–52 weeks (Feron et al. 1979a). Body weight was increased in mice fed a
high-fat diet (not included in Levels of Significant Exposure, LSE, Tables); however, vinyl chloride
exposure had no effect on body weight in mice fed a normal or high-fat diet (Chen et al. 2019; Lang et al.
2018, 2020; Liang et al. 2018; Wahlang et al. 2020).
VINYL CHLORIDE 45
2. HEALTH EFFECTS
No changes in body weight were noted in rats or rabbits exposed to 200 ppm vinyl chloride 7 hours/day,
5 days/week for 6 months (Torkelson et al. 1961) or in mice exposed up to 313 ppm 2 hours/day,
5 days/week for 13 weeks (Jia et al. 2022), 983 ppm 6 hours/day, 5 days/week for 8 weeks (Sharma and
Gehring 1979; Sharma et al. 1980), or up to 1,433.6 ppm 2 hours/day, 5 days/week for 16 weeks (Wang
et al. 2019a). No body weight change was observed in mice given a normal low-fat diet and exposed to
0.8 or 0.85 ppm vinyl chloride for 6 hours/day, 5 days/week for 12 weeks (Chen et al. 2019; Lang et al.
2018, 2020; Liang et al. 2018; Wahlang et al. 2020; Zelko et al. 2022). Exposure to 0.85 ppm vinyl
chloride for 6 hours/day, 5 days/week for 12 weeks did not affect body weight gains of mice fed low-fat
or high-fat diets 9 months after exposure ended (Liu et al. 2023). The vinyl chloride concentration used
in these studies was anticipated to be nontoxic in low-fat diet mice and no other concentrations of vinyl
chloride were used.
No changes in body weight were noted in Wistar rats fed 1.7 mg/kg/day vinyl chloride mixed with PVC
powder in the diet for 149 weeks (Til et al. 1983, 1991).
2.4 RESPIRATORY
Human Studies. Limited information is available on the acute adverse effects from inhalation of vinyl
chloride by humans. Autopsy findings from a man who died after being overcome by vinyl chloride
revealed the irritating nature of a high-level inhalation exposure. The lungs were found to be intensely
hyperemic, and some desquamation of the alveolar epithelium had occurred (Danziger 1960).
Respiratory symptoms, including runny nose, burning sensation in the nose and throat, hoarseness,
shortness of breath, chest tightness, wheezing, burning sensation in the lungs, coughing, and increased
congestion or phlegm, were reported in first responders, refinery workers, and nearby residents following
derailment of a train carrying vinyl chloride (Brinker et al. 2015; Shumate et al. 2017; Wilken et al.
2015).
Reports regarding respiratory effects in workers who are occupationally exposed to vinyl chloride are
contradictory. Several epidemiology studies found no increased incidence of respiratory disease,
respiratory symptom reporting, or pulmonary dysfunction among vinyl chloride workers (Gamble et al.
1976; Laplanche et al. 1987, 1992; NIOSH 1977). However, adverse respiratory effects were reported in
cohort and case-control studies (Lloyd et al. 1984; Wong et al. 1991; Zhu et al. 2005a) and several
occupational health studies, which often had no exposure measurements (Lilis et al. 1975, 1976; Suciu et
al. 1975; Walker 1976). These effects included pharyngeal irritation (Zhu et al. 2005a), increased
VINYL CHLORIDE 46
2. HEALTH EFFECTS
incidence of emphysema (Suciu et al. 1975; Wong et al. 1991), decreased respiratory volume and vital
capacity, respiratory insufficiency (Suciu et al. 1975), decreased respiratory oxygen and carbon dioxide
transfer (Lloyd et al. 1984), pulmonary fibrosis of the linear type (Suciu et al. 1975), abnormal chest
x-rays (Lilis et al. 1975, 1976), and dyspnea (Walker 1976). Interpretation of many of these results is
confounded by the inclusion of smokers among those exposed to vinyl chloride and the concurrent
exposure of many vinyl chloride workers to PVC resin dust, which is known to produce respiratory
lesions (Mastrangelo et al. 1979).
Animal Studies. Brief inhalation of high concentrations of vinyl chloride produced respiratory
inflammation in a variety of animals. A 30-minute exposure of guinea pigs, mice, and rats to
100,000 ppm of vinyl chloride produced hyperemia in all three species (Mastromatteo et al. 1960).
Exposure to higher concentrations (200,000 and 300,000 ppm) produced increased congestion, edema,
and at the highest concentrations, pulmonary hemorrhages in all three species (Mastromatteo et al. 1960).
Tracheal epithelium was also eroded in one guinea pig exposed to 400,000 ppm for 30 minutes
(Mastromatteo et al. 1960). Edema and congestion of the lungs of rats were also observed following a
2-hour exposure to 150,000 ppm (Lester et al. 1963).
Histopathologic examination of mice exposed to 2,500 ppm vinyl chloride 5 hours/day, 5 days/week for
5–6 months revealed proliferation and hypertrophy of the bronchiolar epithelium, hyperplasia of the
alveolar epithelium, hypersecretion of mucin (Suzuki 1978, 1980, 1981), increased endoplasmic
reticulum and free ribosomes in Clara cells, and mobilization of alveolar macrophages (Suzuki 1980).
These changes were observed irrespective of the recovery period (2 or 37 days), indicating that they were
not readily reversible. However, these studies were limited by the small number of animals tested and the
absence of a statistical analysis.
Chronic-duration exposure of rats to 5,000 ppm 7 hours/day, 5 days/week for 12 months produced
hyperplasia of the olfactory epithelium, increased cellularity of the interalveolar septa of the lungs, and an
increased incidence of pulmonary hemorrhage (Feron and Kroes 1979). Interstitial pneumonia and
hemorrhagic lungs were observed in rats exposed to 30,000 ppm of vinyl chloride 4 hours/day,
5 days/week for 12 months (Viola et al. 1971).
VINYL CHLORIDE 47
2. HEALTH EFFECTS
2.5 CARDIOVASCULAR
Human Studies. Cardiovascular symptoms (not further defined) were reported by residents living near
the site of a train derailment resulting in a release of vinyl chloride (Shumate et al. 2017). Occupational
exposure to vinyl chloride has been associated with the development of Raynaud’s phenomenon, a
condition in which the fingers blanch and become numb with discomfort upon exposure to the cold. It
has also been reported in a worker exposed once to a vinyl chloride leak (Ostlere et al. 1992). Most of the
evidence pertaining to Raynaud’s phenomenon in vinyl chloride workers is derived from case reports and
occupational health studies, which often had no exposure measurements and no comparison groups.
Although only a small percentage of vinyl chloride workers develop Raynaud’s phenomenon (Laplanche
et al. 1987, 1992; Lilis et al. 1975; Marsteller et al. 1975; Suciu et al. 1975; Veltman et al. 1975; Walker
1976), the incidence is significantly higher than in unexposed workers (Laplanche et al. 1987, 1992).
Investigation of the peripheral circulation of workers afflicted with Raynaud’s phenomenon has revealed
thickening of the walls of the digital arteries (Harris and Adams 1967), narrowing of the arterial lumen
(Veltman et al. 1975), vascular occlusions (Walker 1976), arterial occlusions (Preston et al. 1976;
Veltman et al. 1975), tortuosity (Preston et al. 1976), hypervascularity (Preston et al. 1976), inflammatory
infiltration of the arterioles (Magnavita et al. 1986), deposition of immune products along the vascular
endothelium (Ward 1976), and impaired capillary microcirculation (Magnavita et al. 1986; Maricq et al.
1976). Some reports indicate that upon removal from exposure, Raynaud’s phenomenon gradually
disappears (Freudiger et al. 1988; Suciu et al. 1975); however, abnormalities of microcirculation, as
measured by capillaroscopy, were shown to persist in vinyl chloride workers 15 years after the cessation
of exposure (Lopez et al. 2013). Genetic polymorphisms of glutathione transferase M1 and glutathione
transferase T1 were not significantly associated with the presence of Raynaud’s disease in a case-control
study of French vinyl chloride workers (Fontana et al. 2006). For further discussion of Raynaud’s
phenomenon, refer to Section 2.14 (Immunological).
Splenomegaly, with evidence of portal hypertension (dilated peritoneal veins and esophageal varices), has
been reported by investigators studying the effects of vinyl chloride exposure (Marsteller et al. 1975). In
addition, hypertension among vinyl chloride workers (NIOSH 1977; Suciu et al. 1975) and significantly
increased mortality rate due to cardiovascular and cerebrovascular disease (Byren et al. 1976) have been
reported. Saad et al. (2017) reported that vinyl chloride workers had increased serum lipoprotein
concentrations compared to healthy unexposed controls. Serum levels of total cholesterol, high-density
lipoprotein (HDL) and low-density lipoprotein (LDL) cholesterol, and triglycerides were similar between
VINYL CHLORIDE 48
2. HEALTH EFFECTS
vinyl chloride workers and controls. Conclusive evidence was not provided for an association of vinyl
chloride with coronary heart disease (Kotseva 1996).
Animal Studies. Investigators studying the anesthetic properties of vinyl chloride in dogs have observed
that doses producing anesthesia (100,000 ppm, Oster et al. 1947; 150,000–900,000 ppm, Carr et al. 1949)
also produced cardiac arrhythmias. Arrhythmias were characterized by intermittent tachycardia, extra
ventricular systoles, vagal beats, ventricular fibrillation, and atrioventricular block. However, the
statistical significance of these effects was not reported. At high concentrations (>150,000 ppm), vinyl
chloride was shown to sensitize the heart to epinephrine, resulting in cardiac arrhythmias in dogs (Carr et
al. 1949). No histopathological changes in the heart were noted in guinea pigs exposed to 400,000 ppm
of vinyl chloride for 30 minutes (Mastromatteo et al. 1960).
Bi et al. (1985) examined relative heart weight in rats after 3 or 6 months of exposure to 0–2,918 ppm
vinyl chloride, 6 hours/day, 6 days/week. Findings did not exhibit a clear dose-response relationship. No
changes in heart weights were reported when immunized rabbits were exposed up to 983 ppm vinyl
chloride 6 hours/day, 5 days/week for 8 weeks (Sharma et al. 1980). Chronic-duration exposure of rats to
5,000 ppm vinyl chloride 7 hours/day, 5 days/week for 1 year resulted in increases in areas of
myodegeneration in the heart and thickening of the walls of arteries (Feron and Kroes 1979). There were
no significant findings reported in the transthoracic echocardiography examination of mice exposed to
0.85 ppm vinyl chloride 6 hours/day, 5 days/week for 12 weeks (Liang et al. 2018). Other cardiovascular
parameters in these mice including gross cardiac dimensions, heart weight to tibia length ratio, left
ventricular mass collected index, intraventricular septal thickness, left ventricular posterior wall, and
cardiomyocyte cross-sectional area were similar to measurements in control mice.
Exposure of LDL receptor-knockout (KO) mice fed a western diet (42% kcal from fat) to 0.8 ppm vinyl
chloride 6 hours/day, 5 days/week for 12 weeks did not affect the atherosclerotic lesion area in the aortic
valves of the innominate artery (Zelko et al. 2022).
Mechanisms. It has been hypothesized that cardiac arrhythmia reported after vinyl chloride exposure
may result from sensitization of the heart to circulatory catecholamines, as occurs with other halogenated
hydrocarbons. This was demonstrated in a dog study where the EC
50
for cardiac sensitization for vinyl
chloride was determined to be 50,000 ppm (Clark and Tinston 1973). Cardiac sensitization by
halogenated hydrocarbons generally occurs at very high air concentrations (0.5–90%) when the
VINYL CHLORIDE 49
2. HEALTH EFFECTS
compounds were tested as anesthetic agents in experimental studies (Brock et al. 2003). Therefore, it
appears unlikely that individuals exposed to low levels of vinyl chloride will experience these effects.
2.6 GASTROINTESTINAL
Human Studies. Gastrointestinal symptoms including nausea and/or vomiting were reported in people
working and living near the site of a train derailment (Shumate et al. 2017; Wilken et al. 2015).
Approximately 32% of the vinyl chloride workers examined by Lilis et al. (1975) reported a history of
“gastritis, ulcers (gastric and duodenal), and upper gastrointestinal bleeding.” Because these subjects
were not compared to workers who had not been exposed to vinyl chloride, the significance of these
findings is unknown. Other symptoms reported by vinyl chloride workers included nausea, abdominal
distension, and heartburn. Loss of appetite and nausea have been reported in a case series of Singapore
workers exposed to 1–21 ppm vinyl chloride (Ho et al. 1991).
Animal Studies. No studies were located regarding gastrointestinal effects in animals exposed to vinyl
chloride.
2.7 HEMATOLOGICAL
Human Studies. Blood tests performed at autopsy of two workers whose deaths were believed to be due
to exposure to extremely high levels of vinyl chloride revealed that blood clotting did not occur (Danziger
1960). Slight-to-severe thrombocytopenia in workers exposed to vinyl chloride was reported in several
occupational health studies, which often had no exposure measurements or a comparison group
(Marsteller et al. 1975; Micu et al. 1985; Veltman et al. 1975). Thrombocytopenia was found in patients
who both did and did not present with splenomegaly (Veltman et al. 1975) but Lilis et al. (1975) found no
increased incidence of thrombocytopenia in their vinyl chloride worker study. A prospective cohort study
of female workers exposed to vinyl chloride at levels ranging from 0.2 to 130.7 ppm showed that the
exposed workers had a significantly lower number of platelets than the nonexposed controls during the
early part of their pregnancies (weeks 8–10) but that this effect had abated by the end of the pregnancy
(34–38 weeks) following a period free from exposure (Bao et al. 1988). Hemoglobin disorders (not
further defined) were diagnosed in a higher number of vinyl chloride-exposed workers compared with
unexposed controls in a cohort study (Zhu et al. 2005a). Splenomegaly was reported in a number of case
reports and occupational health studies (Ho et al. 1991; Marsteller et al. 1975; Popper and Thomas 1975;
Suciu et al. 1975; Veltman et al. 1975). Increased levels of two plasma proteins (α
1
- and α
2
-globulin)
VINYL CHLORIDE 50
2. HEALTH EFFECTS
were reported in case reports and occupational health studies examining the effects of exposure to vinyl
chloride in workers (Harris and Adams 1967; Suciu et al. 1975).
Animal Studies. A brief (30-minute) exposure of guinea pigs to 400,000 ppm vinyl chloride resulted in a
failure of the blood to clot in the animals that died during the exposure (Mastromatteo et al. 1960). Mice
that were exposed to 5,000 ppm (4 hours/day for 6 days) or 10,000 ppm (4 hours/day for 5 days) showed
an increased emergence of basophilic stippled erythrocytes (Kudo et al. 1990). This effect was also noted
in mice that were exposed for 10 weeks to 50 ppm intermittently (4 hours/day for 4–5 days/week) or to
30–40 ppm continuously for 62 days (Kudo et al. 1990). Exposure of rats to either 50,000 ppm for
8 hours/day for 19 consecutive days or 20,000 ppm for 8 hours/day, 5 days/week for 92 days resulted in a
decrease in white blood cells (Lester et al. 1963); this study was not included in Table 2-1 or Figure 2-2
due to colony contamination. Exposure of dogs and rats to 200 ppm for 7 hours/day, 5 days/week, for
6 months had no effect on hematologic values (Torkelson et al. 1961). An 8-week exposure of mice to
983 ppm for 6 hours/day, 5 days/week also had no effect on erythrocyte or leukocyte counts (Sharma and
Gehring 1979). Exposure of rats to 5,000 ppm vinyl chloride for 7 hours/day, 5 days/week for 1 year
produced increased hematopoiesis in the spleen (Feron and Kroes 1979). Blood clotting time was
decreased in rats exposed to 5,000 ppm for 7 hours/day for 1 year (Feron et al. 1979a).
Wistar rats fed 14.1 mg/kg/day for up to 2.7 years showed decreased clotting time of the blood, which
was not observed at 5 mg/kg/day (Feron et al. 1981). No changes in thrombocyte count or prothrombin
times were noted in Wistar rats fed diets containing low concentrations of vinyl chloride in PVC resin
(1.7 mg/kg/day) for 149 weeks (Til et al. 1983, 1991).
No changes in hematological parameters were reported in C57BL/6 mice exposed to 0.8 ppm vinyl
chloride for 6 hours/day, 5 days/week for 12 weeks (Zelko et al. 2022).
2.8 MUSCULOSKELETAL
Human Studies. Case reports and occupational health studies, which often had no exposure
measurements or comparison groups, reported that acroosteolysis, or resorption of the terminal phalanges
of the finger, was observed in a small percentage of workers occupationally exposed to vinyl chloride
(Dinman et al. 1971; Lilis et al. 1975; Marsteller et al. 1975; Sakabe 1975; Veltman et al. 1975; Wilson et
al. 1967). Bone lesions were most often confined to the terminal phalanges of the fingers, but in a few
cases the bones of the toes (Harris and Adams 1967), feet (Preston et al. 1976), sacroiliac joint (Harris
VINYL CHLORIDE 51
2. HEALTH EFFECTS
and Adams 1967), and arms, legs, pelvis, and mandible (Preston et al. 1976) were also involved.
Development of acroosteolysis was most often preceded by Raynaud’s phenomenon (Dinman et al. 1971;
Freudiger et al. 1988; Harris and Adams 1967; Magnavita et al. 1986; Markowitz et al. 1972; Preston et
al. 1976; Sakabe 1975; Veltman et al. 1975; Wilson et al. 1967). In two reports, bone resorption was
observed to progress despite discontinuation of exposure (Markowitz et al. 1972; Preston et al. 1976).
However, in two other reports, improvement was observed after exposure ceased (Veltman et al. 1975;
Wilson et al. 1967). Joint pain was also reported by Lilis et al. (1975).
Animal Studies. Although Sokal et al. (1980) found no alterations in the bones of male rats exposed to
20,000 ppm for 5 hours/day, 5 days/week for 10 months, Viola et al. (1971) observed skeletal changes
(i.e., osteochondroma) in the bones of rats exposed to 30,000 ppm for 4 hours/day, 5 days/week for
12 months.
Mechanisms. Impaired capillary microcirculation has been observed in vinyl chloride workers with
Raynaud’s phenomenon (Magnavita et al. 1986; Maricq et al. 1976). Because impaired microcirculation
in the fingertips has been associated with resorptive bone loss, it has been hypothesized that activation of
osteoclasts may be secondary to vascular insufficiency (Grainger et al. 1980; Ward 1976); however, no
data investigating this possible mechanism are available.
2.9 HEPATIC
Human Studies. A potential association between vinyl chloride exposure and liver toxicity was
evaluated in eight cohort studies, nine cross-sectional studies, four case-control studies (Table 2-3), and
many occupational health case reports and case series (i.e., studies of vinyl chloride workers with no
exposure measurements or relative to a comparison group) (not tabulated). Routine, noninvasive
techniques revealed hepatomegaly (14–37%) in a limited number of workers (Ho et al. 1991; Lilis et al.
1975; Maroni et al. 2003; Marsteller et al. 1975; NIOSH 1977; Suciu et al. 1975). However, when
peritoneoscopy was performed or biopsies were obtained from exposed workers, Marsteller et al. (1975)
found a much higher prevalence of hepatic abnormalities. Only 37% of the workers studied by Marsteller
et al. (1975) were diagnosed with hepatomegaly, but peritoneoscopy revealed a 50% incidence of granular
changes in the liver surface and an 86% incidence of capsular fibrosis with increased numbers of capsular
vessels. Histopathological examination of the biopsied tissue from these workers revealed an 80%
incidence of collagenization of the sinusoidal walls, a 90% incidence of proliferation of cells lining the
sinusoids, a 30% incidence of septal fibrosis, and degeneration of hepatocytes (incidence not specified).

VINYL CHLORIDE 52
2. HEALTH EFFECTS
A number of other investigators observed fibrotic changes in liver tissues obtained from workers exposed
to vinyl chloride or detected by liver ultrasonography of exposed workers (Cave et al. 2010; Falk et al.
1974; Gedigke et al. 1975; Hsiao et al. 2004; Hsieh et al. 2007; Lee et al. 1977b; Maroni et al. 2003;
Popper and Thomas 1975; Tamburro et al. 1984). Steatosis (i.e., fatty liver) and steatohepatitis (i.e., fatty
liver with inflammatory changes) was also observed in studies of exposed workers (Cave et al. 2010;
Hsiao et al. 2004; Maroni et al. 2003; Zhu et al. 2005a).
Table 2-3. Results of Epidemiological Studies Evaluating Exposure to Vinyl
Chloride and Liver Effects (Noncancer)
Reference, study type, and
population
Exposure or biomarker
Outcome evaluated
Result
a
Lee et al. 2020
Cross-sectional, 108 male and
5 female workers (Taiwan)
2,065 µg/m
3
; mean of high-VCM
group
Albumin, AST, ALT, GGT,
total and direct bilirubin,
total cholesterol, TG, ALP
↔
Yuan et al. 2020
Cross-sectional, 447 adult
residents (Taiwan)
Urinary TdGA >232.7 μg/g
creatinine; residents living 10–
20 km from petrochemical
complex
b
FIB-4
↑
Fedeli et al. 2019a
Cohort (mortality), 1,658 male
workers (Italy)
Cumulative exposure
>2,378 ppm-years; workers in
vinyl chloride production and
polymerization facility
Cirrhosis
↑
Wang et al. 2019b
Cross-sectional, 303 school-
aged children (6–13 years)
(Taiwan)
Urinary TdGA ≥160 μg/g
creatinine; children living within
10 km of a petrochemical
complex
AST
↑
ALT, FIB-4, APRI
↔
Mundt et al. 2017
Cohort (mortality), 9,951 vinyl
chloride workers (35 facilities
in the United States)
287 to <2,271 ppm-year (3
rd
and
4
th
quintiles of cumulative
exposure)
Cirrhosis
↑
Cave et al. 2010
Case-control, 16 male, non-
obese, highly-exposed workers
with steatohepatitis, 26 healthy
worker controls, and
11 unexposed, healthy
volunteers (Kentucky, United
States)
11,319 ppm-years, estimated
mean cumulative, long-term
exposure (mean 18.9 years)
CK-18 (whole)
↑
AST, ALT, CK-18
(caspase-cleaved
fragments), TG
↔

VINYL CHLORIDE 53
2. HEALTH EFFECTS
Table 2-3. Results of Epidemiological Studies Evaluating Exposure to Vinyl
Chloride and Liver Effects (Noncancer)
Reference, study type, and
population
Exposure or biomarker
Outcome evaluated
Result
a
Attarchi et al. 2007
Cross-sectional, 52 male PVC
plant workers and 48 male
office workers (Iran)
mean 0.8 ppm, long-term
exposure (mean 9 years)
ALP, GGT
↑
ALT, AST, total and direct
bilirubin
↔
Hsieh et al. 2007
Cohort, 320 male workers in
PVC plants (Taiwan); disease
incidence determined by
ultrasound
Significant exposure-response
trend for 40–400, 400–800, and
>800 ppm-years compare to
<40 ppm-years
Fibrosis (cirrhosis and pre-
cirrhosis)
↑
Maroni and Fanetti 2006
Cohort, 735 male and
22 female workers in vinyl
chloride/PVC plants (Italy)
>1,000 ppm-years, cumulative
exposure, or 500 ppm, historical
maximum yearly average
exposure
GGT, AST, ALT, total and
conjugated bilirubin, TG,
cholesterol, AST/ALT ratio
>1
↔
Zhu et al. 2005a
Cohort, 163 male and
75 female workers at a vinyl
chloride polymerization plant
(China); disease incidence
determined by ultrasound
>15,000 mg, mean cumulative
exposure dose
Liver ultrasonography
abnormality
↑
Fatty liver, hepatic
hemangioma
↔
Hsiao et al. 2004
Cohort, 347 male workers
(Taiwan); disease incidence
determined by ultrasound
Cumulative exposure 2,400 ppm-
months; workers with history of
high exposure jobs
Fibrosis
↑
Pre-cirrhosis
↑
Cirrhosis
↔
Fatty liver
↔
Current exposure ≥10 ppm
AST, ALT, GGT
↔
Mastrangelo et al. 2004
Case-control (nested in a VCM
worker cohort), 40 cases of
cirrhosis, 139 controls without
chronic liver diseases/cancers
(Italy)
>2,500 ppm-years, cumulative
exposure
Cirrhosis
↑
Maroni et al. 2003
Cohort, 735 male and
22 female workers in vinyl
chloride/PVC plants (Italy);
disease incidence determined
by ultrasound
200 ppm (historical maximum
yearly average exposure) or
100–1,000 ppm-years
(cumulative exposure)
Periportal fibrosis
↑
500 ppm, historical maximum
yearly average exposure
Hepatomegaly, steatosis,
GGT, ALT, TG
↔

VINYL CHLORIDE 54
2. HEALTH EFFECTS
Table 2-3. Results of Epidemiological Studies Evaluating Exposure to Vinyl
Chloride and Liver Effects (Noncancer)
Reference, study type, and
population
Exposure or biomarker
Outcome evaluated
Result
a
Ward et al. 2001
Cohort (mortality), 12,700 male
workers in the vinyl-chloride
industry (Italy, Norway,
Sweden, United Kingdom)
≥524 ppm-years, estimated
cumulative exposure
Cirrhosis
↑
Cheng et al. 1999b
Cross-sectional, 251 male
workers in vinyl chloride
manufacturing plants with low
to moderate vinyl chloride
exposure (Taiwan)
0.44–1.63 ppm, range of median
vinyl chloride concentrations
from area sampling (moderate-
VCM-low-EDC group; range of
median EDC concentrations from
area sampling 0.32–0.44 ppm)
c
ALT, AST, GGT
↔
Du and Wang 1998
Case-control, 1,058 male
workers (current and former) at
PVC factories with vinyl
chloride exposure admitted to
hospitals from January 1985 to
March 1994 (Taiwan)
Exposed cases versus
unexposed controls (VCM
workers compared to optical
workers or motorcycle
manufacturers)
Cirrhosis, chronic liver
diseases (unspecified)
↑
Du et al. 1995
Cross-sectional, 244 workers
(7 females, 237 males) in PVC
manufacturing factories
(Taiwan)
56.3 ppm, current mean
exposure for high exposure
group
GGT
↑
AST, ALP, ALT
↔
Liss et al. 1985
Case-control, workers in vinyl
chloride/synthetic rubber
manufacturing plants; 15 cases
of chemical liver injury and
25 healthy worker controls
(United States)
Workers with biopsy evidence of
vinyl chloride-associated liver
damage (50% with exposure
ranking ≥4)
Cholylglycine, conjugates
of cholic acid, indocyanine
green clearance, and
serum bile acids
↑
ALP, ALT, AST and GGT
↔
Tamburro et al. 1984
Cross-sectional, 48 vinyl
chloride monomer workers
(United States); biopsy
samples
Cumulative exposure indices of
≥3.5 (on a scale from 1 to 6)
Focal hepatocyte
hyperplasia (histological
evidence of chemical liver
injury)
↑

VINYL CHLORIDE 55
2. HEALTH EFFECTS
Table 2-3. Results of Epidemiological Studies Evaluating Exposure to Vinyl
Chloride and Liver Effects (Noncancer)
Reference, study type, and
population
Exposure or biomarker
Outcome evaluated
Result
a
Vihko et al. 1984
Cross-sectional, 76 workers
with low to moderate
occupational exposures to
vinyl chloride (location not
reported)
Up to 1 ppm, mean exposure
time 3 years
ALT, chenodeoxycholic
acid (bile acid)
↑
GGT, LDH, conjugated
and total bilirubin, cholic
acid (bile acid)
↔
NIOSH 1977
Cross-sectional, 126 current
and 71 former male workers
with vinyl chloride exposure
(United States)
Current or former workers with
vinyl chloride exposure
(exposure estimates not
reported)
Hepatomegaly
↑
AST, ALP, and total
bilirubin
↔
Former vinyl chloride workers
LDH
↑
a
Up and down arrows were based on statistically significant results only.
b
Used TdGA as a biomarker for vinyl chloride and ethylene dichloride exposure.
c
Workers exposed to vinyl chloride and ethylene dichloride.
↑ = association with increase; ↓ = association with decrease; ↔ = no association; ALP = alkaline phosphatase;
ALT = alanine amino transferase; APRI = AST to platelet ratio index; AST = aspartate amino transferase;
CK-18 = serum cytokeratin 18; EDC = ethylene dichloride; FIB-4 = fibrosis-4 liver fibrosis index model considering
age, AST, ALT, and platelet count as variables; GGT = gamma-glutamyl transferase; LDH = lactate dehydrogenase;
PVC = polyvinyl chloride; TdGA = thiodiglycolic acid; TG = serum triglycerides; VCM = vinyl chloride monomer
Hepatic lesions in workers exposed to vinyl chloride generally include the following features identified by
liver biopsy: hypertrophy and hyperplasia of hepatocytes, activation and hyperplasia of sinusoidal lining
cells, fibrosis of the portal tracts and the septa and intralobular perisinusoidal regions, sinusoidal dilation,
and focal areas of hepatocellular degeneration (Berk et al. 1975; Falk et al. 1974; Gedigke et al. 1975; Ho
et al. 1991; Jones and Smith 1982; Lilis et al. 1975; Liss et al. 1985; Marsteller et al. 1975; NIOSH 1977;
Popper and Thomas 1975; Suciu et al. 1975; Tamburro et al. 1984; Vihko et al. 1984). The incidence and
severity of the effects correlated well with the duration of exposure (Gedigke et al. 1975; Lilis et al. 1975;
NIOSH 1977).
Standard biochemical liver function tests appear to have low sensitivity for detecting liver injury
produced by vinyl chloride (Berk et al. 1975; Cave et al. 2010; Cheng et al. 1999b; Hsiao et al. 2004; Lee
et al. 1977b, 2020; Maroni and Fanetti 2006; Maroni et al. 2003; Marsteller et al. 1975; NIOSH 1977;
Tamburro et al. 1984; Vihko et al. 1984). For example, the values obtained in several standard
biochemical liver function tests (e.g., activities of serum alkaline phosphatase [ALP], aspartate
aminotransferase [AST], alanine aminotransferase [ALT], gamma-glutamyltransferase [GGT]) from
VINYL CHLORIDE 56
2. HEALTH EFFECTS
workers with biopsy or ultrasonographic evidence of vinyl chloride-associated liver damage were not
significantly higher than those from unexposed controls (Cave et al. 2010; Hsiao et al. 2004; Liss et al.
1985). Cytokeratin 18 (CK-18) was elevated in vinyl chloride workers with steatohepatitis (Cave et al.
2010). Serum ALP, ALT, and/or GGT levels were increased in some studies of workers exposed to high
concentrations of vinyl chloride (1–20 ppm) (Du et al. 1995; Ho et al. 1991; Lilis et al. 1975). Serum
ALP and GGT levels were increased by 10 and 29%, respectively, in workers exposed for at least 2 years
to concentrations <1 ppm (Attarchi et al. 2007). Serum bile acids (Berk et al. 1975; Liss et al. 1985)
and/or the results from the indocyanine green clearance test (Liss et al. 1985; Tamburro et al. 1984)
correlated with liver injury. Furthermore, investigators were able to demonstrate that levels of
chenodeoxycholic acid (a serum bile acid) in asymptomatic vinyl chloride workers were elevated when
compared to the 95% interval of values from a healthy reference population (Vihko et al. 1984). The
serum hyaluronic acid concentration was elevated in workers with angiosarcoma of the liver, even when
other liver function tests were normal (McClain et al. 2002). The fibrosis-4 (FIB-4) score, which
evaluates liver fibrosis based on a model considering age, platelet count and AST and ALT levels, was
elevated in residents living near a petrochemical complex in Taiwan (Yuan et al. 2020). Vinyl chloride
exposure in this study was estimated using thiodiglycolic acid as a urinary biomarker. Children with
elevated urinary thiodiglycolic acid concentrations living near the same petrochemical complex did not
exhibit significantly increased FIB-4 scores or an elevated AST to platelet ratio (APRI) (Wang et al.
2019b); however, these indices may not be accurate predictors of liver fibrosis or injury in children
(Alkhouri et al. 2014). AST levels were significantly elevated in highly exposed children, suggesting a
potential for toxicity in this population.
An increase in mortality from liver cirrhosis was demonstrated in several cohort studies of vinyl chloride
workers (Fedeli et al. 2019a; Hsieh et al. 2007; Mastrangelo et al. 2004; Ward et al. 2001). Morbidity
associated with liver cirrhosis was also reported to be elevated among vinyl chloride workers (Du and
Wang 1998). Alcohol intake was not evaluated as a critical confounding factor in these studies.
Mastrangelo et al. (2004) evaluated the possible interaction between alcohol consumption, hepatitis
infection, and liver cirrhosis in a large cohort of vinyl chloride workers. Vinyl chloride was suggested to
be an independent risk factor for liver cirrhosis with a synergistic interaction described for alcohol
consumption and an additive interaction observed for hepatitis infection. Liver ultrasonography revealed
an increase in the incidence of periportal fibrosis in vinyl chloride workers compared to unexposed
workers from the same plants (Maroni et al. 2003). Portal fibrosis and portal hypertension were
considered to contribute to mortality in several studies (Lee et al. 1996; Lelbach 1996). A meta-analysis
of seven studies that included >40,000 vinyl chloride workers did not demonstrate increased mortality
VINYL CHLORIDE 57
2. HEALTH EFFECTS
from liver cirrhosis (Frullanti et al. 2012); however, that may have resulted from cirrhosis not being
included on death certificates when a person died from liver cancer (Fedeli et al. 2019b; Mastrangelo et
al. 2013).
Animal Studies. Brief exposures of animals to extremely high concentrations of vinyl chloride lead to
hepatic damage. For example, acute-duration exposure (30 minutes) of guinea pigs and mice to
300,000 ppm of vinyl chloride produced liver congestion or severe fatty degeneration, while 200,000 ppm
caused fatty infiltration in rats (Mastromatteo et al. 1960). Exposure to 100,000 ppm for 6 hours
produced centrilobular vacuolization and increased alanine serum α-ketoglutarate transaminase activity in
rats (Jaeger et al. 1974). However, exposure of rats to 50,000 ppm for 6 hours produced no observable
effects on the liver (Reynolds et al. 1975a, 1975b). In contrast, a single-concentration study in which
pregnant rats were continuously exposed to 1,500 ppm for 7–9 days during either the first or second
trimester of pregnancy resulted in an increase in the liver-to-body-weight ratio (Ungvary et al. 1978).
Absolute and relative liver weight was also increased (by 9 or 10%, respectively) in pregnant rats exposed
to 2,500 ppm vinyl chloride for 7 hours/day on gestational days (GDs) 6–15 (John et al. 1977, 1981).
In studies with longer durations of exposure, lower concentrations of vinyl chloride have produced
hepatic toxicity. Histopathological signs of hepatotoxicity observed in rats have included fatty liver and
hepatocellular degeneration (Sokal et al. 1980; Torkelson et al. 1961; Wisniewska-Knypl et al. 1980),
swelling of hepatocytes with compression of sinusoids (Lester et al. 1963), dilation of the rough
endoplasmic reticulum (Du et al. 1979), nuclear polymorphism (Sokal et al. 1980), hypertrophy of
smooth endoplasmic reticulum (Thornton et al. 2002; Wisniewska-Knypl et al. 1980), and proliferation of
reticulocytes (Sokal et al. 1980). Changes in metabolic enzyme activities, such as cytochrome P-450,
glucose-6-phosphatase, glutathione reductase, and glucose-6-phosphate dehydrogenase, occurred after
inhalation exposure in rats (Du et al. 1979; Wisniewska-Knypl et al. 1980). Increased liver-to-body-
weight ratio was observed in several studies following intermediate-duration exposure (Bi et al. 1985;
Lester et al. 1963; Sokal et al. 1980; Thornton et al. 2002; Torkelson et al. 1961). Lester et al. (1963) was
not included in Table 2-1 or Figure 2-2 due to parasitic liver cysts present in all animals, suggesting
colony contamination. Histopathological liver lesions in mice have included lipid droplets, eosinophilic
changes, nuclear condensation, steatosis, hepatic edema, cytoplasmic loosening, and hepatocyte nuclear
fragmentation (Jia et al. 2022; Wang et al. 2019a). Mice exposed to vinyl chloride and fed a high-fat diet
experienced liver damage (steatosis), neutrophil infiltration, apoptosis, and oxidative and endoplasmic
reticulum stress compared to exposed mice fed a normal or low-fat diet (Chen et al. 2019; Fujiwara 2018;
Jia et al. 2022; Lang et al. 2018, 2020; Liang et al. 2018; Liu et al. 2023; Wahlang et al. 2020).
VINYL CHLORIDE 58
2. HEALTH EFFECTS
Exposure of rats to 500 ppm for 7 hours/day, 5 days/week for 4.5 months resulted in an increase in liver-
to-body-weight ratio and granular tissue degeneration (Torkelson et al. 1961). An increased liver-to-
body-weight ratio was also found in rats exposed to 100 ppm vinyl chloride for 7 hours/day, 5 days/week
for 6 months (Torkelson et al. 1961). The liver-to-body-weight ratio was increased (14–68%) in a dose-
related manner at concentrations of 11.1, 105.6, and 2,918 ppm vinyl chloride in male rats exposed for
6 hours/day, 6 days/week for 6 months (Bi et al. 1985). In contrast, relative liver weight was decreased in
mice exposed to 983 ppm vinyl chloride for 6 hours/day, 5 days/week for 8 weeks (Sharma and Gehring
1979). No changes in liver weights were reported when immunized rabbits were exposed up to 983 ppm
vinyl chloride 6 hours/day, 5 days/week for 8 weeks (Sharma et al. 1980). Exposure of rats to 500 ppm
for 5 hours/day, 5 days/week for 10 months produced swelling of hepatocytes and proliferation of
reticuloendothelial cells, increased liver weight, and cellular degeneration; at 50 ppm, small lipid droplets
and proliferation of smooth endoplasmic reticulum were noted (Sokal et al. 1980). Histopathological
examination of rats exposed to either 50,000 ppm vinyl chloride for 8 hours/day for 19 consecutive days
or 20,000 ppm vinyl chloride for 8 hours/day, 5 days/week, for 92 days showed hepatocellular
hypertrophy, vacuolization, and sinusoidal compression (Lester et al. 1963); this study was not included
in Table 2-1 or Figure 2-2 due to colony contamination.
Mice exposed to 313 ppm of vinyl chloride for 2 hours/day, 5 days/week for 13 weeks had decreased
absolute liver weight and increased number of fat droplets in the liver (Jia et al. 2022). Histopathological
changes in the liver that included hyperplasia of hepatocytes and activated sinusoidal cells were seen in
mice exposed to 2,500 ppm vinyl chloride 5 hours/day, 5 days/week for up to 6 months (Schaffner 1978).
Centrilobular necrosis and degeneration were noted in rabbits exposed to 200 ppm vinyl chloride
7 hours/day, 5 days/week for 6 months but not at 100 ppm vinyl chloride in this regimen (Torkelson et al.
1961). Exposure of rats to 50 ppm for 5 hours/day, 5 days/week for 10 months produced fatty
degeneration and proliferation of the smooth endoplasmic reticulum (Wisniewska-Knypl et al. 1980). In
contrast, no hepatic effects were seen in mice fed a control diet and exposed to 0.85 ppm vinyl chloride
for 12 weeks (0.85 ppm, 6 hours/day, 5 days/week) examined immediately after the exposure period or
9 months later (Liu et al. 2023). Liver effects were observed in a 2-generation reproductive toxicity study
where rats were exposed to ≥10 ppm vinyl chloride (6 hours/day for a 10-week premating period and a
3-week mating period, through GD 20, and from lactation day 4 through weaning [females only])
(Thornton et al. 2002). Absolute and relative mean liver weights were significantly increased at all
exposure levels in F0 males and in 100- and 1,100-ppm F1 males. Centrilobular hypertrophy, considered
to be a minimal adverse effect, was noted in the livers of all 1,100-ppm male and female F0 and F1 rats,
VINYL CHLORIDE 59
2. HEALTH EFFECTS
most 100-ppm male and female F0 and F1 rats, and 2/30 and 6/30 of the 10-ppm F0 male and F1 female
rats, respectively. Centrilobular hypertrophy was not noted in the 30 female rats of the control group.
Histopathological alterations occurring at 100 and 1,100 ppm included centrilobular hypertrophy and
acidophilic, basophilic, and clear cell foci.
The NOAELs for liver effects in a number of species following a 6-month exposure to vinyl chloride
indicated that mice and rats were the most sensitive (NOAEL of 50 ppm), rabbits were the next most
sensitive (NOAEL of 100 ppm), and dogs and guinea pigs were the least sensitive (NOAEL of >200 ppm)
(Torkelson et al. 1961).
Popper et al. (1981) compared histopathological findings from sections of liver from mice and rats
exposed by Maltoni and Lefemine (1975) with the liver biopsy material obtained from vinyl chloride
workers. Hyperplasia and hypertrophy of hepatocytes and/or sinusoidal cells, with areas of sinusoidal
dilation, were observed in both humans and rodents. The major difference between the species was the
greater degree of fibrosis, seen as reticulin deposition and collagen formation, in the livers of humans.
Also, inflammatory cells were present in the livers of humans but not rodents.
Chronic-duration exposure of rats to vinyl chloride in their feed for 149 weeks produced an increase in
the incidence of several types of microscopic liver lesions in male and female rats (Til et al. 1983, 1991).
Neoplastic and preneoplastic lesions in the liver included several types of foci of cellular alteration (i.e.,
clear-cell, basophilic, eosinophilic, or mixed), neoplastic nodules, hepatocellular carcinoma, and
angiosarcoma. Other liver lesions associated with vinyl chloride exposure included liver-cell
polymorphism and hepatic cysts (Til et al. 1983, 1991). Mottled livers with hemorrhagic patches were
seen in rats gavaged with ≥ 3 mg/kg/day for 2 years (Knight and Gibbons 1987). Chronic-duration oral
exposure of rats fed vinyl chloride daily during a 4-hour period for up to 2.7 years also resulted in areas of
hepatocellular alteration at concentrations as low as 1.7 mg/kg/day (Feron et al. 1981). In this study,
areas of necrosis were observed in the liver of female rats fed 5 mg/kg/day and male rats fed
14.1 mg/kg/day (Feron et al. 1981). At 1.7 mg vinyl chloride/kg/day, there was increased incidence of
hepatic cysts and clear or basophilic foci in female rats with male rats exhibiting the same foci (Til et al.
1983, 1991).
Mechanisms. The mechanisms of vinyl chloride liver toxicity were described by Rusyn et al. (2021)
(Figure 2-4). Vinyl chloride is metabolized to reactive intermediates including chloroethylene oxide and
chloroacetaldehyde. These metabolites produce mitochondrial dysfunction by damaging proteins and

VINYL CHLORIDE 60
2. HEALTH EFFECTS
uncoupling of the electron transport chain, leading to oxidative stress, altered lipid metabolism, and
glycogen depletion resulting in steatohepatitis. Oxidative stress leads to depletion of antioxidants, lipid
peroxidation, and protein damage leading to hepatocellular death and inflammation. Pro-inflammatory
signaling promotes remodeling of the extracellular matrix and fibrosis. Altered lipid metabolism resulting
from mitochondrial dysfunction contributes to steatosis.
Figure 2-4. Key Characteristics of Hepatotoxicity Associated with Vinyl Chloride
Source: Rusyn et al. 2021

VINYL CHLORIDE 61
2. HEALTH EFFECTS
2.10 RENAL
Human Studies. A retrospective mortality study of workers exposed to contaminated drinking water
(vinyl chloride, tetrachloroethylene, trichloroethylene, benzene) at Camp Lejeune in North Carolina did
not show an increase in mortality from kidney disease (Bove et al. 2014). An ecological study evaluating
residential exposure to contaminated groundwater reported an increased risk of decreased estimated
glomerular filtration rate (GFR) and increased proteinuria in residents living near a PVC plant in Taiwan
(Chen and Wu 2017). Groundwater was contaminated with vinyl chloride and other chlorinated solvents
including trichloroethylene, 1,1-dichloroethylene, 1,1-dichloroethane, 1,2-dichloroethane, and
cis-1,2-dichloroethene. No additional human studies were available regarding renal effects of vinyl
chloride exposure.
Animal Studies. Acute-duration exposure of mice and rats to 300,000 ppm of vinyl chloride for
30 minutes resulted in kidney congestion (Mastromatteo et al. 1960). Degenerative changes were
observed in the kidneys of one of five mice exposed to 100,000 or 200,000 ppm of vinyl chloride for
30 minutes (Mastromatteo et al. 1960). Relative kidney weight was increased by 20% in pregnant rats
exposed to >100 ppm vinyl chloride 6 hours/day on GDs 6–19 (Thornton et al. 2002). Exposure of rats to
50,000 ppm for 8 hours/day for 19 consecutive days or 20,000 ppm for 8 hours/day, 5 days/week for
92 days produced no adverse effects on the kidneys (Lester et al. 1963); this study was not included in
Table 2-1 or Figure 2-2 due to colony contamination. However, relative kidney weight was increased in
male rats exposed to 2,918 ppm for 6 hours/day, 6 days/week, for 3 and 12 months or 105.6 ppm vinyl
chloride for 6 hours/day, 6 days/week for 12 months after a 6-month observation period (Bi et al. 1985).
Relative kidney weights were increased in male rats exposed to 500 ppm vinyl chloride for 5 hours/day,
5 days/week, for 10 months, although no histopathological changes in the kidney were noted (Sokal et al.
1980). No changes in kidney weights were reported when mice or immunized rabbits were exposed to
983 ppm vinyl chloride 6 hours/day, 5 days/week for 8 weeks (Sharma and Gehring 1979; Sharma et al.
1980). Urinalysis values were within normal limits in rats and rabbits exposed to 200 ppm vinyl chloride
for up to 7 hours/day, 5 days/week, for 6 months (Torkelson et al. 1961). One year of exposure to
5,000 ppm vinyl chloride for 7 hours/day, 5 days/week produced an increase in the kidney-to-body-
weight ratio (Feron et al. 1979a) and tubular nephrosis in rats (Feron and Kroes 1979).
Renal toxicity was observed in mice where vinyl chloride in aqueous solution (0, 1, or 200 mg/mL) was
applied to the nasal cavity 5 days/week for up to 3 weeks (Hsu et al. 2019). Blood urea nitrogen (BUN)
and creatinine levels were increased at both concentrations and glomerulosclerosis and tubular injury
VINYL CHLORIDE 62
2. HEALTH EFFECTS
were observed. Immunohistochemical analysis showed an increase in markers of fibrosis and autophagy.
Fibrosis and autophagy were also observed in experiments using the HK-2 proximal tubular epithelial cell
line (Hsu et al. 2019).
2.11 DERMAL
Human Studies. Vinyl chloride exists as a liquid when stored under pressure. However, when it is
released from pressurized containers, it rapidly vaporizes to a gas. Thus, the adverse dermal effects
observed after exposure to vinyl chloride are not unique to vinyl chloride but can be expected as a result
of a rapidly evaporating liquid on the skin. The effects are due to tissue freezing rather than direct
toxicity of vinyl chloride. A man who had liquid vinyl chloride sprayed on his hands developed second-
degree burns. At first, the man reported that his hands felt numb. Within a short period, the hands had
developed marked erythema and edema (Harris 1953). Dermatological symptoms (not further specified)
were reported in residents seeking medical attention following derailment of a train carrying vinyl
chloride (Shumate et al. 2017).
Case reports and occupational health studies indicated that exposure to vinyl chloride resulted in
scleroderma-like skin changes on the hands of a small percentage of exposed workers (Freudiger et al.
1988; Lilis et al. 1975; Marsteller et al. 1975; Suciu et al. 1975; Veltman et al. 1975; Walker 1976). The
skin changes were characterized by a thickening of the skin (Lilis et al. 1975; Markowitz et al. 1972;
Ostlere et al. 1992; Preston et al. 1976; Veltman et al. 1975; Walker 1976), decreased elasticity (Lilis et
al. 1975), and edema (Lilis et al. 1975; Suciu et al. 1975) and were almost exclusively observed in
exposed individuals who also suffered from Raynaud's phenomenon. Skin biopsies revealed increased
collagen bundles in the subepidermal layer of the skin (Harris and Adams 1967; Markowitz et al. 1972;
Ostlere et al. 1992; Veltman et al. 1975). Biochemical analyses by Jayson et al. (1976) demonstrated that
a high rate of collagen synthesis was taking place in the affected skin. The skin changes were most often
confined to the hands and wrists, but Jayson et al. (1976) reported scleroderma-like skin changes on the
hands, arms, chest, and face of one afflicted worker.
Animal Studies. Skin changes were observed in rats exposed to 30,000 ppm for 12 months (Viola 1970).
The skin of the paws of the exposed rats showed areas of hyperkeratosis, thickening of the epidermis,
edema, collagen dissociation, and fragmentation of the elastic reticulum. Interpretation of these results is
limited by the absence of a statistical analysis and insufficient information on the treatment of control
animals. Lester et al. (1963) reported that male rats exposed to 50,000 ppm vinyl chloride 8 hours/day for
VINYL CHLORIDE 63
2. HEALTH EFFECTS
19 days had thin coats and scaly tails, while females exposed to the same concentration showed no
effects; this study was not included in Table 2-1 or Figure 2-2 due to colony contamination.
Daily administration of 30 mg/kg of vinyl chloride to rats by gavage for 2 years produced increased
thickness, moisture content, and collagen content of the skin. Newly synthesized intermolecular and
intramolecular collagen crosslinks were also significantly increased (Knight and Gibbons 1987).
2.12 OCULAR
Human Studies. Local burns on the conjunctiva and cornea were observed in a man who died after
exposure to an unknown quantity of vinyl chloride escaping from an open valve (Danziger 1960). First
responders to a train derailment and nearby refinery workers reported irritation, pain, or burning of eyes
(Brinker et al. 2015; Wilken et al. 2015). Ocular symptoms (not further specified) were also reported in
nearby residents seeking medical attention after the train derailment (Shumate et al. 2017).
Animal Studies. No adverse ocular effects were noted in guinea pigs exposed for 30 minutes to up to
400,000 ppm vinyl chloride in inhalation chambers (Mastromatteo et al. 1960).
2.13 ENDOCRINE
Human Studies. A study of workers exposed to vinyl chloride in PVC manufacturing plants reported that
most workers who presented with scleroderma were shown to have thyroid insufficiency detected by
reduced iodine uptake (Suciu et al. 1975).
Animal Studies. No histopathological effects on the adrenals were reported in guinea pigs exposed to
400,000 ppm for 30 minutes (Mastromatteo et al. 1960). No changes in adrenal weights were reported
when immunized rabbits were exposed up to 983 ppm vinyl chloride 6 hours/day, 5 days/week for
8 weeks (Sharma et al. 1980). Rats exposed to 30,000 ppm vinyl chloride 4 hours/day, 5 days/week for
12 months were found to have colloid goiter and markedly increased numbers of perifollicular cells
(Viola 1970).

VINYL CHLORIDE 64
2. HEALTH EFFECTS
2.14 IMMUNOLOGICAL
Human Studies. The potential association between vinyl chloride exposure and immunological toxicity
was evaluated in five cross-sectional studies, three case-control studies (Table 2-4), and many
occupational health studies, case reports, and case series. Male workers exposed to vinyl chloride for an
average of 8 years, with concentrations ranging from 1 to 300 ppm during sampling periods, were found
to have significantly increased percentages of lymphocytes compared to controls (Fucic et al. 1995,
1998). Additionally, 75 out of these 100 workers showed disturbances of mitotic activity in their
lymphocytes. A statistically significant increase in circulating immune complexes was observed in vinyl
chloride workers when compared to the levels in unexposed workers (Bogdanikowa and Zawilska 1984;
Saad et al. 2017). The increase in circulating immune complexes was greatest in women and in those
with duties involving exposure to relatively higher levels of vinyl chloride. Compared to controls, IgG
levels were significantly increased in women exposed to the high levels of vinyl chloride in the same
study (Bogdanikowa and Zawilska 1984). Serum immunoglobulins (IgA, IgG, and IgM) and other
inflammatory markers (i.e., ceruloplasmin, orosomucoid) were elevated in highly exposed male vinyl
chloride workers when compared to a similar worker population exposed to lower concentrations (Bencko
et al. 1988) or an unexposed residential population (Wagnerova et al. 1988). Proinflammatory cytokine
levels (tumor necrosis factor-α, interleukin-1β, interleukin-6, and interleukin-8) were increased in the
serum of vinyl chloride-exposed workers with steatohepatitis when compared with healthy control
workers (Cave et al. 2010).
Table 2-4. Results of Epidemiological Studies Evaluating Exposure to Vinyl
Chloride and Immunological Effects
Reference, study type, and
population
Exposure or biomarker
Outcome evaluated
Result
a
Saad et al. 2017
Cross-sectional, 20 workers
(Egypt)
Exposed versus
unexposed (15 healthy
controls)
Circulating immune complexes,
complement factors C3 and C4
↑
Cave et al. 2010
Case-control, 16 male, non-
obese, highly exposed workers
with steatohepatitis, 26 healthy
worker controls, and
11 unexposed, healthy
volunteers (Kentucky, United
States)
11,319 ppm-years,
estimated mean
cumulative, long-term
exposure (mean
18.9 years)
TNF-α, IL-1β, IL-6, and IL-8
↑

VINYL CHLORIDE 65
2. HEALTH EFFECTS
Table 2-4. Results of Epidemiological Studies Evaluating Exposure to Vinyl
Chloride and Immunological Effects
Reference, study type, and
population
Exposure or biomarker
Outcome evaluated
Result
a
Fucic et al. 1998
Cross-sectional, 121 male
VCM workers, 60 unexposed
controls (Croatia)
300±100 ppm (18.9 years
duration)
Absolute and relative
b
lymphocyte
counts
↑
Fucic et al. 1995
Cross-sectional, 100 male
VCM workers, 100 unexposed
controls (Croatia)
1 ppm (up to 300 ppm for
short periods)
Percent lymphocytes
↑
Bencko et al. 1988
Cross-sectional, 59 male VCM
workers exposed to >4 ppm
compared to 98 male VCM
workers exposed <4ppm
(Czech Republic)
>4 ppm
Serum IgG, IgA, IgM,
ceruloplasmin, orosomucoid
↑
Wagnerova et al. 1988
Cross-sectional, 110 VCM
workers (59 smokers and
51 nonsmokers), 55 age-
matched residential controls
(Czechoslovakia)
Exposed versus
unexposed
Serum IgA, IgG, IgM, lysozyme,
orosomucoid, α
2
-macroglobulin,
ceruloplasmin
↑
Transferrin, α
1
-antitrypsin
↔
Black et al. 1983, 1986
Case-control, 44 workers with
“vinyl chloride disease”
c
,
30 asymptomatic worker
controls, 200 unexposed
controls (United Kingdom)
Exposed versus
unexposed
HLA-DR5 antigen; severity of
disease correlated with HLA-DR3
and HLA-B8 antigens
↑
Antinuclear, anticentromere, anti-
Scl-70 and collagen antibodies
↓
Bogdanikowa and Zawilska
1984
Cross-sectional, 136 vinyl
chloride workers,
41 unexposed controls
(Poland)
Exposed versus
unexposed
Circulating immune complexes,
IgG concentration
↑

VINYL CHLORIDE 66
2. HEALTH EFFECTS
Table 2-4. Results of Epidemiological Studies Evaluating Exposure to Vinyl
Chloride and Immunological Effects
Reference, study type, and
population
Exposure or biomarker
Outcome evaluated
Result
a
Grainger et al. 1980
Case-control, 53 workers with
definite or possible “vinyl
chloride disease”
c
,
35 asymptomatic worker
controls, (location not
specified)
Exposed versus
unexposed
Circulating immune complexes,
cryoglobulinemia, C3 complement
activation, altered IgG structure
↑
a
Up and down arrows were based on statistically significant results only.
b
Relative to the white blood cell count.
c
Symptoms of “vinyl chloride disease” include Reynaud’s phenomenon, scleroderma-like lesions, dyspnea,
arthralgia, and myalgia, as well as radiological evidence of acroosteolysis.
↑ = association with increase; ↓ = association with decrease; ↔ = no association; HLA = human lymphocytic
antigen; Ig = immunoglobulin; IL-1β = interleukin-1β; IL-6 = interleukin-6; IL-8 = interleukin-8; TNF-α = tumor
necrosis factor-α; VCM = vinyl chloride monomer
Studies of workers who developed "vinyl chloride disease," a syndrome consisting of Raynaud's
phenomenon, acroosteolysis, joint and muscle pain, enhanced collagen deposition, stiffness of the hands,
and scleroderma-like skin changes, indicate that this disease may have an immunologic basis. Sera
obtained from patients with varying degrees of severity of symptoms of vinyl chloride disease
demonstrate a close correlation between the disease severity and the frequency of the immunologic
abnormality (Grainger et al. 1980; Langauer-Lewowicka et al. 1976; Ward 1976), although these
symptoms have also been reported without immunological findings (Black et al. 1986; Ostlere et al.
1992). The most frequent immunologic finding in workers with vinyl chloride disease is an increase in
circulating immune complexes or cryoglobulinemia. In workers with the most severe clinical signs, there
also are an increased incidence of B-cell proliferation, hyperimmunoglobulinemia (Ward 1976),
cryoglobulinemia (Grainger et al. 1980), and complement activation (Grainger et al. 1980; Ward 1976).
Evidence of a structurally altered IgG is sometimes observed, and it has been proposed that vinyl chloride
(or a metabolite) binds to IgG (Grainger et al. 1980).
Based on the similarity of vinyl chloride disease and systemic sclerosis, which may be a genetically
linked autoimmune disease, Black et al. (1983, 1986) examined the human lymphocyte antigen (HLA)
phenotypes of patients with vinyl chloride disease. Many autoimmune diseases show statistically
significant associations with certain HLA alleles. These authors found that when compared to unexposed
VINYL CHLORIDE 67
2. HEALTH EFFECTS
controls or asymptomatic controls, workers with vinyl chloride disease were more likely to possess the
HLA-DR5 allele. Furthermore, among those with the disease, the severity of the symptoms was
significantly related to the possession of the HLA-DR3 and B8 alleles. These authors concluded that
susceptibility was increased in the presence of HLA-DR5 or a gene in linkage disequilibrium with it.
Progression was favored in those with the HLA-DR3 and B8 phenotypes. Immune system dysfunction
has also been linked to a case of polymyositis (i.e., muscle fiber necrosis and atrophy) in an exposed
worker where there was involvement of antibodies to histidyl-t-RNA synthetase (Jo-1) (Serratrice et al.
2001). Splenomegaly was reported in a number of case reports and occupational health studies (Ho et al.
1991; Marsteller et al. 1975; Popper and Thomas 1975; Suciu et al. 1975; Veltman et al. 1975).
Animal Studies. No histopathological changes were noted in the spleen or lymph nodes of guinea pigs
exposed to 400,000 ppm vinyl chloride for 30 minutes (Mastromatteo et al. 1960). An increase in the
relative spleen weight was observed in rats exposed to 50 ppm for 5 hours/day, 5 days/week for
10 months (Sokal et al. 1980). Although no dose response was evident, increased relative spleen weight
was also reported by Bi et al. (1985) when rats were exposed to either 11.1 ppm for 6 hours/day,
6 days/week for 6 months or 2,918 ppm for 6 hours/day, 6 days/week for 3 months (Bi et al. 1985).
The immunologic effects of vinyl chloride were also examined in mice and rabbits (Sharma and Gehring
1979; Sharma et al. 1980). Rabbits were injected with a 1:1 mixture of tetanus toxoid and Freud's
complete adjuvant in their footpad or an intradermal injection of tuberculin. Lymphocytes isolated from
the spleens of mice and immunized rabbits exposed to concentrations as low as 10 ppm vinyl chloride
6 hours/day, 5 days/week for 4 weeks had increased spontaneous proliferation and in mice, mitogen-
stimulated responses to phytohemagglutinin and pokeweed mitogen. This increase was not observed
when lymphocytes from unexposed mice were cultured in the presence of vinyl chloride but was observed
in the presence of the vinyl chloride metabolite, thiodiglycolic acid (Sharma and Gehring 1979; Sharma et
al. 1980). Increased absolute and relative thymus weights were also seen in immunized rabbits exposed
to 983 ppm (Sharma et al. 1980). Despite the increased immunoactivity in immunized rabbits exposed to
vinyl chloride, the exposure did not affect antigen-induced immune responses (Sharma et al. 1980). A
2-fold increase in pulmonary interstitial macrophages was reported in male C57BL/6 mice exposed to
0.8 ppm vinyl chloride 6 hours/day, 5 day/week for 12 weeks; however, the levels of alveolar
macrophages, circulating or bronchoalveolar lavage fluid (BALF) immune cells, cytokines or
chemokines, endothelial progenitor cells, or platelet-immune cell aggregates were unaffected by exposure
(Zelko et al. 2022).

VINYL CHLORIDE 68
2. HEALTH EFFECTS
Mechanisms. Vinyl chloride disease exhibits many of the characteristics of autoimmune diseases
(Raynaud's phenomenon and scleroderma). B-cell proliferation, hyperimmunoglobulinemia, and
complement activation, as well as increased circulating immune complexes or cryoglobulinemia, have
been noted in affected workers indicating stimulation of immunological responses (Bogdanikowa and
Zawilska 1984; Grainger et al. 1980; Ward 1976). Mechanisms for the vascular changes, such as those
occurring with Raynaud's phenomenon, have been proposed by Grainger et al. (1980) and Ward (1976).
According to these mechanisms, a reactive vinyl chloride intermediate metabolite, such as
2-chloroethylene oxide or 2-chloroacetaldehyde, binds to a protein such as IgG. The altered protein
initiates an immune response, with deposition of immune products along the vascular endothelium.
Circulating immune complexes are proposed to precipitate in response to low temperatures, and these
precipitates are proposed to cause blockage of the small blood vessels. Scleroderma is an autoimmune
disease of unknown etiology that involves a chronic hardening and contraction of the skin and connective
tissues. It is characterized clinically by cutaneous and visceral fibrosis and can range from limited skin
involvement to extensive cutaneous sclerosis with internal organ changes, including an enlarged and
fibrotic spleen. Fetal cells may be involved in the pathogenesis of scleroderma. An increase in the
number of microchimeric cells of fetal origin was reportedly associated with dermal fibrosis in mice
injected with vinyl chloride (Christner et al. 2000).
2.15 NEUROLOGICAL
Human Studies. Epidemiology studies evaluating neurological effects of vinyl chloride exposure include
two cohort studies, two volunteer studies, and three cross-sectional studies (Table 2-5). Other reports
include three medical surveillance reports following a train derailment plus several occupational health
studies and case reports, which often had no exposure measurements or comparison group (not tabulated).
Table 2-5. Results of Epidemiological Studies Evaluating Exposure to Vinyl
Chloride and Neurological Effects
Reference, study type, and
population
Exposure or biomarker
Outcome evaluated
Result
a
Bove et al. 2014
Cohort (mortality),
8,964 Marine and Navy
personnel stationed at Camp
Lejeune (California, United
States)
>500 µg/L-months
(contaminated drinking
water)
Amyotrophic lateral sclerosis
↔

VINYL CHLORIDE 69
2. HEALTH EFFECTS
Table 2-5. Results of Epidemiological Studies Evaluating Exposure to Vinyl
Chloride and Neurological Effects
Reference, study type, and
population
Exposure or biomarker
Outcome evaluated
Result
a
Zhu et al. 2005a
Cohort, 163 male and
75 female workers at a vinyl
chloride polymerization plant
(China)
>15,000 mg, mean
cumulative exposure dose
Neurasthenia (not further defined)
↑
Perticoni et al. 1986
Cross-sectional, 64 male vinyl
chloride workers (Italy)
Exposed versus unexposed
(not quantified)
Peripheral neuropathy
(denervation-related fasciculations
and fibrillations and increased
duration and amplitude of motor
unit potentials)
↑
NIOSH 1977
Cross-sectional, 126 current
and 71 former male workers
with vinyl chloride exposure
(United States)
Current or former workers
with vinyl chloride exposure
(exposure estimates not
reported)
Headache, loss of consciousness,
depressed reflexes
↑
Spirtas et al. 1975
Cross-sectional, 491 vinyl
chloride and PVC workers
Exposure-response
relationship observed
(exposure estimates from
job categories; low: 0–
10 ppm, high: 20–30 ppm)
Headache, lightheadedness,
dizziness, paresthesia, fatigue
↑
Muscle weakness
↔
Lester et al. 1963
Volunteers, 3 men and
3 women
≥12,000 ppm for 5 minutes
twice a day in periods
separated by 6 hours on
3 consecutive days
Dizziness, headache, nausea
↑
Patty et al. 1930
Volunteers, 2 (gender not
specified) (United States)
25,000 ppm for 3 minutes
Dizziness, disorientation,
headache, burning sensation in
feet
↑
a
Up arrows were based on statistically significant results only.
↑ = association with increase; ↔ = no association
Neurological symptoms, including headache, dizziness, and lightheadedness were reported in first
responders, refinery workers, and nearby residents following derailment of a train carrying vinyl chloride
(Brinker et al. 2015; Shumate et al. 2017; Wilken et al. 2015). No abnormalities were observed by head
CT scan or brain MRI evaluations of nearby residents seeking medical attention (Shumate et al. 2017).
Frequently reported central nervous system symptoms are consistent with the anesthetic properties of
vinyl chloride. A man who had liquid vinyl chloride sprayed on his hands initially reported that his hands

VINYL CHLORIDE 70
2. HEALTH EFFECTS
felt numb (Harris 1953). The most commonly reported central nervous system effects are ataxia or
dizziness (Ho et al. 1991; Langauer-Lewowicka et al. 1983; Lilis et al. 1975; Marsteller et al. 1975;
Shumate et al. 2017; Spirtas et al. 1975; Suciu et al. 1975; Veltman et al. 1975), drowsiness or fatigue
(Langauer-Lewowicka et al. 1983; Spirtas et al. 1975; Suciu et al. 1975; Walker 1976), loss of
consciousness (NIOSH 1977), and/or headache (Brinker et al. 2015; Langauer-Lewowicka et al. 1983;
Lilis et al. 1975; Marsteller et al. 1975; NIOSH 1977; Shumate et al. 2017; Spirtas et al. 1975; Suciu et al.
1975; Veltman et al. 1975; Wilken et al. 2015) and neurasthenia (i.e., lassitude, fatigue, headache, and
irritability) (Zhu et al. 2005a). Other central nervous system effects that were reported by vinyl chloride
workers include euphoria and irritability (Suciu et al. 1975), visual and/or hearing disturbances
(Marsteller et al. 1975), nausea (Marsteller et al. 1975; Spirtas et al. 1975; Wilken et al. 2015), memory
loss (Langauer-Lewowicka et al. 1983; Suciu et al. 1975), plus nervousness and sleep disturbances
(Langauer-Lewowicka et al. 1983; Suciu et al. 1975). Central nervous system tests revealed pyramidal
signs and cerebellar disturbances in some exposed subjects (Langauer-Lewowicka et al. 1983); however,
reliable estimates of exposure levels producing these effects were not available.
Exposure of volunteers to known levels of vinyl chloride provided some indications of the levels of vinyl
chloride associated with the effects noted above. Volunteers exposed to 25,000 ppm vinyl chloride for
3 minutes in a single-exposure study reported experiencing dizziness, disorientation, and burning
sensations in the feet during exposure (Patty et al. 1930). Recovery from these effects was rapid upon
termination of exposure, but the subjects later developed slight headaches, which lasted approximately
30 minutes. Exposure of volunteers to concentrations of vinyl chloride ranging from 4,000 to 20,000 ppm
for 5 minutes twice a day in periods separated by 6 hours on 3 consecutive days was studied by Lester et
al. (1963). No effects were noted at 4,000 ppm. However, at 12,000 ppm, two of six subjects reported
feeling dizzy. The incidence of dizziness increased at higher concentrations. Nausea was experienced at
higher concentrations, and recovery from all effects was rapid upon termination of exposure. Headaches
developed following exposure to 20,000 ppm.
Indications of an exposure-related peripheral neuropathy were observed in a number of the occupational
studies. A peripheral neuropathy, most severe in hands and feet, was diagnosed in 70% of the vinyl
chloride workers examined in a study by Perticoni et al. (1986). The peripheral neuropathy was
manifested as denervation-related fasciculations and fibrillations with increased duration and amplitude of
motor unit potentials (indicating collateral sprouting). Similar effects were observed by Magnavita et al.
(1986) in a case study of a vinyl chloride worker. Other peripheral nervous system symptoms were
reported in occupational health studies of vinyl chloride workers. The symptom most frequently reported
VINYL CHLORIDE 71
2. HEALTH EFFECTS
was tingling (paresthesia) in the extremities (Lilis et al. 1975; Sakabe 1975; Spirtas et al. 1975; Suciu et
al. 1975; Veltman et al. 1975; Walker 1976). Additional peripheral nervous system symptoms included
numbness in the fingers (Lilis et al. 1975; Sakabe 1975), weakness (Langauer-Lewowicka et al. 1983;
Suciu et al. 1975), depressed reflexes (NIOSH 1977), warmth in the extremities (Suciu et al. 1975), and
pain in the fingers (Sakabe 1975). It is unclear whether some of these symptoms were associated with
tissue anoxia due to vascular insufficiency, or whether they represent the direct toxic effects of vinyl
chloride on peripheral nerves.
Animal Studies. Acute-duration exposure to high levels of vinyl chloride in a number of species provides
additional information on the central nervous system effects that are produced. Exposure to 10,000 ppm
for 8 hours (Patty et al. 1930) was observed to be without effects in guinea pigs. Exposure to 25,000 ppm
resulted in ataxia, which progressed to unconsciousness across the 8-hour exposure. As the concentration
was increased, the latency before the animals became unconscious decreased. In a different study,
Mastromatteo et al. (1960) observed the development of unconsciousness within 30 minutes at a vinyl
chloride concentration of 100,000 ppm in guinea pigs. Mice experienced similar signs at approximately
equivalent exposure levels. At 5,000 ppm, vinyl chloride was without effect during a 1-hour exposure.
Exposure to 50,000 ppm produced ataxia and twitching (Hehir et al. 1981), and at 100,000 ppm for
30 minutes, unconsciousness was produced, proceeded by increased motor activity, incoordination,
twitching, and tremors (Mastromatteo et al. 1960). Similar effects in rats were observed by Lester et al.
(1963), Jaeger et al. (1974), and Mastromatteo et al. (1960). In contrast, in one rat study, exposure to
50,000 ppm for 1 hour was without effect (Hehir et al. 1981). No effects were noted in rats exposed to
500 ppm vinyl chloride for 2 weeks (1 hour/day, 5 days/week) or in rats exposed to 50 ppm for 20 weeks
(1 hour/day, 5 days/week) (Hehir et al. 1981). In addition, tolerance developed to the intoxicating effects
of exposure to 50,000 ppm vinyl chloride after five or six 8-hour exposures (Lester et al. 1963); this study
was not included in Table 2-1 or Figure 2-2 due to colony contamination. No changes in brain weights
were reported when immunized rabbits were exposed to 983 ppm vinyl chloride 6 hours/day, 5 days/week
for 8 weeks (Sharma et al. 1980).
Chronic-duration exposure of rats to high levels of vinyl chloride produced damage to nervous tissue.
Rats exposed to 30,000 ppm for 4 hours/day, 5 days/week for 12 months in a single-concentration study
were soporific during the exposure periods (Viola 1970; Viola et al. 1971). Following 10 months of
exposure, the rats had decreased responses to external stimuli and disturbed equilibrium. No animal
studies were located that examined hearing damage after vinyl chloride exposure. Histopathological
examination revealed diffuse degeneration of the brain gray and white matter. Cerebellar degeneration in
VINYL CHLORIDE 72
2. HEALTH EFFECTS
the Purkinje cell layer was pronounced. Peripheral nerve endings were surrounded and infiltrated with
fibrous tissue (Viola 1970; Viola et al. 1971). Nonneoplastic lesions in the brain were not noted in rats
exposed to 5,000 ppm for 7 hours/day, 5 days/week for 12 months in a single-concentration study by
Feron and Kroes (1979).
Mechanisms. Peripheral nervous system symptoms such as paresthesia, numbness, weakness, warmth in
the extremities, and pain in the fingers have been reported after vinyl chloride exposure (Langauer-
Lewowicka et al. 1983; NIOSH 1977; Suciu et al. 1963, 1975). It is not known whether these effects
represent direct adverse effects of vinyl chloride on peripheral nerves or whether they are associated with
tissue anoxia due to vascular insufficiency.
2.16 REPRODUCTIVE
Human Studies. Occupational health studies of vinyl chloride workers suggest that sexual performance
may be affected by vinyl chloride. However, these studies are limited by the lack of quantification of
exposure levels and no comparison group. Sexual impotence was reported by 24% of the workers
examined by Suciu et al. (1975). Approximately 20% of the workers examined by Veltman et al. (1975)
complained of potency troubles. A loss of libido in 35% and impotence and decreased androgen secretion
in 8% of workers exposed at least once to very high levels of vinyl chloride were also reported by Walker
(1976).
In retrospective and prospective studies by Bao et al. (1988), increased incidence and severity of elevated
blood pressure and edema during pregnancy (preeclampsia) were found in female workers exposed to
vinyl chloride when compared to unexposed workers. Company records indicated that exposure levels
ranged from 3.9 to 89.3 ppm during the retrospective study and from 0.2 to 130.7 ppm during the
prospective study.
Animal Studies. A 2-generation reproductive toxicity study was conducted in rats exposed to vinyl
chloride via inhalation (Thornton et al. 2002). Male and female Sprague-Dawley rats were exposed to 0,
10, 100, or 1,100 ppm vinyl chloride 6 hours/day for a 10-week premating period, a 3-week mating
period, through GD 20, and from lactation day 4 through weaning (females only). No adverse effects
were noted in reproductive capability over the two generations at any dose. No effects were seen in body
weight, food consumption, ability to reproduce, gestation index or length, or pre- and postweaning
developmental landmarks. Sperm counts, motility, and morphology were also unaffected by vinyl

VINYL CHLORIDE 73
2. HEALTH EFFECTS
chloride exposure. Changes in liver weights and/or histopathological alterations were seen in F0 and F1
generation male and female rats. For further information regarding the liver toxicity of vinyl chloride,
refer to Section 2.9.
Exposure of rats to >105.6 ppm for 6 hours/day, 6 days/week for up to 12 months produced a significant
increase in the incidence of damage to the seminiferous tubules and depletion of spermatocytes (Bi et al.
1985). At the 6-month interim sacrifice, a significant decrease in relative testicular weight was also
observed at 105.6 ppm. Several methodological limitations have been identified for this study.
Temperature and humidity conditions in the inhalation chambers were not maintained within the normal
range. Inhalation chamber volume and air flow were also not held constant across dose groups.
A significant increase in damage to the spermatogenic epithelium and disorders of spermatogenesis were
found with exposure to 500 ppm vinyl chloride for 5 hours/day, 5 days/week for 10 months (52%
incidence versus 11% incidence in controls) (Sokal et al. 1980). These testicular effects were not
observed in rats exposed to 20,000 ppm. The smaller number of animals in the 20,000-ppm group
(17 versus 28 controls) may have contributed to the lack of statistical significance in this group. No
significant change in testicular weight was found in rats exposed to 500 ppm for 7 hours/day, 5 days/week
for 4.5 months, in dogs, rabbits, or guinea pigs exposed to 200 ppm for 7 hours/day, 5 days/week for
6 months (Torkelson et al. 1961), or in mice exposed to 0.85 ppm vinyl chloride 6 hours/day, 5 days/week
for 12 weeks (Wahlang et al. 2020). No histopathological data on the testes of these animals were
presented.
2.17 DEVELOPMENTAL
Human Studies. The potential association between vinyl chloride exposure and developmental toxicity
was evaluated in one cohort study, one cross-sectional study, six case-control studies, and two ecological
studies (Table 2-6). Although some early studies suggested that members of communities with nearby
vinyl chloride polymerization facilities had significantly greater risk of fetal loss or birth defects (Infante
1976; Infante et al. 1976a, 1976b; NIOSH 1977), most studies failed to demonstrate a correlation between
the developmental toxicity and either parental occupation or proximity to the facility (Bao et al. 1988;
Edmonds et al. 1975, 1978; Rosenman et al. 1989; Theriault et al. 1983). Case-control studies evaluating
exposure to multiple compounds in air and drinking water during pregnancy did not demonstrate an
association between the vinyl chloride concentration and the risk of neural tube defects including spina

VINYL CHLORIDE 74
2. HEALTH EFFECTS
bifida (Ruckart et al. 2013; Swartz et al. 2015), oral clefts (Ruckart et al. 2013), or autism spectrum
disorder (Talbott et al. 2015).
Table 2-6. Results of Epidemiological Studies Evaluating Exposure to Vinyl
Chloride and Developmental Effects
Reference, study type, and
population
Exposure or biomarker
Outcome evaluated
Result
a
Swartz et al. 2015
Case-control, 1,108 cases of
neural tube defects including
spina bifida; 4,132 frequency
matched controls (Texas,
United States)
Ambient air concentration,
95
th
percentile
1.19x10
-1
µg/m
3
Risk of neural tube defects
(including spina bifida)
↔
Talbott et al. 2015
Case-control, 217 cases of
autism spectrum disorder in
children born between 2005
and 2009; 224 frequency
matched controls and
5,007 controls from random
sample of birth certificates
(Pennsylvania, United States)
Ambient air concentration,
75
th
percentile
1.2x10
-4
µg/m
3
Risk of autism spectrum disorder
↔
Ruckart et al. 2013
Case-control, 15 cases of
neural tube defects (spina
bifida and anencephaly),
24 cases of oral clefts (cleft lip
and palate); 524 controls
(North Carolina, United States)
Exposed versus
unexposed comparison
Risk of neural tube defects
↔
Mean high exposure
group, ≥3 ppm in drinking
water
Risk of oral clefts
↔
Rosenman et al. 1989
Case-control, cases of all birth
defects (Plant A: 66,
Plant B: 72), cases of CNS
defects (Plant A: 31,
Plant B: 29); controls
(Plant A: 72, Plant B: 103)
(New Jersey, United States)
Residential distance from
two vinyl chloride
polymerization facilities
Risk of birth defects, risk of CNS
malformations
↔

VINYL CHLORIDE 75
2. HEALTH EFFECTS
Table 2-6. Results of Epidemiological Studies Evaluating Exposure to Vinyl
Chloride and Developmental Effects
Reference, study type, and
population
Exposure or biomarker
Outcome evaluated
Result
a
Bao et al. 1988
Retrospective cohort,
236 female vinyl chloride
workers, 239 unexposed
controls; prospective cohort,
43 female vinyl chloride
workers, 86 unexposed
controls (China)
3.9–89.3 ppm
(retrospective); 0.2–
130.7 ppm (prospective)
Sex ratio, birth weight, birth
height, perinatal mortality,
incidence of congenital
abnormalities
↔
Theriault et al. 1983
Case-control, 68 cases of birth
defects, 68 matched controls
(Canada)
Exposed (residence in a
community with a PVC
plant) versus unexposed
(three comparison
communities)
Risk of birth defects
↔
Edmonds et al. 1978
Case-control study, 46 infants
with CNS birth defects
(18 stillborn), 46 controls
(West Virginia, United States)
Occupation at PVC plant;
residential distance from
the plant
Confirmed cases of
anencephaly, spina bifida,
hydrocephalus and other CNS
malformation (1970–1974)
↔
Infante 1976
Ecological, three communities
with PVC production facilities
(Ohio, United States)
Residence in communities
with PVC plant
Risk of CNS malformations
(three communities combined)
↑
Infante et al. 1976a, 1976b;
NIOSH 1977
Cross-sectional, 70 male
workers (North Carolina,
United States)
Exposed (VCM workers)
versus unexposed (rubber
workers
Fetal death (any conception not
born alive; age-adjusted)
↑
Edmonds et al. 1975
Ecological, hospital birth
registry study (Ohio, United
States)
Distance from PVC
polymerization plants
CNS malformations
(anencephaly, spina bifida)
↔
a
Up arrows were based on statistically significant results only.
↑ = association with increase; ↔ = no association; CNS = central nervous system; PVC = polyvinyl chloride;
VCM = vinyl chloride monomer
The pregnancy outcome for wives of workers employed at a vinyl chloride polymerization facility was
compared to the pregnancy outcome of wives of a control group made up of unexposed rubber workers
VINYL CHLORIDE 76
2. HEALTH EFFECTS
and PVC fabricators believed to be exposed to "very low" levels of vinyl chloride (Infante et al. 1976a,
1976b). Pregnancy outcomes were determined based on the responses given by fathers on a
questionnaire. Infante et al. (1976a, 1976b) and NIOSH (1977) reported a significant excess of fetal loss
in the group whose husbands had been exposed to vinyl chloride. The greatest difference occurred in
wives of men under 30 years of age, where fetal loss was 5.3% for controls and 20.0% for exposed
workers. However, this study has been severely criticized based on the way it was conducted and the
method of statistical analysis used (Hatch et al. 1981; Stallones 1987). Evaluations by Hatch et al. (1981)
and Stallones (1987) concluded that the study failed to demonstrate an association between parental
exposure to vinyl chloride and increased fetal loss.
Additional work by Infante (1976) and Infante et al. (1976b) examined the occurrence of congenital
malformations among populations exposed to emissions from PVC polymerization facilities. A
statistically significant increase in birth defects was observed for three cities in which polymerization
facilities were located when compared to statewide and countywide averages. The greatest increases
were noted for malformations of the central nervous system, upper alimentary tract, and genital organs
and in the incidence of club foot. However, this study has also been criticized based on the ecological
study design (Hatch et al. 1981; Stallones 1987). These authors concluded that the study failed to
demonstrate an association between exposure to emissions and the prevalence of birth defects.
Furthermore, another study that examined the incidence of malformations in one of the cities studied by
Infante (1976) concluded that, although the city had statistically increased incidences of congenital
malformations, no correlation existed based on parental proximity to the polymerization plant or with
parental employment at the plant (Edmonds et al. 1975). In fact, more parents of control infants worked
at the plant or lived closer to the plant than parents of infants with central nervous system malformations.
Additional other studies also examined the prevalence of congenital malformations in populations
exposed to emissions from polymerization facilities (Edmonds et al. 1978; Rosenman et al. 1989;
Theriault et al. 1983). The incidence of central nervous system defects in a West Virginia County with a
polymerization plant was compared to incidences in other regions in the United States with no known
exposure to vinyl chloride (Edmonds et al. 1978). Although the rate of central nervous system defects in
the West Virginia County exceeded that in control areas, no correlation was noted between the increased
central nervous system defects and parental occupation or potential exposure based on proximity to the
plant or prevailing wind patterns. Rosenman et al. (1989) suggested that the risk of central nervous
system defects, but not overall birth defects, was correlated with the amount of emissions from individual
polymerization facilities and with the distance of the residences of affected parents from the facilities;
VINYL CHLORIDE 77
2. HEALTH EFFECTS
however, the findings were not statistically significant, and the study was limited by the small sample
size.
A significantly greater prevalence of birth defects was found in residents of a town with a polymerization
facility than in three matched towns without potential for exposure to vinyl chloride (Theriault et al.
1983). The most commonly reported defects included those of the musculoskeletal, alimentary,
urogenital, and central nervous systems. The incidences were observed to fluctuate with seasonal changes
in emissions. However, no correlations were found between the presence of birth defects and the
proximity of the residence to the plant or parental occupation. Other industrial emissions in the area
evaluated could not be eliminated as potential contributors to the increased incidence of congenital
malformations observed. Additional confounding factors such as nutritional status, smoking, and alcohol
and other drug use were not adjusted for.
Pregnancy outcomes of mothers occupationally exposed to vinyl chloride for >1 year were compared to
those of pregnant workers not exposed to vinyl chloride in retrospective and prospective studies (Bao et
al. 1988). Company records indicated that exposure levels ranged from 3.9 to 89.3 ppm during the
retrospective study and from 0.2 to 130.7 ppm during the prospective study. The study authors concluded
that exposure to vinyl chloride did not correlate with changes in sex ratio, birth weight or body length,
perinatal mortality, or the incidence of congenital abnormalities.
Ruckart et al. (2013) performed a case-control study to evaluate the relationship between exposure to
solvents in contaminated drinking water during pregnancy and neural tube defects, oral clefts, and
childhood hematopoietic cancers. The study included 524 controls, 15 cases of neural tube defects,
24 cases of oral clefts, and 13 cases of cancer. No significant association was seen between vinyl chloride
exposure and these effects. The risk of spina bifida was evaluated in a case-control study using birth
registry data and census tract-level estimates of ambient air concentrations of hazardous air pollutants
(Swartz et al. 2015). Vinyl chloride concentrations were not associated with the risk of spina bifida in
this study. Talbott et al. (2015) evaluated the relationship between modeled concentrations of air toxics
and the risk of autism spectrum disorder. Cases of autism spectrum disorder were recruited from
diagnostic and treatment centers and the control groups consisted of controls that were frequency matched
by child’s year of birth, sex, and race and controls from a random sample of birth certificates. The
estimated vinyl chloride concentrations in air were not associated with increased risk of autism spectrum
disorder.
VINYL CHLORIDE 78
2. HEALTH EFFECTS
Animal Studies. A number of inhalation studies examined the effects of vinyl chloride exposure on
pregnancy outcome in animals. Results of these studies indicate that vinyl chloride produces adverse
developmental effects at concentrations that are also toxic to maternal animals. John et al. (1977, 1981)
exposed rats and rabbits to 0, 500, or 2,500 ppm and mice to 0, 50, or 500 ppm throughout the period of
organogenesis. Separate control groups were used for each of the mice exposure concentrations. Mice
were more sensitive to the effects of vinyl chloride than rats and rabbits. An increase in the mortality rate
was observed in pregnant mice exposed to 500 ppm (John et al. 1977, 1981). Delayed ossification of
skull and sternebrae and unfused sternebrae were noted in fetuses at 500 ppm. Crown-rump length was
increased at 50 ppm but not at 500 ppm. The biological significance of this effect is unknown.
In rats (John et al. 1977, 1981), 500 ppm produced increased crown-rump length and vertebral lumbar
spurs, but these findings were not increased at 2,500 ppm. The only effect observed at 2,500 ppm was an
increased incidence of dilated ureters (fetal incidence of 27 versus 5% in controls).
In rabbits exposed to 500 ppm, fetal animals had delayed ossification of the sternebrae that was not
observed in rabbits at 2,500 ppm. No conclusions may be drawn as to the dose response of these effects.
An embryo-fetal developmental toxicity study was conducted in rats exposed to vinyl chloride via
inhalation (Thornton et al. 2002). Female Sprague-Dawley rats were exposed to 0, 10, 100, or 1,100 ppm
vinyl chloride 6 hours/day on GDs 6–19. No adverse effects were noted in embryo-fetal developmental
parameters including uterine implantation, fetal sex distribution, fetal body weight, and fetal
malformations and variations. Maternal kidney weights were increased relative to total body weight at
100 ppm.
Exposure of rats to either 0 or 1,500 ppm of vinyl chloride during the first, second, or third trimester of
pregnancy was examined (Ungvary et al. 1978). In maternal animals, an increased liver-to-body weight
ratio was observed in those exposed during the first and second trimesters, but no histopathologic
alterations were found. A significant increase in resorptions was observed in animals exposed during the
first trimester of pregnancy. Two central nervous system malformations (microphthalmia and
anophthalmia) were observed in exposed fetuses but not in controls, but the incidence of these
malformations did not reach statistical significance. This study is limited in that only a single
concentration of vinyl chloride was tested, precluding conclusions as to the dose-response relationship of
the effects observed.
VINYL CHLORIDE 79
2. HEALTH EFFECTS
The effects of exposure of rats to vinyl chloride throughout gestation were examined by Mirkova et al.
(1978) and Sal'nikova and Kotsovskaya (1980). An unspecified number of pregnant rats were exposed to
0, 1.9, or 13.9 ppm for 4 hours/day for the 21 days of gestation. Fetuses were examined for abnormalities
just prior to the end of gestation, and offspring were examined at 6 months post-parturition (Sal'nikova
and Kotsovskaya 1980). At 13.9 ppm, a decrease in maternal erythrocyte count was observed. Fetuses
had an increased incidence of hemorrhages at 1.9 and 13.9 ppm and increased edema at 13.9 ppm.
However, the affected organs were not specified. Rats examined at 6 months, following in utero
exposure to 1.9 ppm, were found to have decreased hemoglobin and leukocytes and decreased organ
weights (males: liver, kidneys, spleen; females: lung, liver). In addition to these effects, exposure to
13.9 ppm in utero resulted in an increased hexanol sleep time and a decreased ability of the rats to orient
themselves.
Continuous exposure of an unspecified number of rats to 2.4 ppm of vinyl chloride throughout gestation
resulted in decreased fetal weight and increased early postimplantation loss, hematomas, and
hydrocephaly with intracerebral hematoma. Weanling rats had hepatotoxic effects including decreased
bile secretion and decreased cholic acid content. No histological data on the livers of pups, information
regarding maternal health, or statistical analyses of the data were presented (Mirkova et al. 1978). Both
this study and the report by Sal'nikova and Kotsovskaya (1980) failed to provide information on the
number of animals in each test group.
Vinyl chloride administration to pregnant mice by intraperitoneal injection on GD 6 produced a dose-
related reduction in embryo survival 4 days after injection (percent survival was 96, 86, 67, and 55% at
doses of 0, 200, 400, and 600 mg/kg, respectively). The incidences of morphological abnormalities were
6, 51, and 71% at doses of 200, 400, and 600 mg/kg, respectively. Neural tube defects were the primary
abnormality observed (Quan et al. 2014). The mechanism for this effect appears to be related to
inhibition of neural epithelial cell proliferation and induction of caspase 3-mediated apoptosis. The
developmental toxicity of vinyl chloride was examined using a whole embryo culture system (Zhao et al.
1996). Vinyl chloride induced embryo growth retardation but was not shown to be teratogenic in the rat
in vitro whole embryo culture system.
2.18 OTHER NONCANCER
Human Studies. Epidemiology studies evaluating exposure to vinyl chloride and insulin resistance are
described in Table 2-7. A cross-sectional study of vinyl chloride workers in Taiwan demonstrated an

VINYL CHLORIDE 80
2. HEALTH EFFECTS
exposure-related decrease in the adiponectin/leptin ratio, which may be suggestive of increased insulin
resistance (Lee et al. 2020). No change in serum concentrations of glucose, insulin, adiponectin, or leptin
was observed. Vinyl chloride workers with steatohepatitis also demonstrated measures suggestive of
insulin resistance (increased serum glucose, insulin, and adiponectin) when compared to healthy workers
exposed to vinyl chloride and unexposed healthy volunteers (Cave et al. 2010). Plasma metabolomics
analysis in vinyl chloride workers showed alterations in lipid and amino acid metabolites, which may
contribute to the steatohepatitis (Guardiola et al. 2016).
Table 2-7. Results of Epidemiological Studies Evaluating Exposure to Vinyl
Chloride and Insulin Resistance
Reference, study type, and
population
Exposure or biomarker
Outcome evaluated
Result
a
Cave et al. 2010
Case-control, 16 male, non-
obese, highly exposed workers
with steatohepatitis, 26 healthy
worker controls, and
11 unexposed, healthy
volunteers (Kentucky, United
States)
11,913 ppm-years, estimated
mean cumulative, long-term
exposure (mean 18.9 years)
Serum glucose,
insulin, adiponectin
↑
Serum leptin
↔
Lee et al. 2020
Cross-sectional, 108 male and
5 female workers (Taiwan)
2,065 µg/m
3
; mean of high-VCM
group
Adiponectin/leptin ratio
↓
Serum glucose, insulin,
adiponectin, leptin
↔
a
Up and down arrows were based on statistically significant results only.
↑ = association with increase, ↓ = association with decrease, ↔ = no association; VCM = vinyl chloride monomer
Animal Studies. In C57BL/6J mice exposed to 0.85 ppm vinyl, 5 days/week, 6 hours/day for 12 weeks,
no treatment-related effects were observed on fasting blood glucose levels or glycogen storage (Wahlang
et al. 2020). In other studies, normal findings were observed in tests of oral glucose tolerance (Chen et al.
2019; Lang et al. 2018) and insulin or pyruvate tolerance (Lang et al. 2018). Zelko et al. (2022) reported
no effect on blood glucose or insulin in C57BL/6 mice exposed to 0.8 ppm vinyl, 5 days/week,
6 hours/day for 12 weeks, but did show a 2-fold decrease in glucose tolerance following intraperitoneal
injection of glucose.
VINYL CHLORIDE 81
2. HEALTH EFFECTS
2.19 CANCER
Overview. The development of cancer in humans as a result of vinyl chloride exposure was demonstrated
in a number of studies of workers in the vinyl chloride production industry. The strongest evidence
comes from the greater-than-expected incidences of liver angiosarcoma, a tumor type that is considered to
be very rare in humans (25–30 cases/year in the United States). The latency period for the development
of hepatic angiosarcoma in workers exposed prior to 1974 ranges between 24 and 56 years (Collins et al.
2014; Mundt et al. 2017). Other liver tumors, including hepatocellular carcinoma and
cholangiocarcinoma (commonly referred to as colangiocarcinoma), were also associated with
occupational exposure to vinyl chloride. The latency period for the development of hepatocellular
carcinoma is estimated to range from 32 to 67 years (Mundt et al. 2017).
Studies in several animal species support the conclusion that vinyl chloride is carcinogenic. In rats,
chronic-duration exposure to 5–5,000 ppm vinyl chloride vapors resulted in significantly increased
incidence of mammary gland carcinomas, Zymbal’s gland carcinomas, nephroblastoma, and liver
angiosarcoma compared to controls. Intermediate- and chronic-duration exposures of 50–2,500 ppm
vinyl chloride resulted in significant incidence of liver angiosarcoma, carcinoma, and angioma, lung
adenoma, mammary gland carcinoma, adipose tissue hemangiosarcoma, and hemangiosarcoma of the
subcutis and peritoneum in mice. With the exception of liver angiosarcomas, which were observed in all
species (including humans), there is little consistency in tumor types across species. Chronic-duration
oral administration of 2–6 mg/kg/day of vinyl chloride resulted in the development of neoplastic liver
nodules, hepatocellular carcinoma, and lung and liver angiosarcoma in rats (Feron et al. 1981; Til et al.
1983, 1991).
Studies in rats, mice, and hamsters provide evidence that exposure early in life increases the risk of
hemangiosarcoma in liver, skin, and spleen, stomach angiosarcoma, as well as mammary gland
carcinoma, when compared to the risk associated with exposure after 12 months of age (Drew et al. 1983;
Maltoni et al. 1981). Due to the latency period for vinyl chloride-induced cancer, exposure of animals
during gestation and/or early in life may have increased the likelihood of developing tumors and affected
the type of tumor that develops.
Human Studies. Bosetti et al. (2003) pooled the analyses of worker cohorts from 56 vinyl chloride plants
in North America and Europe. The pooled analysis, which included over 22,000 workers, showed an
elevated risk of liver cancer mortality. While differences between the North American and European
VINYL CHLORIDE 82
2. HEALTH EFFECTS
cohorts were observed for soft tissue sarcoma and brain cancer, no significant excess in mortality from
these cancers was seen in the pooled data. Deaths from lung and laryngeal cancer were lower than
expected, and no excess mortality from lymphoid and hematopoietic system cancers was observed.
Boffetta et al. (2003) performed a meta-analysis including the multicenter cohort studies from North
America and Europe as well as six smaller studies from the former Soviet Union, France, Canada,
Germany, China, and Taiwan. The meta-analysis confirmed the elevated risk of liver cancer mortality
among vinyl chloride workers. It also reported excess mortality from multiple types of liver cancer
including angiosarcoma, hepatocellular carcinoma, and other liver tumors with unspecified
histopathology. Boffetta et al. (2003) also reported a possible increase in the risk for soft-tissue sarcoma,
especially in North American workers; however, misclassification of the diagnosed cause of death may
have contributed to this result (i.e., angiosarcoma of the liver classified as a soft tissue sarcoma). A meta-
analysis that included three occupational cohorts and 12,816 participants reported an association between
cumulative exposure to vinyl chloride and increased mortality from liver angiosarcoma and soft tissue
sarcoma (Edwards et al. 2021). Similar to the pooled results from Bosetti et al. (2003), Boffetta et al.
(2003) reported that no increase was observed in mortality from lung or brain cancers. A strong
association was not observed between vinyl chloride exposure and lymphatic/hematopoietic system
cancers; however, this negative conclusion was considered premature due to the heterogeneity of the
study results (Boffetta et al. 2003).
Epidemiology studies evaluating the risk of selected types of cancer associated with vinyl chloride
exposure are presented in Table 2-8 (ecological studies and case reports are not tabulated). The most
compelling evidence for the carcinogenic potential of vinyl chloride in humans comes from many reports
of greater-than-expected incidences of angiosarcoma of the liver in workers occupationally exposed to
vinyl chloride (Table 2-8).
Approximately 30 years after the introduction of vinyl chloride for use in the industrial production of
PVC, it became apparent that workers exposed to high levels of vinyl chloride had an unusually high
incidence of angiosarcoma of the liver. Investigators identified an increased likelihood of developing
hepatic angiosarcoma among those exposed to the highest levels of vinyl chloride and those exposed to
vinyl chloride for the longest duration (Fortwengler et al. 1999; Fox and Collier 1977; Infante et al.
1976b; Jones et al. 1988; Mundt et al. 2017; Rinsky et al. 1988; Weber et al. 1981; Wong et al. 1991; Wu
et al. 1989). Mundt et al. (2017) demonstrated a strong association between mortality from angiosarcoma
of the liver and exposure to cumulative vinyl chloride concentrations of ≥865 ppm-years. An increase in

VINYL CHLORIDE 83
2. HEALTH EFFECTS
hepatobiliary cancer mortality was observed in workers exposed to vinyl chloride for ≥16 years (Carreón
et al. 2014).
Angiosarcoma of the liver was not found in residents living in the vicinity of vinyl chloride sites unless
they were also exposed to high concentrations of vinyl chloride in the workplace (Elliott and
Kleinschmidt 1997). Lewis et al. (2003) reported the occurrence of angiosarcoma of the liver in retirees
from a PVC production plant in Louisville, Kentucky. This incidence increase is reported primarily for
those workers employed prior to 1960, suggesting that those exposed to the highest concentrations of
vinyl chloride remain at risk for developing cancer for the remainder of their lives. The reported latency
period for workers diagnosed prior to 1975 was 12–28 years, while those diagnosed after 1975 showed a
latency of 27–47 years. Examination of >73,000 death certificates of North American workers employed
between 1940 and 2008 showed a mean latency for death from angiosarcoma of the liver of 37 years
(range of 24–56 years) (Collins et al. 2014). Workers with the first exposure occurring after 1974 did not
develop angiosarcoma of the liver (Collins et al. 2014). The median latency for angiosarcoma deaths in
vinyl chloride workers from 35 facilities in the United States was 36 years (ranging from 14 to 56 years)
(Mundt et al. 2017). Plasma metabolomics analysis of vinyl chloride workers who developed
angiosarcoma showed upregulation of taurocholate, bradykinin, and fibrin degradation product 2
(Guardiola et al. 2021).
Table 2-8. Summary of Epidemiological Studies Evaluating Possible
Associations between Vinyl Chloride Exposure and
Risk of Selected Cancer Types
Cancer type
Association
a
No association
b
Liver and biliary (angiosarcoma,
hepatocellular carcinoma,
cholangiocarcinoma)
Scarselli et al. 2022
c
Guardiola et al. 2021
d
Fedeli et al. 2019a
c
Mundt et al. 2017
c
Carreón et al. 2014
c
Collins et al. 2014
c
Hsieh et al. 2011
c
Gennaro et al. 2008
c
Mastrangelo et al. 2004
d
Lewis et al. 2003
c
Maroni et al. 2003
c
Wong et al. 2002a
c
, 2003a
d
Ward et al. 2001
c
Cheng et al. 1999a
e
Fortwengler et al. 1999
c
Du and Wang 1998
d
Elliott and Kleinschmidt 1997
f,g
Laplanche et al. 1992
c
Marsh et al. 2021
c,h
Marsh et al. 2007a
c,h
Marsh et al. 2007b
c,h

VINYL CHLORIDE 84
2. HEALTH EFFECTS
Table 2-8. Summary of Epidemiological Studies Evaluating Possible
Associations between Vinyl Chloride Exposure and
Risk of Selected Cancer Types
Cancer type
Association
a
No association
b
Simonato et al. 1991
c
Wong et al. 1991
c
Pirastu et al. 1990
c
Teta et al. 1990
c
Wu et al. 1989
c
Jones et al. 1988
c
Rinsky et al. 1988
c
Forman et al. 1985
d
Theriault and Allard 1981
c
Weber et al. 1981
c
Fox and Collier 1977
c
Byren et al. 1976
c
Infante et al. 1976b
c
Waxweiler et al. 1976
c
Monson et al. 1975
c
Brain and central nervous system
Rodrigues et al. 2020
d
Wong et al. 1991
c,i
Cooper 1981
c,i
Waxweiler et al. 1976
c,i
Monson et al. 1975
c
Mundt et al. 2017
c
Pan et al. 2005
d
Lewis and Rempala 2003
d
Lewis et al. 2003
c
Lewis 2001
c
Ward et al. 2001
c
Mundt et al. 2000
c
Simonato et al. 1991
c
Wu et al. 1989
c,i
Jones et al. 1988
c
Thomas et al. 1987
d
Fox and Collier 1977
c
Byren et al. 1976
c
Tabershaw and Gaffey 1974
c,i
Lung and respiratory tract
(large-
cell undifferentiated carcinoma or
adenocarcinoma)
Girardi et al. 2022
c
Gennaro et al. 2008
c
Mastrangelo et al. 2003
d
Belli et al. 1987
c
Heldaas et al. 1984
c
Infante et al. 1976b
c
Waxweiler et al. 1976
c
Monson et al. 1975
c
Mundt et al. 2017
c
Hsieh et al. 2011
c
Scelo et al. 2004
d
Wong et al. 2002a
c
Wong et al. 1991
c
Ward et al. 2001
c
Mundt et al. 2000
c
Cheng et al. 1999a
e
Du and Wang 1998
d
Simonato et al. 1991
c
Hagmar et al. 1990
c
Wu et al. 1989
c
Jones et al. 1988
c
Cooper 1981
c
Buffler et al. 1979
c
Fox and Collier 1977
c
Connective and other soft tissues
(including soft tissue sarcoma)
Mundt et al. 2017
c
Mundt et al. 2000
c
Ward et al. 2001
c

VINYL CHLORIDE 85
2. HEALTH EFFECTS
Table 2-8. Summary of Epidemiological Studies Evaluating Possible
Associations between Vinyl Chloride Exposure and
Risk of Selected Cancer Types
Cancer type
Association
a
No association
b
Lymphatic/hematopoietic system
(including leukemias, myelomas and
lymphomas)
Poynter et al. 2017
e
Hsieh et al. 2011
c
Wong et al. 2002a
c
Du and Wang 1998
d
Rinsky et al. 1988
c
Smulevich et al. 1988
c
Weber et al. 1981
c
Monson et al. 1975
c
Mundt et al. 2017
c
Bove et al. 2014
c
Carreón et al. 2014
c
Ruckart et al. 2013
d
Ward et al. 2001
c
Mundt et al. 2000
c
Cheng et al. 1999a
e
Infante et al. 1976b
c
Jones et al. 1988
c
Wong et al. 1991
c
a
Significant association between exposure and cancer incidence or mortality.
b
No significant association between exposure and cancer incidence or mortality.
c
Cohort studies.
d
Case-control studies.
e
Cross-sectional study.
f
Ecological studies.
g
Association was reported for exposed workers, but not residents living near sites.
h
Exposure to vinyl chloride was relatively low (<2 ppm-year).
i
Studies based on workers from the same cohort from a Chemical Manufacturers Association (CMA) study (Wong
and Whorton 1993).
Histopathological examination of liver tissue from humans with hepatic angiosarcoma led to the
hypothesis that angiosarcoma develops as a result of hyperplastic changes in sinusoidal cells. In liver
parenchyma, areas of transition to angiosarcoma contained greatly increased numbers of sinusoidal cells
with greatly expanded sinusoidal spaces. Hepatic cells were replaced by fibrous tissue-forming
trabeculae. These areas also showed infiltration of angiosarcoma cells. In fully developed angiosarcoma,
multiple areas with nodules of angiosarcoma cells were noted, the centers of which exhibited hemorrhagic
necrosis (Popper et al. 1981). Case reports suggest that vinyl chloride can also produce malignant
hemangiopericytomas (Hozo et al. 1997, 2000) and epithelioid hemangioendotheliomas (Gelin et al.
1989) in the liver (both are vascular tumors similar to angiosarcomas), and adrenal
epithelioidangiosarcoma (Criscuolo et al. 2014).
Other liver tumors, including hepatocellular carcinoma and cholangiocellular carcinoma, have also been
associated with occupational exposure to vinyl chloride (Cheng et al. 1999a; Du and Wang 1998; Fedeli
et al. 2019a; Hsieh et al. 2011; Lelbach 1996; Mundt et al. 2017; Saurin et al. 1997; Ward et al. 2001;
Weihrauch et al. 2000; Wong et al. 2002a, 2003a). The latency period for the development of
hepatocellular carcinoma was estimated to range from 32 to 67 years in a study of vinyl chloride workers
VINYL CHLORIDE 86
2. HE
ALTH EFFECTS
in the United States (Mundt et al. 2017). The risk of developing liver cancer was elevated in those with a
history of Hepatitis B viral infection (Du and Wang 1998; Wong et al. 2003a).
M
astrangelo et al. (2004) evaluated the possible interaction between alcohol consumption, hepatitis
infection, and hepatocellular carcinoma in a large cohort of vinyl chloride workers. Vinyl chloride was
suggested to be an independent risk factor for hepatocellular carcinoma with a synergistic interaction
described for alcohol consumption and an additive interaction for hepatitis infection. Sequential
development of hepatocellular carcinoma followed by later development of angiosarcoma of the liver was
demonstrated in the case report of a worker exposed to high concentrations of vinyl chloride (4,100 ppm-
years) (Guido et al. 2016). Mortality from liver cancer was not elevated by vinyl chloride in a study of
workers exposed to low concentrations of vinyl chloride (<2 ppm-years) (Marsh et al. 2007a, 2007b,
2021). An ecological study in Texas associated exposure to vinyl chloride in polluted ambient air and the
incidence of hepatocellular carcinoma (Cicalese et al. 2017); however, several letters to the editor from
the vinyl industry described significant methodological limitations of this study (Gennissen et al. 2018;
Krock 2018; Marsh and Towle 2018). Therefore, Cicalese et al. (2017) was not included in Table 2-8.
An ecological study, funded by the vinyl industry, did not report an association between Texas county-
level ambient air concentrations of vinyl chloride and liver cancer incidence or mortality (Towle et al.
2021).
Ot
her tumor types have statistically significant increases in mortality rates among vinyl chloride workers,
in at least some studies. They include cancer of the brain and central nervous system, the lung and
respiratory tract, connective and other soft tissues, plus the lymphatic/hematopoietic system (Table 2-8).
In general, follow-up mortality studies at polymer production plants indicate that liver cancer mortality
remained elevated while brain cancer mortality was markedly reduced when recent studies are compared
to the earlier studies. Increased brain cancer incidence was not associated with vinyl chloride exposure in
these later studies (Lewis 2001; Lewis and Rempala 2003; Lewis et al. 2003; Mundt et al. 2000, 2017;
Ward et al. 2001). A recent case-control study of brain and other CNS cancers in semiconductor workers
showed an association between cumulative vinyl chloride exposure (1965–1999) and cancer risk
(Rodrigues et al. 2020).
An association between respiratory tract cancer and vinyl chloride exposure has not been consistently
observed (Table 2-8). Although smoking history was not considered in these studies, Waxweiler et al.
(1976) noted that the types of respiratory tract cancer most frequently recorded were large-cell
undifferentiated lung carcinoma or adenocarcinoma that are not usually associated with smoking but can
VINYL CHLORIDE 87
2. HE
ALTH EFFECTS
be influenced by the smoking status of the exposed individual. Increased risk of lung cancer was also
associated with exposure to high concentrations of PVC dust particles (Girardi et al. 2022; Mastrangelo et
al. 2003; Waxweiler et al. 1976).
A si
gnificant increase in cancers of connective and other soft tissues was observed in some, but not all
follow up mortality studies (Table 2-8). A meta-analysis of five occupational exposure studies suggested
a weak association between vinyl chloride exposure and pancreatic cancer (Ojajarvi et al. 2001).
However, no association was observed between vinyl chloride exposure and mortality from pancreatic
cancer in the updated mortality studies of vinyl chloride workers (Carreón et al. 2014; Fedeli et al.
2019a).
No
consistent findings were noted regarding the association between cancers of the lymphatic/
hematopoietic system and exposure to vinyl chloride (i.e., both positive and negative findings were
reported, and the conclusions of the pooled and meta-analysis differed) (Table 2-8; Boffetta et al. 2003;
Bosetti et al. 2003).
An
increased incidence of malignant melanoma among vinyl chloride workers has been reported (Heldaas
et al. 1984, 1987), but the significance of this finding has been disputed (ten Berge 1987). A follow up to
the original Heldaas et al. (1984, 1987) studies reported only one additional case of melanoma between
1985 and 1993, weakening the proposed association between vinyl chloride exposure and the
development of malignant melanoma (Langard et al. 2000). Follow-up mortality studies have not
demonstrated an association between vinyl chloride exposure and risk of melanoma (Mundt et al. 2017;
Ward et al. 2001).
F
ew studies directly address the incidence of cancer in women occupationally exposed to vinyl chloride.
One study found that women employed in the production of vinyl chloride and PVC had a significantly
greater chance of developing leukemia or lymphomas (Smulevich et al. 1988). In the same study, the
subgroup of women who were exposed to the highest levels of vinyl chloride had increased incidences of
stomach cancer and the highest incidences of leukemia and lymphoma. In this study, there was no
significant increase in any type of cancer in exposed males, irrespective of their level of exposure.
Increased breast cancer risk was associated with exposure to vinyl chloride as a hazardous air pollutant in
California (Garcia et al. 2015).
VINYL CHLORIDE 88
2. HEALTH EFFECTS
The human epidemiology data demonstrate a clear association between vinyl chloride exposure and liver
cancer (i.e., angiosarcoma and hepatocellular carcinoma). Although other cancers have been previously
reported for vinyl chloride workers (i.e., respiratory tract cancer, brain cancer, soft tissue cancers,
lymphatic/hematopoietic system cancers, malignant melanoma), more recent follow-up studies and
pooled and meta-analysis studies do not demonstrate a consistent association between vinyl chloride
exposure and tumor formation in these organs or tissue-systems (Boffetta et al. 2003; Bosetti et al. 2003;
Table 2-8).
Animal Studies. Studies in several animal species support the conclusion that vinyl chloride is
carcinogenic. A large series of experiments was performed by Maltoni et al. (1981) using rats (Sprague-
Dawley and Wistar), mice, and hamsters. In one group of studies, Maltoni et al. (1981) exposed Sprague-
Dawley rats to vinyl chloride for 52 weeks at concentrations ranging from 1 to 30,000 ppm. Animals
were examined at the time of their spontaneous death. Statistically significant increases were noted in the
incidence of mammary gland carcinomas, Zymbal gland carcinomas, nephroblastoma, and liver
angiosarcoma. Exposure of Swiss mice to 50 ppm vinyl chloride for 4 hours/day, 5 days/week for
30 weeks also appeared to increase the incidence of liver angiosarcoma and angioma (Maltoni et al.
1981). Maltoni et al. (1981) also reported that decreasing the duration of exposure decreased the
incidence of vinyl chloride-related tumors (nephroblastomas, liver angiosarcomas, Zymbal gland
carcinomas, and to some extent, neuroblastomas).
Some variation in the target organs that developed tumors was observed when different species were
exposed to vinyl chloride (Maltoni et al. 1981). Whereas angiosarcomas of the liver were reported to
occur in rats, mice, and hamsters, mammary gland carcinomas were found only in rats and mice. Zymbal
gland carcinomas, neuroblastomas, and nephroblastomas were found only in rats; lung tumors were found
only in mice; and melanomas, acoustical duct epithelial tumors, plus leukemias were found only in
hamsters.
Other inhalation experiments support the carcinogenicity of vinyl chloride. Rats and mice exposed to 0,
50, 250, or 1,000 ppm for 6 hours/day, 5 days/week for 6 months (Hong et al. 1981) or up to 12 months
(Lee et al. 1977a, 1978) had a significantly increased incidence of hemangiosarcoma of the liver at
≥250 ppm. In a 2-generation study in rats, pre-neoplastic liver lesions (i.e., foci of hepatocellular
alteration, hepatocellular foci) were observed in F1 males at 100 ppm and F1 males and F1 females at
1,100 ppm (6 hours/day for 16–19 weeks) (Thornton et al. 2002). Increases in bronchio-alveolar
adenoma of the lung and mammary gland tumors (adenocarcinomas, squamous and anaplastic cell
VINYL CHLORIDE 89
2. HEALTH EFFECTS
carcinomas) were also observed in mice at ≥50 ppm, (Lee et al. 1977a, 1978). Mice exposed to 50 or
500 ppm vinyl chloride for 6 hours/day, 5 days/week for 6 months or 1 year had an increased incidence of
lung adenoma, as well as hemangiosarcoma of fat tissue in various organs (Holmberg et al. 1976). Only
one liver hemangiosarcoma was noted.
Male rats exposed to concentrations as low as 105.6 ppm for 6 hours/day, 6 days/week, for 12 months had
significantly increased incidence of cancer, including angiosarcoma of the liver and lung, when sacrificed
at 18 months (Bi et al. 1985). Rats exposed to 30,000 ppm vinyl chloride 4 hours/day, 5 days/week, for
12 months had an increased incidence of epidermoid carcinoma of the skin, adenocarcinoma of the lungs,
and osteochondroma in the bones (Viola et al. 1971), while rats exposed to 5,000 ppm for 52 weeks had
primary tumors in the brain, lung, Zymbal gland, and nasal cavity (Feron and Kroes 1979). However,
these studies (Feron and Kroes 1979; Viola et al. 1971) are limited by the absence of statistical analysis of
the data. Female mice exposed to 50 ppm vinyl chloride for 6 months showed increased incidence of
hemangiosarcoma of the subcutis, peritoneum, and skin, as well as lung and mammary gland carcinomas
(Drew et al. 1983).
In a preliminary study with a limited number of animals, alveogenic lung tumors developed in 26 of
27 mice exposed to 2,500 or 6,000 ppm for 5–6 months (Suzuki 1978). A concentration-related increase
in the incidence of alveogenic tumors was observed in a study in which a larger number of mice were
exposed to 0–600 ppm for 4 weeks and then observed for up to 40 weeks postexposure (Suzuki 1983).
The lowest concentration at which multiple foci tumors were observed was 100 ppm (Suzuki 1983). A
significant increase in the incidence of pulmonary adenomas was reported in mice exposed to 50 ppm,
6 hours/day, 5 days/week for 6 months (Adkins et al. 1986). An increase in bronchioalveolar adenoma
was observed in a lifespan study of mice that were exposed once to 5,000 ppm for only 1 hour (Hehir et
al. 1981).
Some data suggest that exposure of animals during gestation and/or early post-birth may increase the
likelihood and the type of tumor that develops (Drew et al. 1983; Maltoni et al. 1981). Maltoni et al.
(1981) evaluated the effect of vinyl chloride dosing on liver carcinogenicity in Sprague-Dawley rats. Rats
were exposed to 0, 6,000, or 10,000 ppm vinyl chloride for 100 hours, beginning either at 1 day or at
13 weeks of age. The incidence of angiosarcoma of the liver in newborn rats exposed for only 5 weeks
was higher than the incidence observed in rats exposed for 52 weeks beginning at 13 weeks. Hepatoma
incidence was approximately 50% in newborn rats exposed for 5 weeks but did not occur in rats exposed
for 52 weeks after maturity.
VINYL CHLORIDE 90
2. HEALTH EFFECTS
When hamsters, mice, and rats were exposed to vinyl chloride for periods of 6–24 months starting at
various time-points after weaning, the incidence of tumors such as hemangiosarcoma of the liver, skin,
and spleen, and angiosarcoma of the stomach was greater when animals were exposed for 12 months
immediately after weaning than if animals had no exposures for 12 months and were then exposed for the
subsequent 12 months (Drew et al. 1983). Maltoni and Cotti (1988) also exposed pregnant rats to
2,500 ppm vinyl chloride starting on GD 12 and continued to expose both maternal animals and offspring
for a total of 76 weeks. Hepatocellular carcinoma, hepatic angiosarcoma, and neuroblastoma were
increased in treated animals compared to controls. The incidence of hepatocarcinoma was reported to be
much higher in offspring than in maternal animals. In contrast, the incidence and latency period of
neuroblastomas and hepatic angiosarcomas was similar between offspring and their parents.
Mammary gland carcinoma was significantly increased when 2- or 8-month-old hamsters, but not 14- or
20-month-old hamsters, were exposed to 200 ppm vinyl chloride for 6 months (Drew et al. 1983).
Fibroadenoma of the mammary gland was increased in female rats exposed to 100 ppm of vinyl chloride
for 6 hours/day, 5 days/week, over 6–24 months (Drew et al. 1983). When pregnant rats were exposed to
6,000 ppm vinyl chloride from GD 12 through 18, the incidence of mammary gland carcinomas, Zymbal
gland carcinomas, and forestomach epithelial tumors was reported to be greater in the transplacentally-
exposed animals than in the maternal animals (Maltoni et al. 1981). At 10,000 ppm in this study, more
nephroblastomas were observed in transplacentally exposed animals than the maternal animals (Maltoni
et al. 1981); however, there was no unexposed control group.
Many of the tumors that were observed in the Drew et al. (1983) and Maltoni et al. (1981) studies were
also observed in a study performed by Froment et al. (1994). In this study, Sprague-Dawley pups were
exposed to 500 ppm vinyl chloride 8 hours/day, 6 days/week, on postpartum days 3–28. After weaning,
22 animals/gender were exposed for an additional 2 weeks, for a total exposure duration of 33 days. Rats
were observed daily until death or development of tumors, and the surviving rats were sacrificed at
19 months. All livers from exposed animals that appeared normal at gross examination were found to
contain multiple nodular hyperplastic foci of hepatocytes. Liver tumors that were found in exposed
animals included angiosarcomas, hepatocellular carcinomas, and benign cholangiomas. Other tumors
found included pulmonary angiosarcoma (probably metastatic), nephroblastoma, abdominal angiomyoma,
leukemia, Zymbal gland carcinoma, pituitary adenoma, mammary carcinoma, and mammary fibroma.
Tumor incidence was not reported in control animals. Only one concentration (500 ppm) of vinyl
VINYL CHLORIDE 91
2. HEALTH EFFECTS
chloride was used because the purpose of the study was to examine the genotoxic impact of vinyl chloride
in the liver tumors produced by exposure.
Vinyl chloride induced preneoplastic foci in newborn rats, but not in mature rats (Laib et al. 1985). A
study with newborn male or female Wistar rats exposed to 2,000 ppm vinyl chloride indicated that the
induction of preneoplastic hepatocellular lesions in rats by vinyl chloride is restricted to an early stage in
the life of the animals. The early life stage sensitivity to the induction of tumors in animals exposed to
vinyl chloride appears to be related to the induction by vinyl chloride of hepatic adenosine-5’-triphos-
phatase (ATPase) deficient enzyme altered foci, which are putative precursors of hepatocellular
carcinoma.
Five studies were located that examined the carcinogenic potential of vinyl chloride in animals when
administered by the oral route. In two Wistar rat studies, vinyl chloride was added to the diet for up to
149 weeks by adding a PVC powder containing a high level of the monomer (Feron et al. 1981; Til et al.
1983, 1991). To limit volatilization of vinyl chloride from the diet, the rats were allowed access to the
diet for only 4 hours/day. The actual intake of vinyl chloride in these reports was calculated by taking
into consideration both the food consumption data and the rate of vinyl chloride evaporation. Statistically
significant increases in angiosarcoma were observed in the 2.7-year study by Feron et al. (1981) at
5mg/kg/day in males and 14.1 mg/kg/day in females. In the same study, statistically significant increases
in neoplastic nodules of the liver were also observed at a concentration of 5 mg/kg/day in males and as
low as 1.7 mg/kg/day in females (Feron et al. 1981). In the 149-week study by Til et al. (1983, 1991),
statistically significant increases in hepatocellular carcinoma were observed in males and hepatic
neoplastic nodules in females at 1.7 mg/kg/day. A few animals exposed to 1.7 mg/kg/day in this study
developed hepatic angiosarcoma. An increased incidence of Zymbal gland tumors was also observed in
the study by Feron et al. (1981). Although the increase was not statistically significant, the tumors were
considered to be treatment related based on the historical rarity of this type of tumor. Conversely, Til et
al. (1983, 1991) did not observe any Zymbal tumors in rats fed ≤1.7 mg vinyl chloride/kg/day for
149 weeks. Wistar rats gavaged with 300 mg/kg/day developed liver tumors, predominantly
angiosarcomas, within 60 days of exposure (Knight and Gibbons 1987). Liver tumors were also observed
in rats exposed to a lower dose for a longer period of time (30 mg/kg/day for 2 years) (Knight and
Gibbons 1987).
Two studies were located in which vinyl chloride was administered to Sprague-Dawley rats by gavage for
52 weeks. In one of these studies, a statistically significant increase in the incidence of hepatic
VINYL CHLORIDE 92
2. HEALTH EFFECTS
angiosarcomas was observed at doses as low as 16.65 mg/kg/day in females and 50 mg/kg/day in males.
Zymbal gland tumors at 16.65 and 50 mg/kg/day, even though not statistically significant, were
considered to be treatment related because of the rarity of this type of tumor (Maltoni et al. 1981). Lower
doses of vinyl chloride were also tested in a similar study where hepatic angiosarcomas were observed at
doses as low as 0.3 mg/kg/day and Zymbal gland tumors at 1 mg/kg/day. Although neither of these
findings reached statistical significance, the tumors were considered to be treatment related because
historically they rarely occurred in the rat colony (Maltoni et al. 1981).
Mechanisms of Cancer. The metabolism of vinyl chloride to its highly reactive metabolites, the
observance of deoxyribonucleic acid (DNA) adduction in mechanistic studies, and the observed
carcinogenicity resulting from a single, high level inhalation exposure in animals, suggest that the primary
mechanism for vinyl chloride carcinogenicity involves direct interaction with DNA rather than secondary
responses to cytotoxicity. 2-Chloroethylene oxide and 2-chloroacetaldehyde can both react with DNA
nucleotide bases. 2-Chloroethylene oxide is the more potent mutagen and may be the ultimate
carcinogenic metabolite of vinyl chloride (Chiang et al. 1997). The mutation profile for the DNA adducts
formed by the reactive metabolites of vinyl chloride (2-chloroethylene oxide and 2-chloroacetaldehyde)
includes the four cyclic etheno-adducts: 1,N
6
-ethenoadenine, 3,N
4
-ethenocytosine, 3,N
2
-ethenoguanine,
and 1,N
2
-ethenoguanine (Akasaka et al. 1997; Chiang et al. 1997; Dosanjh et al. 1994; Guichard et al.
1996; Matsuda et al. 1995; Pandya and Moriya 1996; Zhang et al. 1995; Zielinski and Hergenhahn 2001).
The role of etheno-adducts in the carcinogenesis of vinyl chloride was reviewed in several publications
(Albertini et al. 2003; Barbin 1998, 2000; Dogliotti 2006; Guengerich and Ghodke 2021; Kielhorn et al.
2000; Laib 1986; Rioux and Delaney 2020; Whysner et al. 1996). These adducts lead to base-pair
transitions during transcription and DNA crosslinks (Cullinan et al. 1997; Pandya and Moriya 1996;
Singer 1996; Singer et al. 1987). Such mutations have resulted in the mutation of ras oncogenes such as
those found in hepatic angiosarcoma tumors of workers exposed to high levels of vinyl chloride. In
addition, mutations in the p53 tumor suppressor gene identified in vinyl chloride workers are associated
with a variety of tumor types. Mutations of the p53 gene in vinyl chloride-exposed rats were similar to
those reported in humans (Section 2.20).
The mechanisms for the clastogenic effects of vinyl chloride exposure were examined by Fucic et al.
(1990a). Since chromatid and bichromatid breaks most frequently occurred in the terminal A, B, and C
group chromosomes, these investigators suggested that vinyl chloride or its metabolites might interact
with specific sites along chromosomes, thereby fragmenting the gene. This implies that the
carcinogenicity induced by vinyl chloride can be explained in part by its nonrandom interaction with
VINYL CHLORIDE 93
2. HEALTH EFFECTS
particular genes. Epigenetic processes that may contribute to vinyl chloride induced cancer formation
include aberrant DNA methylation (Chappell et al. 2016) and cell cycle deregulation (Pan et al. 2021).
Cancer Weight-of-Evidence Determination. The Department of Health and Human Services NTP
classified vinyl chloride as “known to be a human carcinogen” (NTP 2021) and IARC concluded that
there is sufficient evidence for carcinogenicity in humans and animals to classify vinyl chloride as a
Category 1 carcinogen (carcinogenic to humans) (IARC 2012). The IARC Working Group (IARC 2012)
concluded that vinyl chloride causes both liver angiosarcomas and hepatocellular carcinomas and found
suggestive evidence for an increased risk of malignant neoplasia of soft and connective tissue. No
association was found between vinyl chloride exposure and lung cancer, and the evidence for an increased
risk for brain cancer, lymphatic and hematopoietic cancers, and melanoma was characterized as weak.
The EPA weight-of-evidence characterization for vinyl chloride classifies it as a known human
carcinogen by the inhalation route of exposure based on human epidemiological data (EPA 2000). By
analogy, vinyl chloride is carcinogenic by the oral route because of the positive animal bioassay results
and the pharmacokinetic data that support extrapolation across exposure routes. Vinyl chloride is also
considered highly likely to be carcinogenic by the dermal route because it is well absorbed and acts
systemically (EPA 2000). However, the animal data suggest that dermal absorption of vinyl chloride gas
is not likely to be significant (Hefner et al. 1975a). Because the epidemiological evidence does not
provide sufficient exposure and incidence data to quantify risk based solely on the human data, the EPA
cancer potency factors for inhalation and oral exposure were calculated based on animal data. An
inhalation unit risk of 8.8x10
-6
per μg/m
3
for continuous lifetime exposure initiated at birth was estimated
(EPA 2000) based on the incidence of liver tumors in the rat inhalation study by Maltoni et al. (1981).
An inhalation unit risk of 4.4x10
-6
per μg/m
3.
for continuous lifetime exposure during adulthood was also
estimated by EPA (2000) based on the same study (Maltoni et al. 1981).
2.20 GENOTOXICITY
Vinyl chloride is mutagenic and clastogenic in both in vitro and in vivo test systems. Tables 2-9 and 2-10
list the key in vitro and in vivo genotoxicity studies, respectively, for vinyl chloride.

VINYL CHLORIDE 94
2. HEALTH EFFECTS
Table 2-9. Genotoxicity of Vinyl Chloride In Vitro
Species (test system)
Endpoint
Result
Reference
With
activation
Without
activation
Salmonella typhimurium
Reverse mutation
+
–
Rannug et al. 1974
+
+
Bartsch et al. 1975,
1976
+
+
Andrews et al. 1976
+
+
Simmon et al. 1977
Not tested
–
Elmore et al. 1976
+
+
Poncelet et al. 1980
+
+
de Meester et al. 1980
+
+
Victorin and Stahlberg
1988
+
Not tested
McCann et al. 1975
+
+
Rannug et al. 1976
S. typhimurium TA100,
TA1535
Base-pair substitution
+
+
du Pont 1992a, 1992b
+
Not tested
Malaveille et al. 1975
Escherichia coli
Not applicable
+
Jacobsen et al. 1989
E. coli transfected with
human plasmid DNA
DNA repair
Not applicable
+
Kowalczyk et al. 2006
E. coli transfected with
plasmid DNA
Mutation and DNA
repair
Not applicable
+
Maciejewska et al.
2010
Saccharomyces cerevisiae
Not tested
–
Shahin 1976
Gene conversion
+
Not tested
Loprieno et al. 1976
Schizosaccharomyces
pombe
Forward mutation
+
–
Loprieno et al. 1977
+
Not tested
Loprieno et al. 1976
D7RAD yeast
Gene conversion
+
_
Eckardt et al. 1981
Chinese hamster ovary
cells
Mutation
Not applicable
+
Huberman et al. 1975
+
Not tested
Drevon et al. 1978
+
–
du Pont 1992c
Chinese hamster lung cells
Chromosomal
aberration
+
–
Asakura et al. 2008
Bacillus subtilis
Rec-repair
Not tested
–
Elmore et al. 1976
Rat liver microsomes
RNA alkylation
Not applicable
+
Laib and Bolt 1977
QT6 (avian cells)
Inhibition of DNA
synthesis
Not applicable
+
Kandala et al. 1990
African green monkey
fibroblast cell line (COS-7)
Mutation spectra after
transfection with DNA
adducts of vinyl
chloride
Not applicable
+
Fernandes et al. 2005

VINYL CHLORIDE 95
2. HEALTH EFFECTS
Table 2-9. Genotoxicity of Vinyl Chloride In Vitro
Species (test system)
Endpoint
Result
Reference
Human plasmid DNA
Mutation
Not applicable
+
Kowalczyk et al. 2006
Human lymphoblast
Micronuclei
Not applicable
+
Feng et al. 2014
– = negative result; + = positive result; DNA = deoxyribonucleic acid; RNA = ribonucleic acid
Table 2-10. Genotoxicity of Vinyl Chloride In Vivo
Species (exposure route)
Endpoint
Results
Reference
Mouse (inhalation)
Dominant lethal
–
Anderson et al. 1976
Micronuclei
+
Richardson et al. 1983
Rat (inhalation)
Dominant lethal
–
Short et al. 1977
–
Anderson et al. 1976
–
Purchase et al. 1975
Chromosomal aberration
+
Anderson and Richardson 1981
Hamster (inhalation or i.p.
injection)
Chromosomal aberration
+
Fleig and Thiess 1978
Human lymphocytes from
exposed workers
Sister chromatid exchange
–
Hansteen et al. 1978
+
Fucic et al. 1990a
+
Fucic et al. 1992
+
Fucic et al. 1995
+
Fucic et al. 1996a
+
Fucic et al. 1996b
+
Kucerova et al. 1979
+
Sinués et al. 1991
+
Zhao et al. 1994
DNA damage
+
Awara et al. 1998
+
Du et al. 1995
+
Lei et al. 2004
+
Kumar et al. 2013
+
Zhu et al. 2005b
+
Zhu et al. 2008
Micronuclei
+
Feng et al. 2017
+
Fucic et al. 1990a
+
Garaj-Vrhovac et al. 1990
+
Ji et al. 2010
+
Jiao et al. 2012
+
Kumar et al. 2013
+
Li et al. 2013
+
Qiu et al. 2008

VINYL CHLORIDE 96
2. HEALTH EFFECTS
Table 2-10. Genotoxicity of Vinyl Chloride In Vivo
Species (exposure route)
Endpoint
Results
Reference
+
Qiu et al. 2011a
+
Qiu et al. 2011b
+
Sinués et al. 1991
+
Vaglenov et al. 1999
+
Wang et al. 2010a
+
Wang et al. 2011
+
Wang et al. 2013a
+
Wang et al. 2013b
+
Wen-Bin et al. 2009
+
Wu et al. 2013
+
Zheng et al. 2017
Chromosomal aberration
_
Picciano et al. 1977
+
Anderson et al. 1980, 1981
+
Anderson 1999
+
Becker et al. 2001
+
Ducatman et al. 1975
+
Fleig and Thiess 1978
+
Fucic et al. 1990a, 1990b
+
Fucic et al. 1992
+
Fucic et al. 1995
+
Fucic et al. 1996a
+
Fucic et al. 1996b
+
Funes-Cravioto et al. 1975
+
Garaj-Vrhovac et al. 1990
+
Hansteen et al. 1978
+
Heath et al. 1977
+
Hrivnak et al. 1990
+
Hüttner et al. 1998
+
Hüttner et al. 1999
+
Hüttner and Nikolova 1998
+
Kucerova et al. 1979
+
Kumar et al. 2013
+
Purchase et al. 1978
+
Vaglenov et al. 1999

VINYL CHLORIDE 97
2. HEALTH EFFECTS
Table 2-10. Genotoxicity of Vinyl Chloride In Vivo
Species (exposure route)
Endpoint
Results
Reference
Rat (inhalation)
DNA alkylation
+
Bolt et al. 1986 (liver)
+
Ciroussel et al. 1990 (liver, lungs
brain)
+
Eberle et al. 1989 (liver, lung)
+
Green and Hathway 1978 (liver)
+
Gwinner et al. 1983 (liver)
+
Laib 1986 (liver)
+
Singer et al. 1987 (liver)
Mouse (inhalation)
DNA alkylation
+
Osterman-Golkar et al. 1977
DNA damage
+
Walles et al. 1988
Rat (inhalation)
DNA adduct
+
Bolt et al. 1986 (liver)
+
Ciroussel et al. 1990 (liver, lungs
brain)
+
Eberle et al. 1989 (liver, lung)
+
Fedtke et al. 1990 (liver, lung,
kidney, brain, spleen)
+
Morinello et al. 2002a, 2002b
(liver, brain)
+
Swenberg et al. 1992 (liver)
Rat (i.p. injection)
DNA damage
+
Qiu et al. 2019 (liver)
– = negative result; + = positive result; i.p. = intraperitoneal; DNA = deoxyribonucleic acid
Concentrations of vinyl chloride tested in vitro range from 0.275% (Shahin 1976) to 40% (du Pont
1992a). Shahin (1976) reported negative results for 0.275 and 0.55% vinyl chloride in Saccharomyces
cerevisiae. In Salmonella typhimurium, a doubling of the number of revertant colonies was reported to
occur at a concentration of about 5% vinyl chloride (Victorin and Stahlberg 1988). Vinyl chloride was
found to be mutagenic in Chinese hamster ovary cells and yeast (Drevon et al. 1978; du Pont 1992c;
Eckardt et al. 1981; Loprieno et al. 1976). A 5-hour exposure to 4,600 ppm vinyl chloride did not cause
mutagenicity in the mammalian spot test (Peter and Ungvary 1980).
There is evidence that in S. typhimurium, Escherichia coli, and Bacillus subtilis, it is the oxidation of
vinyl chloride to its reactive intermediates, 2-chloroethylene oxide and 2-chloroacetaldehyde, that leads to
its mutagenicity (Bartsch et al. 1976, 1979; Hussain and Osterman-Golkar 1976; Jacobsen et al. 1989;
Laumbach et al. 1977; McCann et al. 1975; Rannug et al. 1976). The S-9 fraction from surgically
obtained human liver specimens was shown to metabolize vinyl chloride to electrophiles that were
mutagenic to S. typhimurium TA1530 (Sabadie et al. 1980). Mutagenicity assays were performed by
VINYL CHLORIDE 98
2. HEALTH EFFECTS
exposing the plates containing S. typhimurium and 150 μL human S-9 fraction to a gaseous mixture of
20% vinyl chloride in air for 4 hours. The gaseous mixture was removed after the exposure, leaving a
vinyl chloride concentration of 4x10
-3
M in the aqueous phase of the plates. Incubation was continued for
an additional 48 hours. When compared with the number of revertant colonies per plate resulting from
identically prepared S-9 fractions from female strain BD IV rats, the human S-9 fractions mutations
averaged 84% of those mediated by rat S-9. A 9-fold individual variation was observed among human
S-9 samples.
The chloroacetaldehyde metabolite of vinyl chloride appears to be less genotoxic in yeast and Chinese
hamster V79 cells than 2-chloroethylene oxide (Huberman et al. 1975; Loprieno et al. 1977) and has been
shown to inhibit DNA synthesis in avian cells (Kandala et al. 1990). However, 2-chloroacetaldehyde can
react directly with single-stranded DNA, producing DNA base changes and subsequent reversion when
the DNA was inserted into E. coli via a phage technique (Jacobsen et al. 1989). Other studies found
2-chloroacetaldehyde to be mutagenic in human fibroblast cells using shuttle vectors (Matsuda et al.
1995).
Vinyl chloride produced chromosome aberrations in a gas exposure system using Chinese hamster lung
cells (Asakura et al. 2008). DNA adducts of vinyl chloride were shown to be mutagenic following
transfection into COS-7 mammalian cells (Fernandes et al. 2005). Chloroacetaldehyde, a metabolite of
vinyl chloride, produced sequence specific mutations in the p53 gene region of human DNA (Kowalczyk
et al. 2006). DNA repair kinetics, evaluated following transfection of human plasmid DNA into E. coli,
were also sequence specific with rapid repair occurring in some locations and delayed repair occurring at
mutation hotspots (Kowalczyk et al. 2006). Repair of chloroacetaldehyde-induced mutations in E. coli
was shown to be mediated by the AlkB protein, which is produced as part of an adaptive response to
alkylating agents in these bacteria (Maciejewska et al. 2010).
Genotoxicity studies of vinyl chloride in humans include assays evaluating micronuclei, chromosome
aberrations, or DNA damage in cultured human lymphocytes of occupationally exposed workers. Studies
completed through the mid-1980s generally found a statistically significant increase in the frequency of
chromosomal aberrations, usually of the chromatid type (i.e., affecting only one of the two strands formed
upon DNA replication), but also including some other chromosomal-type defects such as inversions,
rings, and translocations, which affect the entire chromosome (Anderson 1999, 2000; Anderson et al.
1981; Fleig and Thiess 1978; Fucic et al. 1990a; Heath et al. 1977). Total chromosomal aberrations and
chromatid type aberrations were increased in vinyl chloride workers with exposure durations of >8 years,
VINYL CHLORIDE 99
2. HE
ALTH EFFECTS
compared with workers exposed for a shorter time period and unexposed controls (Kumar et al. 2013).
An increase in chromosomal aberrations was also observed following an accidental environmental
exposure to vinyl chloride (Becker et al. 2001; Hüttner and Nikolova 1998; Hüttner et al. 1998, 1999).
Micronuclei frequency was significantly increased in vinyl chloride workers compared to control workers
(Feng et al. 2017; Fucic et al. 1990a; Garaj-Vrhovac et al. 1990; Ji et al. 2010; Jiao et al. 2012; Kumar et
al. 2013; Sinués et al. 1991; Wang et al. 2010a, 2011, 2013a, 2013b; Wu et al. 2013; Zheng et al. 2017).
The increase in micronuclei frequency was generally associated with cumulative exposure to vinyl
chloride in the cited studies. Female workers were shown to be more susceptible to the increase in
micronuclei frequency than male workers (Wang et al. 2013a). An increase in chromosome aberrations
and micronuclei was correlated with both the air concentration of vinyl chloride and the excretion of
thiodiglycolic acid in the urine of exposed workers at a plastic plant (Vaglenov et al. 1999).
I
ncreased sister chromatid exchanges were reported in occupationally exposed workers (Fucic et al.
1990a, 1992, 1995; Kucerova et al. 1979; Sinués et al. 1991; Zhao et al. 1996). Sister chromatid
exchange frequencies were significantly increased compared to those of the controls at 0.003–7.3 ppm
vinyl chloride (Sinués et al. 1991). A positive correlation between frequency of chromosomal
aberrations, length of exposure, and history of exposure to excursion levels (up to 2,000 ppm) was
reported by Purchase et al. (1978) after examination of a cohort of 57 vinyl chloride workers, 19 on-site
controls, and 5 off-site controls. The exposures for this cohort ranged from 1,000 ppm between 1945 and
1955 to 5 ppm in the years after 1975. These authors also reported an effect of vinyl chloride on
chromosomal aberrations in the individuals who reported smoking. Smoking and the presence of an
aldehyde dehydrogenase 2 genotype was associated with an increase in the frequency of sister chromatid
exchange among vinyl chloride workers (Wong et al. 1998).
DNA si
ngle strand breaks were increased in lymphocytes from workers exposed to vinyl chloride
concentrations >5 ppm (Kumar et al. 2013; Lei et al. 2004). A correlation was observed between the
severity of DNA damage and the duration of exposure (Awara et al. 1998). The level of single-strand
breaks was also significantly associated with levels of the urinary biomarker, thiodiglycolic acid (Lei et
al. 2004). DNA single strand breaks present in human lymphocytes from exposed workers were quickly
repaired following cessation of exposure (Du et al. 1995). Induction of single strand breaks in liver DNA
was also observed in mice after inhalation of vinyl chloride (Walles et al. 1988).
T
he reversibility of chromosome damage was reported for several worker populations following cessation
or reduction of exposure to vinyl chloride. The increase of chromosome aberrations observed in workers
VINYL CHLORIDE 100
2. HEALTH EFFECTS
exposed to 50 ppm returned to normal within 42 months after exposure levels were reduced to <5 ppm
(Anderson et al. 1980). Another study demonstrated a statistically significant increase in aberrations in
workers exposed to vinyl chloride concentrations of approximately 25 ppm. Following a reduction in
exposure to 1 ppm, vinyl chloride chromosomal aberrations returned to control values (Hansteen et al.
1978). A 9-year follow-up study of an occupationally exposed population demonstrated a decrease in
chromosome aberrations and sister chromatid exchange frequencies over time, corresponding to a
decrease in vinyl chloride air concentrations at the plant (Fucic et al. 1996a, 1996b).
The reversibility of clastogenic effects was not observed in a study of 12 current and 3 retired plastics
industry workers who had been exposed to vinyl chloride while employed for periods of 1.5–35 years
(Fucic et al. 1992). Sister chromatid exchange frequencies were significantly higher in the workers
exposed to concentrations up to 2,000 ppm than in the controls. These findings showed no significant
decrease in sister chromatid exchange frequencies in the participants following periods of 8 days to
10 years after exposure (Fucic et al. 1992).
Other papers on human subjects focused on specific mechanisms involved in producing the clastogenic
effects of vinyl chloride. A cohort of 67 workers exposed to approximately 5 ppm for an average of
15 years was reported to have a nonrandom distribution of chromatid and bichromatid DNA strand breaks
(Fucic et al. 1990b). The most frequently affected areas of the genome were the terminal segments of the
A, B, and C group chromosomes, suggesting that vinyl chloride or its metabolites interact more
frequently with specific sites along the chromosome than would be expected. The study authors
presented no correlation with particular fragile sites (gene sequences more prone to breakage than normal)
or oncogene locations known to occur at these terminal segments. The implication is that the
carcinogenicity of vinyl chloride could be at least partially explained by its nonrandom interaction with
particular genes. The workers were also periodically exposed to vinyl chloride concentrations as high as
2,000 ppm for short periods. No specific information was given as to the frequency or duration of the
high vinyl chloride concentration events.
Male workers (n=20) employed for 2–14 years at a vinyl chloride polymerization plant and exposed to
concentrations of vinyl chloride of 1 ppm (with occasional peaks of 300 ppm) underwent cytogenetic
testing (Fucic et al. 1995). The test results were compared to those from 20 unexposed male controls.
The exposed individuals had higher percentages of chromosome aberrations, primarily chromatid breaks
than the controls. Sister chromatid exchange frequencies were also increased in the exposed workers (4–
22 per cell) compared to controls (4–7 per cell). Significant changes in mitotic activity were noted among
VINYL CHLORIDE 101
2. HEALTH EFFECTS
exposed workers; values for second mitosis events were lower than controls and values for a third mitosis
event were higher than controls (Fucic et al. 1995, 1997). Chromosome aberrations were not increased in
workers exposed to <5 ppm vinyl chloride; however, the average exposure duration for this study was less
than 1 year (Picciano et al. 1977).
Polymorphisms of genes involved in metabolism (CYP2E1, glutathione S-transferase pi 1 [GSTP1],
aldehyde dehydrogenase 2 [ALDH2]), DNA repair (human 8-oxoguanine glycosylase 1 [hOGG1],
O6-methylguanine-DNA methyltransferase [MGMT], X-ray repair cross complementing group 1
[XRCC1], xeroderma pigmentosum complement groups A, C, D, and E [XPA, XPC, XPD, XPF],
thymine-DNA glycosylase [TDG], apurinic/apyrimidinic endonuclease 1 [APE1]), apoptosis (MDM2,
BCL2), and cell cycle control (p53, p21) are associated with increased micronuclei and sister chromatid
exchange frequency in vinyl chloride workers (Feng et al. 2017; Ji et al. 2010; Li et al. 2013; Qiu et al.
2008, 2011a; Wang et al. 2010a, 2010b, 2013b; Wen-Bin et al. 2009; Wong et al. 2003b). Increased
micronuclei frequency was also associated with altered promoter methylation of MGMT in vinyl
chloride-exposed workers (Wu et al. 2013). Qiu et al. (2011b) found an increase in p21 mRNA
expression in workers exposed to vinyl chloride; however, there was no correlation with the frequency of
micronuclei measured in these workers. Polymorphisms of CYP2E1, XRCC1, and XPD were also
associated with susceptibility to DNA damage (single-strand breaks in lymphocyte DNA) of vinyl
chloride-exposed workers (Zhu et al. 2005b, 2008). Genetic polymorphisms of the XRCC1 DNA repair
gene were also associated with an increase in the retention of etheno-DNA adducts in lymphoblast cell
lines derived from vinyl chloride workers (Li et al. 2006, 2009a). The occurrence of mutation biomarkers
in serum was correlated with polymorphisms of the DNA repair genes XRCC1 (mutant p53) and excision
repair cross complementation group 2 (ERCC2)/XPD (mutant p53 and ras-p21) in vinyl chloride workers
(Li et al. 2006, 2009b). The presence of a polymorphism for CYP2E1 (variant c2 allele) was also
associated with the occurrence of mutant p53 and ras-p21 serum biomarkers (Schindler et al. 2007).
Polymorphisms of other genes involved in vinyl chloride metabolism (microsomal epoxide hydrolase
[mEH], glutathione S-transferase mu 1 [GSTM1], glutathione S-transferase theta 1 [GSTT1]) were not
associated with mutant p21 or p53 biomarkers in vinyl chloride workers (Li et al. 2005a, 2005b;
Schindler et al. 2007).
Animal studies of rats and mice exposed via inhalation to vinyl chloride concentrated on identifying the
direct effects of vinyl chloride and its metabolites on DNA. Vinyl chloride is metabolized by cytochrome
P450 mixed function oxidases (CYP) to form an epoxide intermediate, 2-chloroethylene oxide, which
spontaneously rearranges to form 2-chloroacetaldehyde (Section 3.1.3, Metabolism). Reactive
VINYL CHLORIDE 102
2. HEALTH EFFECTS
metabolites of vinyl chloride can be transported intercellularly from parenchymal cells to the
nonparenchymal cells (Kuchenmeister et al. 1996). Many studies have characterized the mutation profile
associated with DNA adducts formed by the reactive metabolites of vinyl chloride (Akasaka et al. 1997;
Chiang et al. 1997; Dosanjh et al. 1994; Guichard et al. 1996; Matsuda et al. 1995; Pandya and Moriya
1996; Zhang et al. 1995; Zielinski and Hergenhahn 2001). The four primary mutagenic DNA adducts
formed by the reactive metabolites of vinyl chloride are cyclic etheno-adducts that include
1,N
6
-ethenoadenine, 3,N
4
-ethenocytosine, N
2
,3-ethenoguanine, and 1,N
2
-ethenoguanine. These adducts
can induce base-pair (i.e., purine-to-purine or pyrimidine-to-pyrimidine exchange) transitions during
transcription (Cullinan et al. 1997; Oesch and Doerjer 1982; Pandya and Moriya 1996; Singer 1996;
Singer et al. 1987). 1,N
6
-Ethenoadenine adducts reduce the binding of topoisomerase I to DNA, affecting
DNA replication and transcription (Pourquier et al. 1998). The adduct, 7-(2’-oxoethyl) guanine, is
extensively formed in mammalian liver (Laib et al. 1981); however, it is quickly recognized and removed
by DNA repair mechanisms. Etheno-adducts are less abundant, but more persistent because they are
poorly repaired (Brandt-Rauf et al. 2000a; Whysner et al. 1996).
The presence of etheno-nucleosides has been reported following inhalation exposure to vinyl chloride in
rats (Bolt et al. 1986; Ciroussel et al. 1990; Eberle et al. 1989; Fedtke et al. 1990; Morinello et al. 2002a,
2002b; Swenberg et al. 1992). Immature rats exposed in vivo formed 6 times more of this nucleoside
adduct, which correlated with the age-related sensitivity to carcinogenesis in these animals (Ciroussel et
al. 1990). This age-related sensitivity to DNA adduct formation was also noted in an inhalation study of
lactating rats and their 10-day-old pups exposed 4 hours/day, for 5 days to 600 ppm of vinyl chloride
(Fedtke et al. 1990). Concentrations of two adducts found in the liver of the pups were 4-fold higher than
those found in the liver of the dams. Increased alkylation of liver DNA and increased cell proliferation
were reported by Laib et al. (1989) following exposure to 600 ppm vinyl chloride for 6 hours. Young rats
were apparently more susceptible to the effects of vinyl chloride, but only three male adults and two
female adults were used for comparison. In a similar study comparing three newborn rats to two adult
rats, exposure to 2,000 ppm vinyl chloride 8 hours/day, 5 days/week for 10 weeks resulted in
hepatocellular foci that were deficient in nucleoside-5-triphosphatase in newborns animals only (Laib et
al. 1979). The concentration of ethenoguanine adducts was 2–3-fold greater in weanling rats as compared
to adult rats exposed at the same dose for the time period (0, 10, 100, or 1,100 ppm, 6 hours/day for
5 days) (Morinello et al. 2002a). Rats exposed to 2,000 ppm vinyl chloride for 8 hours/day, 5 days/week,
for 3 weeks beginning at 7 days of age demonstrated hepatocellular ATPase-deficient foci and alkylation
of liver DNA (Gwinner et al. 1983). A study in rats exposed to 1,100 ppm vinyl chloride for 6 hours/day,
5 days/week for 1 or 4 weeks demonstrated that ethenoguanine adducts are not formed in the adult rat
VINYL CHLORIDE 103
2. HEALTH EFFECTS
brain (Morinello et al. 2002b). This differential induction of DNA adducts (brain versus liver) may relate
to the direct effect of reactive intermediates at the site of metabolite generation.
The role of etheno-adducts in the carcinogenesis of vinyl chloride was reviewed by a number of
researchers (Albertini et al. 2003; Barbin 1998, 1999, 2000; Gros et al. 2003; Kielhorn et al. 2000; Laib
1986; Mutlu et al. 2010, 2012; Nivard and Vogel 1999; Pottenger et al. 2014; Swenberg et al. 2011;
Whysner et al. 1996). Both 2-chloroethylene oxide and 2-chloroacetaldehyde can react with DNA
nucleotide bases; however, 2-chloroethylene oxide is a more potent mutagen and may be the ultimate
carcinogenic metabolite of vinyl chloride (Chiang et al. 1997). Etheno-adducts mainly lead to base pair
substitution mutations. Mutations in specific genes (i.e., ras oncogenes, p53 tumor suppressor gene)
identified in vinyl chloride-induced liver tumors in rats and humans are discussed in further detail below.
Exocyclic DNA adducts are excised from the DNA by glycosylase enzymes that contribute to genetic
stability (Laval and Saparbaev 2001). The four primary cyclic adducts formed in DNA by the vinyl
chloride metabolite, chloroacetaldehyde, are released by human glycosylase enzymes (Dosanjh et al.
1994; Singer and Hang 1999). The expression of the DNA repair enzyme N-methylpurine-DNA-
glycosylase was shown to be deficient in nonparenchymal cells of the rat liver, the target cells for vinyl
chloride-induced angiosarcomas (Holt et al. 2000; Swenberg et al. 1999). However, there were no
differences observed in the formation of ethenoguanine adducts in hepatocytes and nonparenchymal cells
immediately following vinyl chloride exposure (Morinello et al. 2002a). Together, these data suggest that
cellular differences in DNA repair capacity may play a role in vinyl chloride-induced carcinogenesis. It is
important to note that endogenously formed etheno-adducts are also present in humans and laboratory
animals due to a reaction between DNA and lipid peroxidation by-products. The background incidence of
etheno-adducts should be considered when evaluating exposure to chemicals like vinyl chloride (Albertini
et al. 2003; Bartsch and Nair 2000; Gonzalez-Reche et al. 2002; Swenberg et al. 2000; Watson et al.
1999; Yang et al. 2000; Zielinski and Hergenhahn 2001). A stable isotope method using [
13
C
2
]-labeled
vinyl chloride was used to determine the half-life of etheno-guanidine adducts following inhalation
exposure in rats, which allowed for a distinction between endogenous and exogenous adducts (Mutlu et
al. 2010, 2012; Swenberg et al. 2011).
Members of the ras gene family, including Ha-ras, Ki-ras, and N-ras, may be responsible for the control
of cell proliferation and differentiation (Froment et al. 1994). DNA adducts formed by vinyl chloride
metabolites can produce point mutations in these genes. Mutations of the Ki-ras-2 gene were found in
hepatic angiosarcomas of workers exposed to high levels of vinyl chloride; this specific gene was shown
to be activated by a GC-AT transition at codons 12 and 13 (Brandt-Rauf et al. 1995; Guido et al. 2016;
VINYL CHLORIDE 104
2. HEALTH EFFECTS
Marion et al. 1991; Weihrauch et al. 2002). Similar mutations of Ki-ras-2 were found in hepatocellular
carcinomas of workers exposed to vinyl chloride (Weihrauch et al. 2001a, 2001b). Hypermethylation of
the p16 gene was also associated with Ki-ras-2 mutation in hepatocellular carcinomas from exposed
workers (Weihrauch et al. 2001b).
Mutation of the Ki-ras-2 gene results in the expression of a mutant p21 protein. This mutant oncoprotein
was detected in serum samples taken from vinyl chloride workers with angiosarcoma of the liver (DeVivo
et al. 1994; Marion 1998). Mutant p21 protein was also detected in the serum or plasma of exposed
workers without liver tumors and a relationship between the frequency of the mutant protein in serum and
the intensity of vinyl chloride exposure was demonstrated in several studies (Brandt-Rauf et al. 1995;
DeVivo et al. 1994; Li et al. 1998; Luo et al. 1998, 2003; Marion 1998).
Rat liver tumors induced by exposure to 500 ppm vinyl chloride were examined for mutations of the
Ha-ras, Ki-ras, and N-ras genes (Boivin-Angele et al. 2000; Froment et al. 1994; Marion and Boivin-
Angele 1999). In contrast to the studies in humans, the Ki-ras gene mutation does not occur in rats or
mice with angiosarcoma of the liver induced by vinyl chloride exposure. Rats with hepatocellular
carcinoma demonstrated a AT–TA transversion of base 2 of codon 61 of the Ha-ras gene. However, this
mutation was not detected in rodent angiosarcoma of the liver, suggesting that there might be cell-specific
factors that affect the ras gene. Other mutations in codons 13 and 36 of the N-ras A gene were found in
two out of five of the liver angiosarcomas examined (Froment et al. 1994).
The p53 tumor suppressor gene is mutated in a variety of human cancers (Staib et al. 2003; Trivers et al.
1995). A study was performed to examine the p53 tumor suppressor genes and the murine double min-
2 (MDM2) proto-oncogenes from tumors of five vinyl chloride workers, four with angiosarcoma of the
liver and one with hepatocellular carcinoma (Hollstein et al. 1994). The p53 tumor suppressor gene was
being tested for mutation, while the MDM2 proto-oncogene was being tested for amplification. No
amplification of the MDM2 gene was detected; however, adenosine-to-thymidine missense mutations
were found in exons 5–8 (codons 249 and 255) of the p53 gene in two of the angiosarcoma cases. In
another study, tumors (angiosarcoma of the liver) from three of six vinyl chloride workers also had
adenosine-to-thymidine missense mutations in the p53 gene (codons 249, 255, and 179) (Trivers et al.
1995). Data from a study of angiosarcoma of the liver resulting from endogenous or unknown sources
(i.e., no vinyl chloride exposure) indicated that p53 mutations were uncommon, providing support for the
specificity of p53 mutations with vinyl chloride exposure in cases of angiosarcoma of the liver (Soini et
al. 1995). The p53 gene mutation pattern in rat liver tumors (angiosarcoma and hepatocellular carcinoma)
VINYL CHLORIDE 105
2. HEALTH EFFECTS
was shown to be similar to that observed in human tumors from vinyl chloride-exposed workers (Barbin
et al. 1997; Marion and Boivin-Angele 1999). In a different study, mutations of the p53 gene were found
in hepatocellular carcinomas from workers exposed to vinyl chloride; however, no correlation with vinyl
chloride exposure occurred and the mutation pattern was thought to reflect endogenous mechanisms (e.g.,
deamination of 5-methylcytosine) rather that chemical mutagenesis (Weihrauch et al. 2000). A
p53 mutation at codon 179 was detected in myofibroblast-type cells isolated from a liver tumor in an
exposed worker (Boivin et al. 1997). Ki-ras mutations were not observed in these cells. Vinyl chloride
mutations of the p53 gene produce conformational effects in the expressed p53 protein that affect its
function (Cheng et al. 1999a).
Mutant p53 protein and/or anti-p53 antibodies were detected in the serum and plasma of vinyl chloride-
exposed workers (Luo et al. 1999; Marion 1998; Smith et al. 1998; Trivers et al. 1995). A relationship
between the frequency of the mutant protein or p53 antibodies in serum/plasma and the vinyl chloride
exposure concentration was demonstrated in these studies. Polymorphisms of the genes for vinyl chloride
metabolism (CYP2E1) and DNA repair (x-ray cross-complementing group 1) are associated with a
greater risk of p53 gene mutation and over-expression of p53 mutant protein (Li et al. 2003a; Wong et al.
2002b).
Rat studies suggest that gap junctional intercellular communication mediated by connexin 37 is disturbed
in angiosarcoma of the liver; however, mutation of the connexin 37 gene is rare (Saito et al. 1997). The
incidence of hypoxanthine-guanine-phosphoribosyl-transferase (HPRT) mutants was not consistently
elevated in workers exposed to vinyl chloride (Hüttner and Holzapfel 1996; Liber et al. 1999). HPRT
mutants were also not increased in humans accidentally exposed to vinyl chloride (Becker et al. 2001).
Vinyl chloride has not been shown to be positive for dominant lethal effects in rats exposed to up to
30,000 ppm, for 6 hours/day for 5 days (Anderson et al. 1976; Purchase et al. 1975; Short et al. 1977).
The studies showed no evidence of pre- or post-implantation loss among the untreated females mated to
the exposed males. These results indicate that no germinal mutations were produced by these acute-
duration exposures. Vinyl chloride induces somatic and sex-linked recessive lethal mutations in
Drosophila but does not induce dominant lethal mutations (Ballering et al. 1996; Giri 1995; Magnusson
and Ramel 1978).
Vinyl chloride is mutagenic in S. typhimurium (Andrews et al. 1976; Bartsch et al. 1975, 1976; de
Meester et al. 1980; Elmore et al. 1976; Malaveille et al. 1975; Poncelet et al. 1980; Simmon et al. 1977),
VINYL CHLORIDE 106
2. HEALTH EFFECTS
but only in strains reverted by base-pair substitution by alkylating agents rather than by frameshift
mutations (Bartsch et al. 1976; du Pont 1992a, 1992b). Metabolic activation is necessary for any
mutagenic activity in this system (Rannug et al. 1974) or for a maximal response (Simmon et al. 1977).
In addition, vinyl chloride is mutagenic in the gaseous phase, but not when it is dissolved in water
(Poncelet et al. 1980). The negative findings for vinyl chloride dissolved in water are most likely due to
methodological problems associated with rapid evaporation and therefore do not reflect a lack of
mutagenic potential.
Summary. There are substantial data on clastogenesis in humans exposed to vinyl chloride that indicate
that this chemical acts as a potent genotoxicant (Anderson 2000; Anderson et al. 1980; Awara et al. 1998;
Becker et al. 2001; Ducatman et al. 1975; Fucic et al. 1990a, 1990b, 1992, 1995; Funes-Cravioto et al.
1975; Hansteen et al. 1978; Hrivnak et al. 1990; Hüttner and Nikolova 1998; Hüttner et al. 1998, 1999;
Kucerova et al. 1979; Marion et al. 1991; Purchase et al. 1978; Sinués et al. 1991; Wong et al. 1998; Zhao
et al. 1996). Reversibility of chromosome damage has been reported for several populations of workers
following a cessation or reduction of exposure to vinyl chloride (Anderson et al. 1980; Fucic et al. 1996a,
1996b; Hansteen et al. 1978). Findings in humans are supported by both animal studies and in vitro
studies that show positive genotoxicity in a variety of microbial organisms, cultured cell lines, and
isolated nucleic acid assays (Anderson and Richardson 1981; Andrews et al. 1976; Bartsch 1976; Bartsch
et al. 1976; Bolt et al. 1986; Ciroussel et al. 1990; de Meester et al. 1980; Eberle et al. 1989; Froment et
al. 1994; Green and Hathway 1978; Gwinner et al. 1983; Hansteen et al. 1978; Huberman et al. 1975;
Jacobsen et al. 1989; Kandala et al. 1990; Laib and Bolt 1977; Laib et al. 1989; Loprieno et al. 1977;
McCann et al. 1975; Osterman-Golkar et al. 1977; Poncelet et al. 1980; Rannug et al. 1974, 1976;
Simmon et al. 1977; Singer et al. 1987; Victorin and Stahlberg 1988; Walles et al. 1988). The role that
etheno-adducts play in the carcinogenesis of vinyl chloride has been extensively studied (Albertini et al.
2003, Barbin 1998, 1999, 2000; Kielhorn et al. 2000; Nivard and Vogel 1999; Whysner et al. 1996).
Both 2-chloroethylene oxide and 2-chloroacetaldehyde can react with DNA nucleotide bases; however,
2-chloroethylene oxide is a more potent mutagen and may be the ultimate carcinogenic metabolite of
vinyl chloride (Chiang et al. 1997). Etheno-adducts generate mainly base pair substitution mutations.
Mutations in specific genes (i.e., ras oncogenes, p53 tumor suppressor gene) have been identified in vinyl
chloride-induced liver tumors in rats and humans (Barbin et al. 1997; Brandt-Rauf et al. 1995; Hollstein et
al. 1994; Marion and Boivin-Angele 1999; Marion et al. 1991; Trivers et al. 1995; Weihrauch et al. 2002).
Immunological techniques were used to detect the presence of Asp13p21 (oncoprotein for mutation of the
Ki-ras gene), p53 mutant protein, and p53 antibodies in the serum of exposed workers (Brandt-Rauf et al.
2000a, 2000b; Marion 1998). Statistical analyses suggest a relationship between vinyl chloride exposure
VINYL CHLORIDE 107
2. HEALTH EFFECTS
and the presence of these serum biomarkers; however, the predictive value of the biomarkers for
development of cancer is not known.
VINYL CHLORIDE 108
CHAPTER 3. TOXICOKINETICS, SUSCEPTIBLE POPULATIONS,
BIOMARKERS, CHEMICAL INTERACTIONS
3.1 TOXICOKINETICS
Human studies of vinyl chloride provide limited quantitative information on absorption, metabolism, and
excretion. Vinyl chloride toxicokinetics have been studied in nonhuman primates (e.g., rhesus monkeys)
and rodents, with most of the quantitative information derived from studies conducted in rats. An
overview of these data is summarized below.
• Studies in humans and animals indicate that vinyl chloride is readily absorbed through the lungs
following inhalation. Animal studies demonstrate that vinyl chloride is rapidly and almost
completely absorbed from the gastrointestinal tract after oral exposure. A single study in
monkeys suggests that dermal absorption of vinyl chloride gas is not likely to be significant.
• No human studies were identified that provided reliable information about the distribution of
vinyl chloride in tissues other than blood.
• Animal studies indicate that the distribution of vinyl chloride is rapid and widespread; however,
storage in the body is limited because of rapid metabolism and excretion. Metabolites of vinyl
chloride can be found in the liver, kidney, spleen, skin, and brain, but tissue concentrations do not
increase following repeated exposure.
• Vinyl chloride can cross the placenta after inhalation exposure in rat dams.
• Metabolism in humans and experimental animals occurs via the oxidation of vinyl chloride by
CYP to form an epoxide intermediate, 2-chloroethylene oxide, which spontaneously rearranges to
form 2-chloroacetaldehyde. Intermediates are detoxified primarily via glutathione conjugation
and conjugates are excreted in urine as substituted cysteine derivatives.
• Metabolism follows Michaelis-Menten kinetics in rats, with enzyme saturation near 100 ppm in
air or between 1 and 100 mg/kg/day for a single gavage dose.
• Vinyl chloride metabolites are excreted primarily in the urine following oral or inhalation
exposure to low doses. At higher doses where metabolic saturation has been exceeded, vinyl
chloride is exhaled as the parent compound.
3.1.1 Absorption
Inhalation absorption of vinyl chloride is rapid in humans. Five young adult male volunteers were
exposed to vinyl chloride concentrations of 2.9, 5.1, 11.7, or 23.5 ppm by way of a gas mask for 6 hours
(Krajewski et al. 1980). Retention was estimated by measuring the difference between inhaled and
exhaled concentrations. An average retention of 42% was estimated. Although the results varied among
VINYL CHLORIDE 109
3. TOXICOKINETICS, SUSCEPTIBLE POPULATIONS, BIOMARKERS, CHEMICAL INTERACTIONS
the individuals tested, the percentage retained was independent of the concentration inhaled. Since
retention did not change with increasing vinyl chloride concentrations, it appears that saturation of the
major pathway of overall metabolism did not occur in this exposure regimen.
Animal data demonstrate that the inhalation absorption of vinyl chloride occurs readily and rapidly.
Physiologically based pharmacokinetic (PBPK) models developed to provide quantitative estimates of
uptake are discussed in Section 3.1.5. Peak blood levels occurred at 30 minutes in rats exposed (head
only) to 7,000 ppm (Withey 1976). On removal from the vinyl chloride-containing atmosphere, blood
levels fell rapidly. After 2 hours, concentrations were barely detectable.
Several studies in rats indicate that vinyl chloride is rapidly and virtually completely absorbed from the
gastrointestinal tract following oral exposure. Peak blood levels of vinyl chloride were observed within
10–20 minutes after gavage dosing of rats with vinyl chloride in an aqueous solution (single doses of 44–
92 mg/kg) (Withey 1976). Peak blood levels varied from 6 to >40 μg/mL. Data from another study in
which rats were administered single gavage doses of 0.05, 1, and 100 mg/kg vinyl chloride labelled with
radioactive carbon (
14
C-vinyl chloride) (in corn oil) suggested that absorption of vinyl chloride was nearly
complete (Watanabe et al. 1976a).
The fraction of the administered dose recovered in the feces, roughly indicative of the proportion
unabsorbed, ranged from 0.47 to 2.39%; total recovery ranged from 82.3 to 91.3%. Loss of radioactivity
might be attributed either to experimental error or to incomplete sampling of the carcass. Fecal excretion
was measured in rats fed 0, 1.8, 5.6, and 17.0 mg/kg/day of vinyl chloride monomer (from powdered PVC
containing a high level of the monomer) (Feron et al. 1981). Fecal excretion accounted for 8, 10, and
17% of the vinyl chloride present in the low-, middle-, and high-dose groups, respectively. The
investigators hypothesized that the vinyl chloride recovered from the feces was encapsulated by PVC,
thereby not available to the rats for absorption, and that absorption of bioavailable vinyl chloride was
virtually complete.
No studies were located regarding absorption in humans after dermal exposure to vinyl chloride. Animal
data suggest that dermal absorption of vinyl chloride gas is not likely to be significant. Dermal
absorption was measured in two rhesus monkeys that received full body (except head) exposure to vinyl
chloride gas. It was estimated that 0.031 and 0.023% of the total available vinyl chloride was absorbed at
800 and 7,000 ppm, respectively, after a 2–2.5-hour exposure (Hefner et al. 1975a). The investigators
concluded that, after short-term exposure to high concentrations, dermal absorption was far less

VINYL CHLORIDE 110
3. TOXICOKINETICS, SUSCEPTIBLE POPULATIONS, BIOMARKERS, CHEMICAL INTERACTIONS
significant than inhalation absorption. No information is available regarding dermal absorption of vinyl
chloride from liquid or solid media.
3.1.2 Distribution
Representative vinyl chloride partition coefficients for humans, rats, mice, and hamsters are provided in
Table 3-1. These partition coefficients were obtained for use in PBPK models. They were estimated
using a vial equilibration technique (U.S. Air Force 1990). Further details about how the values were
obtained, including the number of experiments completed and whether the errors shown are standard
deviations or standard errors, were not provided. In general, concentrations of vinyl chloride found in fat
are higher than would be found in other tissues. Partition coefficients for vinyl chloride range from 10 to
20 (fat/air) and from 1 to 3 (muscle/air, blood/air, and liver/air). In animal studies, females have shown
greater partitioning from air to fat than males.
Table 3-1. Vinyl Chloride Partition Coefficients
Species
Strain
Sex
Partition coefficient
Blood/air
Liver/air
Muscle/air
Fat/air
Rat
CDBR
a
M
1.79±0.216
3.0±0.407
2.18±0.470
14.6±0.917
F
2.12±0.437
1.66±0.429
1.28±0.245
19.2±0.96
F-344
a
M
1.60±0.328
1.99±1.96
2.06±0.703
11.8±0.811
F
1.55±0.11
2.05±0.17
2.39±0.46
21.1±1.3
Wistar
a
M
2.10±0.313
2.69±0.555
2.72±0.575
10.2±1.61
F
1.62±0.0664
1.48±0.28
1.06±0.221
22.3±0.542
Sprague-
Dawley
b
M
2.4±0.5
–
–
–
Mouse
B6C3F1
a
M
2.83±0.22
–
–
–
F
2.56±0.14
–
–
–
CD-1
a
M
2.27±0.0725
–
–
–
F
2.37±0.16
–
–
–
Hamster
Golden Syrian
a
M
2.74±0.151
3.38±0.362
2.56±0.457
14.3±5.32
F
2.21±0.47
1.31±0.28
1.96±0.28
21.10±2.01
Human
c
NA
NR
1.16
–
–
–
a
U.S. Air Force 1990; values determined using vial equilibration method.
b
Barton et al. 1995.
c
EPA 1987.
– = no data; F = female; M = male; NA = not applicable; NR = not reported
VINYL CHLORIDE 111
3. TOXICOKINETICS, SUSCEPTIBLE POPULATIONS, BIOMARKERS, CHEMICAL INTERACTIONS
Tissue/blood partition coefficients in male Sprague-Dawley rats measured using a vial equilibration
method were reported as 10±3 for fat/blood, 0.4±0.2 for muscle/blood, 0.7±0.3 for liver/blood, and
0.7±0.4 for kidney/blood (Barton et al. 1995).
Data from rat studies suggest that the distribution of inhaled vinyl chloride is rapid and widespread, but
storage of vinyl chloride in the body is limited by rapid metabolism and excretion. In rats exposed to
14
C-vinyl chloride and pretreated with 6-nitro-1,2,3-benzothiadiazole to block metabolism of vinyl
chloride by microsomal CYP oxidation pathways, the highest levels of radiolabel were located in the fat,
with lesser amounts in the blood, liver, kidney, muscle, and spleen. When metabolism was not blocked,
the highest levels of radiolabeled metabolites were located in the liver and kidney (Buchter et al. 1977).
Immediately after a 5-hour exposure to
14
C-vinyl chloride at 50 ppm, tissue levels of
14
C-activity,
expressed as the percentage incorporated per gram of tissue, were highest in the kidney (2.13%) and liver
(1.86%), with lower levels in the spleen (0.73%) and brain (0.17%) (Bolt et al. 1976a). Radioactivity in
tissue was measured in rats 72 hours after exposure to 10 or 1,000 ppm
14
C-vinyl chloride for 6 hours. In
order of decreasing concentration for rats exposed to 10 ppm,
14
C-labeled compounds (expressed as
percentage present as nonvolatile metabolites), were measured in the liver (0.14), kidney (0.08), skin
(0.07), lung (0.07), muscle (0.05), carcass (0.05), plasma (0.05), and fat (0.03). For rats exposed to
1,000 ppm, the tissue radiolabel percentages were: liver (0.15), skin (0.12), kidney (0.06), carcass (0.05),
lung (0.05), muscle (0.04), fat (not detected), and plasma (not detected) (Watanabe et al. 1976b).
There was no difference in the routes or rate of excretion between repeated-dose versus single-dose
exposure of rats to 5,000 ppm of
14
C-vinyl chloride (Watanabe et al. 1978a). The concentration of
radiolabel detected in tissues 72 hours after exposure revealed no statistically significant difference
between rats exposed once or repeatedly to vinyl chloride. Percentages of radioactivity after 72 hours
measured in tissues are as follows (for single and repeated doses, respectively): liver (0.12 and 0.16),
kidney (0.06 and 0.07), skin (0.05 and 0.08), carcass (0.03 and 0.04), and fat (not detected and not
detected).
Placental transfer of vinyl chloride can occur rapidly in rats. Female rats exposed to approximately 0,
2,000, 7,000, or 13,000 ppm vinyl chloride for 2.5 hours on GD 18 showed high concentrations of vinyl
chloride in maternal and fetal blood and amniotic fluid (Ungvary et al. 1978). Vinyl chloride
concentrations in maternal blood were 19.02, 32.40, and 48.43 μg/mL, respectively, while fetal blood
concentrations were 12.80, 22.67, and 30.52 μg/mL, respectively. Vinyl chloride concentrations in
VINYL CHLORIDE 112
3. TOXICOKINETICS, SUSCEPTIBLE POPULATIONS, BIOMARKERS, CHEMICAL INTERACTIONS
amniotic fluid were 0, 4.27, 4.93, and 13.50 μg/mL at 0, 2,000, 7,000, and 13,000 ppm vinyl chloride,
respectively (Ungvary et al. 1978).
The level of
14
C-nonvolatile metabolites was measured in tissues of rats 72 hours after single gavage
doses (0.05–100 mg/kg) of
14
C-vinyl chloride in corn oil (Watanabe et al. 1976a). The highest levels of
radioactivity for each dose level occurred in the liver. These levels were 2–5 times higher than in the
other tissues examined (skin, plasma, muscle, lung, fat, and carcass).
3.1.3 Metabolism
Vinyl chloride metabolism in humans is attributed to the CYP monooxygenases in the liver (Ivanetich et
al. 1977; Sabadie et al. 1980; Salmon 1976). The proposed metabolic pathways for vinyl chloride are
shown in Figure 3-1. Data obtained in rats indicate that metabolic pathways are consistent for both
inhalation and oral exposure (Bartsch et al. 1976, 1979; Green and Hathway 1975, 1977; Hathway 1977;
Watanabe and Gehring 1976; Watanabe et al. 1976a). Metabolism occurs via the oxidation of vinyl
chloride by CYP to form an epoxide intermediate, 2-chloroethylene oxide, which spontaneously
rearranges to form 2-chloroacetaldehyde (Guengerich et al. 1979, 1981; Gwinner et al. 1983; Laib 1982).
2-Chloroethylene oxide can also be detoxified by epoxide hydrolase to yield glycolaldehyde (IARC
2012). These intermediates are detoxified mainly through conjugation with glutathione catalyzed by
glutathione S-transferase. The conjugated products are excreted in urine as substituted cysteine
derivatives and include thiodiglycolic acid, S-formylmethylcysteine, and N-acetyl-S-(2-hydroxyethyl)
cysteine (Bolt et al. 1980; Hefner et al. 1975b). Urinary metabolites identified in rats exposed by
inhalation include polar compounds at low exposure concentrations (Hefner et al. 1975b; Watanabe et al.
1976b) and 2-chloroacetic acid at high exposure concentrations (Hefner et al. 1975b). Mitochondrial
aldehyde dehydrogenase 2 (ALDH2) may also play a role in detoxifying 2-chloroacetaldehyde (Chen et
al. 2019). Activation of ALDH2 with an agonist (Alda-1) was shown to attenuate liver injury and reduce
oxidative stress in mice exposed to vinyl chloride (Chen et al. 2019).
Metabolic saturation was not demonstrated in volunteers exposed to vinyl chloride at concentrations of
2.9, 5.1, 11.7, and 23.5 ppm for 6 hours (Krajewski et al. 1980). In rats, metabolism follows Michaelis-
Menten kinetics, with enzyme saturation near 100 ppm in air or between 1 and 100 mg/kg/day for a single
gavage dose (Hefner et al. 1975b; Watanabe et al. 1976a).

VINYL CHLORIDE 113
3. TOXICOKINETICS, SUSCEPTIBLE POPULATIONS, BIOMARKERS, CHEMICAL INTERACTIONS
Isolated rat liver cells converted
14
C-vinyl chloride into nonvolatile metabolites (Hultmark et al. 1979),
indicating that the in vitro liver cell microsomal metabolism was NADPH-dependent and probably
involved CYP. Pretreatment with 6-nitro-1,2,3-benzothiadiazole, an inhibitor of some microsomal CYP
oxidation pathways, was sufficient to totally block the metabolism of vinyl chloride in rats exposed to
0.45 ppm in a closed system for 5 hours (Bolt et al. 1977). This observation suggests that metabolism of
vinyl chloride proceeds primarily through a CYP pathway with likely production of an epoxide
intermediate.
Figure 3-1. Proposed Metabolic Pathways for Vinyl Chloride*
**Excreted in urine.
Sources: Bolt et al. (1980); Hefner et al. (1975b); IARC (2012); Park et al. (1993); Plugge and Safe
(1977)
CH
2
CH
O
Cl
OH CH
2
C
O
H
2-chloroethylene oxide
(1-chlorooxirane)
+ glutathione
G-S-CH
2
-CHO
S-formylmethyl
glutathione
cys-S-CH
2
-CHO
S-formylmethyl
cysteine**
cys-S-CH
2
-CH
2
OH
S-(2-hydroxyethyl)-cysteine
N-Ac-cys-S-CH
2
-CH
2
OH
N-acetyl-S-(2-hydroxyethyl) cysteine**
mixed function oxidase
ClHC=CH
2
vinyl chloride
ClH
2
C-CHO
2-chloroacetaldehyde
aldehyde
dehydrogenase
ClH
2
C-CHOOH
2-chloroacetic acid**
+ glutathione
G-S-CH
2
-COOH
S-carboxymethyl glutathione
cys-S-CH
2
-COOH
S-carboxymethyl cysteine
NH
3
ammonia
(transamination)
CO
2
carbon dioxide
(oxidative decarboxylation)
HOOC-CH
2
-S-CH
2
-COOH
thiodiglycolic acid (thiodiacetic acid)**
Glycolaldehyde
VINYL CHLORIDE 114
3. TOXICOKINETICS, SUSCEPTIBLE POPULATIONS, BIOMARKERS, CHEMICAL INTERACTIONS
Inhalation exposure of rats to high concentrations of vinyl chloride was associated with a reduction in the
liver nonprotein sulfhydryl functional group concentration (Barton et al. 1995). A reduction in these
functional groups is expected since there are limited amounts of liver glutathione and/or cysteine to
conjugate the metabolites of vinyl chloride. (Bolt et al. 1976b; Hefner et al. 1975b; Jedrychowski et al.
1984; Watanabe et al. 1978b).
Saturation of metabolic pathways was observed in rats and monkeys that were exposed in a closed system
to
14
C-vinyl chloride (Bolt et al. 1977; Buchter et al. 1980; Filser and Bolt 1979). In Wistar rats,
metabolic saturation was determined to occur at approximately 250 ppm, and a metabolic rate (V
max
) of
110 μmol/hour/kg was estimated (Bolt et al. 1977; Filser and Bolt 1979). Kinetic constants of
58 μmol/hour/kg for V
max
and 1 μM for the K
m
in male Sprague-Dawley rats were also reported (Barton et
al. 1995). In an experiment using rhesus monkeys, metabolic saturation occurred at 200 ppm, with a V
max
of 50 μmol/hour/kg (Buchter et al. 1980). The V
max
of 50 μmol/hour/kg estimated using rhesus monkeys
was suggested as a closer approximation of metabolism in humans than the value of 110 μmol/hour/kg
estimated for rats by Filser and Bolt (1979).
Kinetic constants for vinyl chloride metabolism were derived from in vitro studies in rat liver microsomes
(el Ghissassi et al. 1998). Metabolism followed Michaelis-Menton kinetics with a K
m
of 7.42 μM and a
V
max
of 4,674 pmol/mg protein/minute. Inhibitor studies using chemical and immunological inhibitors
demonstrate that vinyl chloride is metabolized primarily by CYP2E1.
Urinary metabolites identified from rats ingesting
14
C-vinyl chloride are consistent with the metabolic
pathways postulated for inhalation exposure, in particular with the formation of 2-chloroethylene oxide
and 2-chloroacetaldehyde. Metabolites identified include N-acetyl-S-(2-hydroxyethyl) cysteine,
2-chloroacetic acid, and thiodiglycolic acid (Green and Hathway 1975, 1977; Watanabe and Gehring
1976; Watanabe et al. 1976a). Metabolic saturation appears to occur with a single gavage dose of
between 1 and 100 mg/kg/day (Watanabe et al. 1976a).
Several investigators observed the binding of nonvolatile metabolites of
14
C-vinyl chloride to liver
macromolecules both in vitro and in rats exposed by inhalation (Guengerich and Watanabe 1979;
Guengerich et al. 1979, 1981; Kappus et al. 1976; Watanabe et al. 1978a, 1978b). In single-exposure
experiments at different concentrations, the extent of macromolecular binding increased proportionately
to the amount of vinyl chloride metabolized and disproportionately to the exposure concentration
(Watanabe et al. 1978b). The extent of macromolecular binding was increased by repeated exposure to
VINYL CHLORIDE 115
3. TOXICOKINETICS, SUSCEPTIBLE POPULATIONS, BIOMARKERS, CHEMICAL INTERACTIONS
vinyl chloride (Watanabe et al. 1978a) and by pretreatment with phenobarbital (Guengerich and
Watanabe 1979). Macromolecular binding was attributed to the reactive intermediate 2-chloroethylene
oxide, which binds to DNA and RNA, and to its rearrangement product, 2-chloroacetaldehyde that can
form an adduct with some amino acid side-chains, altering the protein conformation (Guengerich and
Watanabe 1979; Guengerich et al. 1979, 1981; Kappus et al. 1976; Watanabe et al. 1978a, 1978b).
3.1.4 Excretion
Studies demonstrated that the primary route of vinyl chloride excretion is dose-dependent (Krajewski et
al. 1980; Watanabe and Gehring 1976; Watanabe et al. 1976b, 1978a). Vinyl chloride metabolites are
excreted primarily in the urine following oral and inhalation exposure at low doses or concentrations. In
humans exposed by inhalation, exhalation of vinyl chloride was a minor pathway of elimination even at
low concentrations (Krajewski et al. 1980). Animal studies have shown that at higher doses where
metabolic saturation has been exceeded, vinyl chloride is exhaled as the parent compound (Watanabe and
Gehring 1976; Watanabe et al. 1976b, 1978a).
Human data suggest that exhalation of unmetabolized vinyl chloride is not an important pathway of
elimination at low exposure concentrations. The mean concentration in expired air for humans exposed
for 6 hours to air containing 2.9–23.5 ppm ranged from 0.21 to 1.11 ppm, representing from 7.23 to
4.73% of the inhaled amounts, respectively (Krajewski et al. 1980). After dermal exposure in monkeys,
most of the minimal vinyl chloride absorbed was excreted in exhaled air (Hefner et al. 1975a).
The mode of excretion of vinyl chloride and its metabolites following inhalation exposure of animals to
different concentrations reflects the saturation of metabolic pathways. The cumulative excretion of
radioactivity over a 72-hour postexposure period was measured in rats exposed to 10–1,000 ppm
(Watanabe and Gehring 1976; Watanabe et al. 1976b) or 5,000 ppm (Watanabe et al. 1978a)
14
C-vinyl
chloride for 6 hours. Radioactivity expired as carbon dioxide or vinyl chloride, excreted in the urine and
feces, and retained in the carcass was expressed as a percentage of the total radioactivity recovered. The
results suggest that metabolism was nearly complete at 10 ppm because <2% of the recovered
radioactivity occurred as unchanged parent compound. The predominant route for excretion of
radioactive metabolites was through the urine, accounting for about 70% of the recovered radioactivity.
At 1,000 ppm, the fraction of unchanged vinyl chloride increased to 12.3% and urinary radioactivity
decreased to 56.3%, indicating that metabolism was saturated at this concentration.
VINYL CHLORIDE 116
3. TOXICOKINETICS, SUSCEPTIBLE POPULATIONS, BIOMARKERS, CHEMICAL INTERACTIONS
Increasing vinyl chloride concentrations may have different effects for animals and humans. In humans
exposed to low concentrations, a higher percentage of unmetabolized vinyl chloride was found in expired
breath (Krajewski et al. 1980). This is the opposite of what is observed in animals, wherein there is a
trend for a greater percentage of vinyl chloride being exhaled at higher concentrations. In rats exposed to
5,000 ppm for 6 hours, more than half of the recovered radioactivity appeared as unchanged vinyl
chloride in expired air, and urinary excretion accounted for about 27% of the recovered activity
(Watanabe et al. 1978a). Generally, there was little change in the proportion of recovered radioactivity
excreted in the feces or exhaled as carbon dioxide. The percentage of the radioactivity retained in the
carcass and tissues of rats appeared to be somewhat decreased at 5,000 ppm compared with 10 and
1,000 ppm, suggesting preferential retention of metabolites rather than unchanged vinyl chloride
(Watanabe and Gehring 1976; Watanabe et al. 1978a, 1976b). However, it is possible that a reversal of
this trend would occur in humans if concentrations were increased to those used in the animal studies or
to concentrations closer to the K
m
for human metabolism.
Pulmonary excretion of unaltered vinyl chloride in rats followed first-order kinetics regardless of
exposure concentrations, with half-lives of 20.4, 22.4, and 30 minutes following 6-hour exposures at 10,
1,000, and 5,000 ppm, respectively (Watanabe et al. 1976b). After oral exposure, pulmonary excretion of
vinyl chloride appeared to be monophasic at <1.0 mg/kg, with a half-life of about 55–58 minutes
(Watanabe et al. 1976a). At 100 mg/kg, pulmonary excretion of vinyl chloride was biphasic, with half-
lives of 14.4 and 40.8 minutes for the rapid and slower phases, respectively. Exhalation of unchanged
vinyl chloride was generally complete within 3–4 hours; however, excretion of metabolites in urine
continued for days (Green and Hathway 1975).
The urinary excretion of radioactivity was biphasic, with the second or slow phase accounting for <3% of
the total urinary excretion (Cheng et al. 2001; Watanabe et al. 1976a). Estimated half-lives for the rapid
(first-order) phase were 4.6, 4.1, and 4.5 hours at 10, 1,000, and 5,000 ppm, respectively (Cheng et al.
2001) and 4.5–4.6 hours for oral doses of 0.05–100 mg/kg (Watanabe et al. 1976a). Single oral doses of
14
C-vinyl chloride (0.05, 0.25, 1.0, 20, 100, and 450 mg/kg) were administered to rats, and the excretion
of radioactivity was monitored over a 72-hour period (Green and Hathway 1975; Watanabe and Gehring
1976; Watanabe et al. 1976a). A striking increase in exhalation of unchanged vinyl chloride and
compensatory decreases in urinary and fecal excretion of radioactivity and exhalation of carbon dioxide
were observed at >20 mg/kg, suggesting that metabolic saturation had occurred at that dosage. At
<1.0 mg/kg, the predominant route of elimination was urinary excretion of polar metabolites.
VINYL CHLORIDE 117
3. TOXICOKINETICS, SUSCEPTIBLE POPULATIONS, BIOMARKERS, CHEMICAL INTERACTIONS
Urinary metabolites included N-acetyl-S-(2-hydroxyethyl) cysteine, thiodiglycolic acid, and possibly
S-(2-hydroxyethyl) cysteine (Watanabe et al. 1976b). Identification of these metabolites of vinyl chloride
in the urine indicates that vinyl chloride is transformed in the body to a reactive metabolite, which is then
detoxified by reaction with glutathione (GSH, gamma-glutamylcysteinylglycine). Subsequently, the
glutamic acid and glycine moieties of the tripeptide are cleaved, and the cysteine conjugate of the reactive
metabolite of vinyl chloride is either acetylated or further oxidized and excreted. Thiodiglycolic acid is
the major metabolite of vinyl chloride detected in the urine of exposed workers (Cheng et al. 2001).
Urinary thiodiglycolic acid levels were correlated with vinyl chloride levels in air at concentrations
>5 ppm; however, this correlation appears to be more variable at lower vinyl chloride concentrations in
air (Chen et al. 2019).
Metabolites identified in the urine of orally treated rats were consistent with the formation of
2-chloroethylene oxide and 2-chloroacetaldehyde (Green and Hathway 1977; Watanabe et al. 1976a), as
postulated for metabolism following inhalation exposure. The major metabolites were identified as
thiodiglycolic acid, N-acetyl-S-(2-hydroxyethyl) cysteine, N-acetyl-S-(2-chloroethyl)cysteine, and
S-(2-chloroethyl)cysteine (Green and Hathway 1977; Watanabe et al. 1976a). Minor metabolites included
urea, glutamic acid, and 2-chloroacetic acid (Green and Hathway 1975).
Dermal exposure of high concentrations of vinyl chloride gas resulted in most excreted in expired air for
the small fraction that was absorbed. Hefner et al. (1975a) reported that two rhesus monkeys received
whole-body (except head) exposure to vinyl chloride gas (800 and 7,000 ppm) for 2–2.5 hours and most
was excreted in expired air (Hefner et al. 1975a). The percentages of absorbed vinyl chloride that were
exhaled were 0.028% at 700 ppm and 0.014% at 8,000 ppm (Hefner et al. 1975a).
The elimination of radioactivity following intraperitoneal administration of
14
C-vinyl chloride to rats
resembles the pattern observed following inhalation or oral administration. Following an intraperitoneal
dose of 0.25 mg/kg, exhalation of unchanged vinyl chloride, exhalation of carbon dioxide, and urinary
and fecal excretion of radioactivity accounted for 43.2, 11.0, 43.1, and 1.8% of the administered dose,
respectively (Green and Hathway 1975). At 450 mg/kg, exhaled vinyl chloride increased to 96.2% of the
administered dose, carbon dioxide decreased to 0.7%, urinary radioactivity decreased to 2.6%, and fecal
radioactivity decreased to 0.1%.
Doses administered intravenously were eliminated very rapidly and almost entirely by exhalation of
unchanged vinyl chloride. Green and Hathway (1975) administered a 0.25-mg/kg intravenous dose of
VINYL CHLORIDE 118
3. TOXICOKINETICS, SUSCEPTIBLE POPULATIONS, BIOMARKERS, CHEMICAL INTERACTIONS
14
C-vinyl chloride to rats and recovered 80% of the dose within 2 minutes and 99% within 1 hour as
unchanged compound in expired air.
3.1.5 Physiologically Based Pharmacokinetic (PBPK)/Pharmacodynamic (PD) Models
Models are simplified representations of a system with the intent of reproducing or simulating its
structure, function, and behavior. PBPK models are more firmly grounded in principles of biology and
biochemistry. They use mathematical descriptions of the processes determining uptake and disposition of
chemical substances as a function of their physicochemical, biochemical, and physiological
characteristics (Andersen and Krishnan 1994; Clewell 1995; Mumtaz et al. 2012a; Sweeney and Gearhart
2020). PBPK models have been developed for both organic and inorganic pollutants (Ruiz et al. 2011)
and are increasingly used in risk assessments, primarily to predict the concentration of potentially toxic
moieties of a chemical that will be delivered to any given target tissue following various combinations of
route, dose level, and test species (Mumtaz et al. 2012b; Ruiz et al. 2011; Sweeney and Gearhart 2020;
Tan et al. 2020). PBPK models can also be used to more accurately extrapolate from animal to human,
high dose to low dose, route to route, and various exposure scenarios and to study pollutant mixtures (El-
Masri et al. 2004). Physiologically based pharmacodynamic (PBPD) models use mathematical
descriptions of the dose-response function to quantitatively describe the relationship between target tissue
dose and toxic endpoints (Clewell 1995).
PBPK models are available for vinyl chloride. These models predict the metabolism and distribution of
vinyl chloride. The overall results and individual models are discussed in this section in terms of their use
in risk assessment, tissue dosimetry, and dose, route, and species extrapolations.
3.1.5.1 EPA (1987) Animal Models
EPA (1987) developed a PBPK model to estimate the metabolized dose of vinyl chloride when coupled to
a multistage model to estimate cancer risk in animals. This PBPK model consists of four compartments:
the liver, fat, highly perfused tissue, and poorly perfused tissue. All metabolism is assumed to occur in
the liver by one saturable (reflecting Michaelis-Menten kinetics) first-order metabolism pathway.
The dose delivery provided by the vinyl chloride model developed by EPA (1987) was validated by the
U.S. Air Force (1990) study and by additional vinyl chloride metabolism studies conducted in rats. At

VINYL CHLORIDE 119
3. TOXICOKINETICS, SUSCEPTIBLE POPULATIONS, BIOMARKERS, CHEMICAL INTERACTIONS
low concentrations, this model fit in vivo data in rats by Gehring et al. (1978) well, but at concentrations
above 25 ppm, the model predicted a greater level of vinyl chloride metabolism than was observed.
3.1.5.2 U.S. Air Force (1990) Rat, Mouse, and Hamster Models
The U.S. Air Force (1990) modified the EPA (1987) model to improve the fit with actual data,
particularly as it relates to glutathione depletion and doses above 25 ppm. In the first modification, both
vinyl chloride and the epoxide metabolite were assumed to react with glutathione. This model had
difficulty predicting glutathione depletion at high doses; for example, it predicted glutathione depletions
higher than observed at 4,600–5,800 ppm vinyl chloride concentrations. The second alternative model
assumed that only the product of the first-order metabolism reacted with glutathione. It also predicted
glutathione depletions higher than observed at high exposure concentrations. To improve the model, the
investigators suggested the addition of a low-affinity glutathione pathway.
Using vinyl chloride concentration data obtained from Wright-Patterson Air Force Base (AFB), the U.S.
Air Force (1990) extended the first glutathione conjugation model, developed in rats, to different strains
of rats, mice, and hamsters. Vinyl chloride gas uptake experiments were completed in which animals
were exposed to various concentrations of vinyl chloride in closed chambers for up to 6 hours, and the
disappearance of vinyl chloride was monitored. The glutathione content of the animals was also
measured immediately after exposure. Using data from these studies with the physiologic parameters
shown in Table 3-2, the investigators estimated metabolic parameters for vinyl chloride and the rate
constant for the conjugation of vinyl chloride with glutathione (Table 3-3). Using the metabolic
parameters determined from the gas uptake experiments, the model predictions showed good agreement
with the actual data for all of the animal strains tested.
Table 3-2. Physiological Parameters Used to Estimate Parameters from Vinyl
Chloride Gas Uptake Experiments
a
Parameter
Rats
Mice
Hamsters
Ventilation rate (L/hour/body weight
0.74
)
14
23–35
b
13
Total cardiac output (L/hour/body weight
0.74
)
14
23–35
b
13
Blood flow to the liver (fraction of total cardiac output)
0.25
0.24
0.24
Blood flow to highly perfused tissue (fraction of total cardiac output)
0.51
0.52
0.52
Blood flow to fat (fraction of total cardiac output)
0.09
c
0.05
0.09
Blood flow to poorly perfused tissue (fraction of total cardiac output)
0.15
c
0.20
0.15
Volume of tissue (L/body weight)
0.04
0.04
0.04

VINYL CHLORIDE 120
3. TOXICOKINETICS, SUSCEPTIBLE POPULATIONS, BIOMARKERS, CHEMICAL INTERACTIONS
Table 3-2. Physiological Parameters Used to Estimate Parameters from Vinyl
Chloride Gas Uptake Experiments
a
Parameter
Rats
Mice
Hamsters
Volume of highly perfused tissue (L/body weight)
0.05
0.05
0.05
Volume of fat tissue (L/body weight)
0.07–0.1
d
0.04
0.07
Volume of poorly perfused tissue (L/body weight)
0.72–0.75
d
0.78
0.75
a
U.S. Air Force (1990); units of body weight were not provided.
b
Ventilation rates and total cardiac outputs were 23 for male B6C3F1 mice, 25 for female B6C3F1 mice, 28 for
female CD-1 mice, and 35 for male CD-1 mice.
c
Male Wistar rats blood flow to fat = 0.08 and blood flow to slowly perfused tissue = 0.16.
d
Female F-344 and female Wistar rats had volume of fat tissue = 0.07 and volume of slowly perfused tissue = 0.75;
male F-344 and female Wistar rats had volume of fat tissue = 0.08 and volume of slowly perfused tissue = 0.74;
male Wistar rats and male CDBR rats had volume of fat tissue = 0.1 and volume of slowly perfused tissue = 0.72.
Table 3-3. Estimates of Metabolic Parameters Obtained from Gas Uptake
Experiments
Species
Strain
Sex
V
max
/body weight
0.7
(mg/hour/body
weight
0.7
)
Kfc
(body weight
0.3
/
hour)
Kgsc
(body weight
0.3
/hour/μmol/L
GSH)
Rat
CDBR
M
2.50
0.63
ND
F
2.47
1.00
0.000241
F-344
M
3.17
1.08
0.000249
F
2.95
1.03
0.000227
Wistar
M
3.11
0.45
0.000093
F
2.97
1.55
0.00040
Mouse
B6C3F1
M
5.89
5.5
0.000827
F
5.53
8.93
0.001670
CD-1
M
6.99
5.1
0.000563
F
5.54
6.62
0.000809
Hamster
Golden
Syrian
M
4.94
1.67
0.000330
F
4.76
2.06
ND
Source: U.S. Air Force 1990
F = female; GSH = glutathione; Kfc = first order of epoxide formation; Kgsc = rate constant for conjugation of vinyl
chloride with glutathione; M = male; ND = not determined; V
max
= maximum velocity of reaction
It does not appear that the investigators further validated the Wright-Patterson AFB model with data from
studies other than those used to determine the metabolic parameters. This model was not used to estimate
metabolized doses for humans because the investigators indicated that human data to estimate all of the
required parameters were not available. They suggested that allometry may have to be used to estimate
some of the parameters for humans.
VINYL CHLORIDE 121
3. TOXICOKINETICS, SUSCEPTIBLE POPULATIONS, BIOMARKERS, CHEMICAL INTERACTIONS
3.1.5.3 Clewell et al. (1995) Human Models
Clewell et al. (1995) used PBPK modeling coupled with a linearized multistage model to predict human
cancer risk. The model again had four compartments as described for the EPA (1987) study, and the
same EPA physiologic parameters were used. Partition coefficients were from in vitro experiments and
are shown in Table 3-1. Metabolism was modeled by two saturable pathways: one high affinity, low
capacity (P4502E1), and one low affinity, high capacity (2C11/6 and 1A1/2). The metabolic parameters
used were not provided, but they were estimated from the U.S. Air Force (1990) model. This model
assumed that the metabolites (chloroethylene oxide and chloroacetaldehyde) were further degraded to
carbon dioxide, reacted with glutathione, or reacted with DNA. The parameters (not stated) for the
degradation reactions of chloroethylene oxide and chloroacetaldehyde were estimated from vinylidene
chloride data (D’Souza and Andersen 1988) using appropriate allometric scaling.
Based on the Clewell et al. (1995) PBPK model and a linearized multistage model using liver
angiosarcoma data from animal studies, the human risk estimates for lifetime exposure to 1 ppb vinyl
chloride ranged from 1.1 to 15.7/million persons. Based on the incidence of liver angiosarcoma in human
epidemiological studies, the risk estimates for lifetime exposure to 1 ppb vinyl chloride were 0.4–
4.22/million persons. Clewell et al. (1995) indicated that the risk estimates in the occupational exposure
range using PBPK modeling are about 30–50 times lower than estimates using external dose calculations
based on the linearized multistage model.
Clewell et al. (2001) further refined the PBPK model for vinyl chloride. This model was applied by the
EPA to develop quantitative toxicity values for vinyl chloride (i.e., reference dose [RfD], reference
concentration [RfC], inhalation unit risk, oral slope factor) (EPA 2000). The model had four
compartments and metabolism was modeled by two saturable pathways: one high affinity, low capacity
(P4502E1), and one low affinity, high capacity (2C11/6 and 1A1/2). A description of glutathione kinetics
was also included in the model. Cancer risk estimates in the occupational exposure range calculated
using the PBPK model were consistent with risk estimates from epidemiological studies and were
approximately 80-fold lower than cancer risk estimates from animal studies without PBPK modeling.
The inhalation portion of the PBPK model is well documented with experimental inhalation data
sufficient to ensure a high degree of confidence in the derived dose metrics. Less confidence is associated
with the oral dose metrics due to the limited experimental data available (EPA 2000).
VINYL CHLORIDE 122
3. TOXICOKINETICS, SUSCEPTIBLE POPULATIONS, BIOMARKERS, CHEMICAL INTERACTIONS
The Clewell et al. (2001) model was also applied to evaluate the potential impact of age- and sex-specific
pharmacokinetic differences on the dosimetry of vinyl chloride (Clewell et al. 2004). The rate of
metabolite production per volume of liver was estimated to rise rapidly from birth until about
age 16 years, after which it remains relatively constant before rising again late in life. Other factors that
may affect vinyl chloride toxicity at early life stages include the presence of fetal P450s and the level of
glutathione transferase.
The PBPK model described in Clewell et al. (2001) and EPA (2000) was used to derive the chronic-
duration oral MRL. For more information on ATSDR’s use of the Clewell model, refer to Appendix A.
3.1.5.4 Reitz et al. (1996) Rat, Mouse, and Human Models
Reitz et al. (1996) also developed a PBPK model that coupled measures of delivered dose in rats to a
linearized multistage model to predict the incidence of hepatic angiosarcoma in mice and humans. The
model incorporated four compartments: fat, muscle, rapidly perfused tissues, and liver. Physiological
parameters in the model were based on similar ones used in an earlier multispecies PBPK model
developed for methylene chloride. Partition coefficients were estimated by vial equilibration techniques
similar to those described in the U.S. Air Force (1990) study. Metabolic rate constants were obtained
from in vivo gas uptake experiments performed at Wright-Patterson AFB.
Based on the PBPK-based procedure utilized by Reitz et al. (1996), the predicted human risk estimates
ranged from about 200 cases of angiosarcoma per 100,000 (for workers employed 10 years at a plant
where the time-weighted average [TWA] was 50 ppm) to almost 4,000 cases/100,000 in workers
employed for 20 years in a plant where the TWA was 2,000 ppm. The predictions of human risk were
compared with the data reported by Simonato et al. (1991). The predictions of angiosarcoma incidence in
humans were almost an order of magnitude higher than actually observed in exposed human populations
and were more than two orders of magnitude lower than risk estimations that did not utilize
pharmacokinetic data.
3.1.5.5 Other Models
Yoon et al. (2007) evaluated the impact of assuming extrahepatic metabolism by CYP2E1 in PBPK
models for vinyl chloride inhalation. The study concluded that predictions for the rat and human models
were not significantly affected by the inclusion of extrahepatic metabolism by CYP2E1 in the kidney and
lung. Chiu and White (2006) described the development of a simplified steady-state solution of a generic
VINYL CHLORIDE 123
3. TOXICOKINETICS, SUSCEPTIBLE POPULATIONS, BIOMARKERS, CHEMICAL INTERACTIONS
PBPK model for volatile organic compounds. This steady-state analysis was shown to produce similar
results to the full PBPK model reported in the EPA (2000) risk assessment for vinyl chloride. Mumtaz et
al. (2012a) developed a generic seven-compartment PBPK model, which added compartments for blood,
kidney, and skin. A comparison of the results of this model to the Clewell et al. (2001) model showed
that both models adequately predicted blood concentrations during, and immediately following, exposure.
3.1.6 Animal-to-Human Extrapolations
Limited information is available regarding the toxicokinetic differences between species. Toxicokinetic
data in humans are limited (Krajewski et al. 1980; Sabadie et al. 1980). A primate study suggested that
metabolism may saturate at lower concentrations in primates (>300–400 ppm) than in rats (Buchter et al.
1980).
PBPK models were also developed to predict the metabolism and distribution of vinyl chloride in
laboratory animals and humans (Section 3.1.5). The most recent PBPK model for vinyl chloride (Clewell
et al. 2001) was applied by EPA to develop quantitative toxicity values for vinyl chloride (RfD, RfC,
inhalation unit risk, oral slope factor) (EPA 2000). The model has four compartments and metabolism
was modeled by two saturable pathways: one high affinity, low capacity (P4502E1), and one low
affinity, high capacity (2C11/6 and 1A1/2). A description of glutathione kinetics was also included in the
model. Cancer risk estimates calculated using the PBPK model were consistent with risk estimates from
epidemiological studies.
There appears to be a correlation of vinyl chloride toxicity between humans and animals with regard to
respiratory, cardiovascular, hematological, hepatic, dermal, immunological, neurological, reproductive
and cancer effects. Renal effects of vinyl chloride, including increased relative kidney weight and an
increase in severity of tubular nephrosis, are reported in several rat studies (Bi et al. 1985; Feron and
Kroes 1979; Feron et al. 1979a). However, kidney toxicity was only observed in a single human study of
exposure to multiple chlorinated solvents in drinking water (Chen and Wu 2017). Evidence for
developmental effects of vinyl chloride has not been reliably demonstrated in epidemiology studies (Bao
et al. 1988; Edmonds et al. 1975, 1978; Rosenman et al. 1989; Ruckart et al. 2013; Swartz et al. 2015;
Talbott et al. 2015; Theriault et al. 1983) but did occur in studies of several animal species (John et al.
1977, 1981).
VINYL CHLORIDE 124
3. TOXICOKINETICS, SUSCEPTIBLE POPULATIONS, BIOMARKERS, CHEMICAL INTERACTIONS
3.2 CHILDREN AND OTHER POPULATIONS THAT ARE UNUSUALLY SUSCEPTIBLE
This section discusses potential health effects from exposures during the period from conception to
maturity at 18 years of age in humans. Potential effects on offspring resulting from exposures of parental
germ cells are considered, as well as any indirect effects on the fetus and neonate resulting from maternal
exposure during gestation and lactation. Children may be more or less susceptible than adults to health
effects from exposure to hazardous substances and the relationship may change with developmental age.
This section also discusses unusually susceptible populations. A susceptible population may exhibit
different or enhanced responses to certain chemicals than most persons exposed to the same level of these
chemicals in the environment. Factors involved with increased susceptibility may include genetic
makeup, age, health and nutritional status, and exposure to other toxic substances (e.g., cigarette smoke).
These parameters can reduce detoxification or excretion or compromise organ function.
Populations at greater exposure risk to unusually high exposure levels to vinyl chloride are discussed in
Section 5.7, Populations with Potentially High Exposures.
Data suggest that fetuses, infants, and young children are susceptible to the toxic effects of vinyl chloride.
Some epidemiologic studies (Infante et al. 1976a, 1976b; NIOSH 1977) suggested an association between
fetal death and birth defects and parental vinyl chloride exposure. However, the design and analysis of
these studies has been criticized (Hatch et al. 1981; Stallones 1987). Some inhalation studies with
animals have suggested that vinyl chloride is a developmental toxicant (e.g., produces delayed
ossification) at doses that also produce maternal toxicity (John et al. 1977, 1981; Mirkova et al. 1978;
Sal’nikova and Kotsovskaya 1980; Ungvary et al. 1978). However, no adverse effects on embryo-fetal
development were noted in a rat inhalation study (Thornton et al. 2002).
Vinyl chloride can cross the placenta and enter the blood of the fetus (Ungvary et al. 1978). Studies by
Drew et al. (1983) and Maltoni et al. (1981) have shown that animals exposed by inhalation prior to
adolescence or during pregnancy may have a greater death rate and increased likelihood of developing
cancer than adult animals exposed for similar periods. This may relate to the length of the induction
period of hepatic angiosarcoma rather than to an increased susceptibility of the young, per se. Lifetime
cancer risk was also dependent on the age of the animals at the time of exposure to vinyl chloride. Refer
to Section 2.19 for more details on studies addressing cancer and age of vinyl chloride exposure.
VINYL CHLORIDE 125
3. TOXICOKINETICS, SUSCEPTIBLE POPULATIONS, BIOMARKERS, CHEMICAL INTERACTIONS
It is also possible that there are explanations for these findings. Cogliano and Parker (1992) suggested
that in the multistage model of carcinogenesis, carcinogens that induce an initial transition early in the life
of an animal would be more effective since there would be a longer period of time remaining in the
lifespan for completion of the remaining transitions. Their empirical model of the effect of age at
exposure on the development of cancer suggests that there is an age-sensitive period of exposure to vinyl
chloride.
An age-related increase in DNA adduct formation was noted in an inhalation study of lactating rats and
their 10-day-old pups exposed to 600 ppm of vinyl chloride, 4 hours/day for 5 days (Fedtke et al. 1990).
Concentrations of two adducts found in livers of pups were 4-fold higher than those found in livers of
dams; however, pups were exposed to contaminated breast milk in addition to air concentrations of vinyl
chloride. In another study, immature rats exposed to vinyl chloride formed 6 times more etheno-
nucleosides compared with adults (Ciroussel et al. 1990). The concentration of ethenoguanine adducts
was 2–3-fold greater in weanling rats as compared to adult rats exposed at the same dose for the time
period (0, 10, 100, or 1,100 ppm, 6 hours/day for 5 days) (Morinello et al. 2002a).
Taken together, the studies cited above suggest an early life stage sensitivity to vinyl chloride
carcinogenicity (Cogliano et al. 1996). EPA has recommended an adjustment of the cancer risk estimates
to account for early life-stage sensitivity to vinyl chloride (EPA 2000; Ginsberg 2003).
The toxicokinetic behavior of vinyl chloride in children is expected to be similar to that in adults (Clewell
et al. 2004; EPA 2000; Gentry et al. 2003). Urinary metabolites of vinyl chloride and other volatile
compounds have been measured in preterm infants in a neonatal intensive care unit (El-Metwally et al.
2018). An evaluation of pharmacokinetic differences across life stages suggests that the largest difference
in pharmacokinetics occurs during the perinatal period (Gentry et al. 2003). The most important factor
appears to be the potential for decreased clearance due to immature metabolic enzymes systems. For
instance, clearance is hampered in the embryonic liver because CYP2E1 is not expressed but rapidly
increases during the first 24 hours after birth. Between the developmental ages of 1 and 10 years,
children’s CYP2E1 protein levels and enzyme activity are comparable to adults (EPA 2000).
Young children appear to have the capacity of metabolizing vinyl chloride to reactive intermediates that
form DNA adducts that lead to cancer. A PBPK model was applied to evaluate the potential impact of
age- and sex-specific pharmacokinetic differences on the dosimetry of vinyl chloride (Clewell et al.
2004). The rate of metabolite production per volume of liver was estimated to rise rapidly from birth
VINYL CHLORIDE 126
3. TOXICOKINETICS, SUSCEPTIBLE POPULATIONS, BIOMARKERS, CHEMICAL INTERACTIONS
until about age 16, after which it remains relatively constant before rising again late in life. The data on
CYP2E1 levels in the developing organism suggests that early life stage sensitivity to vinyl chloride-
induced cancer is not solely due to an increase in the production of reactive intermediates via this
isozyme. Fetal CYP isoforms may play a role in metabolism of vinyl chloride to reactive intermediates in
the fetus and neonate. Glutathione conjugation may also differ in the developing organism. DNA repair
capacity and other pharmacodynamic factors may also be associated with an early life stage susceptibility
to cancer.
Individuals with comorbidities (e.g., obesity and liver disease) and genetic polymorphisms of HLA-DR5,
HLA-DR3, and B8 alleles are unusually susceptible to the effects of vinyl chloride. Lifestyle factors such
as exposure to organochlorine pesticides, consuming high-calorie diets, ethanol, or barbiturates, or taking
Antabuse for alcoholism may make people have increased susceptibility to vinyl chloride effects.
Irregular heart rhythms, impaired peripheral circulation, and systemic sclerosis (Section 3.3.2) may also
increase susceptibility.
Mice fed a high-fat diet are more susceptible to liver injury induced by low concentrations of vinyl
chloride. High-fat diet mice exposed to 0.85 ppm vinyl chloride for 12 weeks showed liver damage,
neutrophil infiltration, non-parenchymal cell apoptosis, mitochondrial dysfunction, and oxidative and
endoplasmic reticulum stress compared to mice fed a normal or low-fat diet (Chen et al. 2019; Fujiwara
2018; Lang et al. 2018, 2020; Liang et al. 2018; Liu et al. 2023; Wahlang et al. 2020). High-fat diet mice
exposed to ≥63 ppm of vinyl chloride (2 hours/day, 5 days/week for 13 weeks) also showed steatosis,
oxidative and endoplasmic reticulum stress in the liver, and upregulated expression of de novo
lipogenesis-related proteins (Jia et al. 2022). Liu et al. (2023) reported that high-fat diet mice exposed to
0.85 ppm vinyl chloride for 6 hours/day, 5 days/week for 12 weeks had an increase in the number of
hepatic tumors observed 9 months after exposure had ended, compared to mice fed a low-fat diet.
Mice injected with lipopolysaccharide or fed diets high in fat and exposed orally to 2-chloroethanol also
experienced enhanced liver injury when compared to mice fed a normal or low-fat diet (Anders et al.
2016a, 2016b; Kaelin et al. 2020; Lang et al. 2019). This effect was attenuated by rapamycin, which
protects against mitochondrial damage and subsequent oxidative stress (Lang et al. 2019). Mitochondrial
ALDH2 may also play a role in detoxifying 2-chloroacetaldehyde (Chen et al. 2019). Activation of
ALDH2 with an agonist (Alda-1) was shown to attenuate liver injury and reduce oxidative stress in high-
fat diet mice exposed to vinyl chloride (Chen et al. 2019).
VINYL CHLORIDE 127
3. TOXICOKINETICS, SUSCEPTIBLE POPULATIONS, BIOMARKERS, CHEMICAL INTERACTIONS
Vinyl chloride is metabolized in the liver in a multistep process. The prevalence of liver ultrasound
abnormalities (not further defined) was associated with polymorphism of the CYP2E1 gene (c1c2/c2c2
genotype) (Zhu et al. 2005a). A genetic polymorphism of CYP2E1 (increase in CYP2E1 c2c2 genotype)
was also associated with liver fibrosis, diagnosed by ultrasonography in 13 of 320 workers employed in
five PVC manufacturing plants (Hsieh et al. 2007). No association was found between liver effects and
genetic polymorphisms of glutathione transferase or aldehyde dehydrogenase in these studies.
Polymorphisms of genes involved in metabolism (CYP2E1, GSTP1, ALDH2), DNA repair (hOGG1,
MGMT, XRCC1, XPA, XPC, XPD, XPF, TDG, APE1), apoptosis (MDM2, BCL2) and cell cycle control
(p53, p21) have been associated with increased micronuclei, sister chromatid exchange frequency, DNA
damage and retention of DNA adducts in vinyl chloride workers (Feng et al. 2017; Ji et al. 2010; Li et al.
2006, 2009a, 2013; Qiu et al. 2008, 2011a; Wang et al. 2010a, 2010b, 2013b; Wen-Bin et al. 2009; Wong
et al. 2003b; Zhu et al. 2005b, 2008). The occurrence of the mutation biomarkers in serum was correlated
with polymorphisms of the DNA repair genes XRCC1 (mutant p53), excision repair cross
complementation group 2 (ERCC2)/XPD (mutant p53 and ras-p21) and ALDH2 and CYP2E1 in vinyl
chloride workers (Li et al. 2003b, 2006, 2009b). The presence of a polymorphism for CYP2E1 (variant
c2 allele) was also associated with the occurrence of mutant p53 and ras-p21 serum biomarkers (Schindler
et al. 2007). The risk of developing liver cancer also appeared to be elevated in those with a history of
Hepatitis B viral infection (Du and Wang 1998; Wong et al. 2003b).
Vinyl chloride workers with genetic polymorphisms of genes related to metabolism, DNA repair, and cell
cycle control may be more susceptible to liver toxicity and liver cancer. A polymorphism of the CYP2E1
gene was associated with an increase in liver abnormalities evaluated by ultrasound (Hsieh et al. 2007;
Zhu et al. 2005a). Genetic polymorphisms of several genes were associated with increased micronuclei
frequency, DNA damage, retention of DNA adducts, and an increase in tumor biomarkers in serum (Ji et
al. 2010; Li et al. 2006, 2009a; Qiu et al. 2008, 2011a; Schindler et al. 2007; Wang et al. 2010a, 2010b,
2013b; Wen-Bin et al. 2009; Zhu et al. 2005b, 2008). The risk of developing liver cancer also appears
elevated in those with a history of Hepatitis B viral infection (Du and Wang 1998; Wong et al. 2003b).
Work by Black et al. (1983, 1986) has shown that persons with the HLA allele, HLA-DR5, may have an
increased likelihood of developing vinyl chloride disease, and those with the alleles, HLA-DR3 and B8,
may have an increased severity of the disease.
Phenobarbital and Aroclor 1254 increase mixed function oxidase (MFO) activity and have been shown to
greatly increase the hepatotoxicity of vinyl chloride (Conolly and Jaeger 1979; Conolly et al. 1978; Jaeger
et al. 1974, 1977; Jedrychowski et al. 1985; Reynolds et al. 1975a, 1975b). The intermediary metabolites
VINYL CHLORIDE 128
3. TOXICOKINETICS, SUSCEPTIBLE POPULATIONS, BIOMARKERS, CHEMICAL INTERACTIONS
of vinyl chloride, 2-chloroethylene oxide and 2-chloroacetaldehyde, have been suggested to be
responsible for some of the adverse effects produced by vinyl chloride. Thus, individuals taking
barbiturates or who might be exposed to organochlorine pesticides that are known to induce microsomal
enzymes (such as Aroclor 1254) would be expected to be at increased risk for developing vinyl chloride-
induced hepatotoxicity.
Radike et al. (1981) demonstrated that ethanol-consuming rats exposed to vinyl chloride had an increased
incidence of cancer and an earlier death rate than animals exposed to vinyl chloride in the absence of
ethanol. Some persons consume the agent, Antabuse, to curb the desire for alcohol. In its role as a
therapeutic agent, Antabuse blocks aldehyde dehydrogenase and causes a build-up of acetaldehyde, which
is emetic, in the body when alcohol is consumed. If persons taking Antabuse are exposed to vinyl
chloride, the alternative metabolic pathway for vinyl chloride metabolism will be blocked, causing more
vinyl chloride to be metabolized to the toxic metabolite, 2-chloroethylene oxide. Thus, these persons may
be at increased risk for hepatotoxicity, cancer, and death at an early age.
3.3 BIOMARKERS OF EXPOSURE AND EFFECT
Biomarkers are broadly defined as indicators signaling events in biologic systems or samples. They have
been classified as biomarkers of exposure, biomarkers of effect, and biomarkers of susceptibility
(NAS/NRC 2006).
A biomarker of exposure is a xenobiotic substance or its metabolite(s) or the product of an interaction
between a xenobiotic agent and some target molecule(s) or cell(s) that is measured within a compartment
of an organism (NAS/NRC 2006). The preferred biomarkers of exposure are generally the substance
itself, substance-specific metabolites in readily obtainable body fluid(s), or excreta. Biomarkers of
exposure to vinyl chloride are discussed in Section 3.3.1.
Biomarkers of effect are defined as any measurable biochemical, physiologic, or other alteration within an
organism that (depending on magnitude) can be recognized as an established or potential health
impairment or disease (NAS/NRC 2006). This definition encompasses biochemical or cellular signals of
tissue dysfunction (e.g., increased liver enzyme activity or pathologic changes in female genital epithelial
cells), as well as physiologic signs of dysfunction such as increased blood pressure or decreased lung
capacity. Note that these markers are not often substance specific. They also may not be directly
VINYL CHLORIDE 129
3. TOXICOKINETICS, SUSCEPTIBLE POPULATIONS, BIOMARKERS, CHEMICAL INTERACTIONS
adverse, but can indicate potential health impairment (e.g., DNA adducts). Biomarkers of effect caused
by vinyl chloride are discussed in Section 3.3.2.
A biomarker of susceptibility is an indicator of an inherent or acquired limitation of an organism's ability
to respond to the challenge of exposure to a specific xenobiotic substance. It can be an intrinsic genetic or
other characteristic or a preexisting disease that results in an increase in absorbed dose, a decrease in the
biologically effective dose, or a target tissue response. If biomarkers of susceptibility exist, they are
discussed in Section 3.2, Children and Other Populations that are Unusually Susceptible.
3.3.1 Biomarkers of Exposure
The only exposure biomarker specific to vinyl chloride is the measurement of this compound in expired
air. Other exposure biomarkers are not specific to vinyl chloride exposure only. As such, there is limited
utility in urine tests for thiodiglycolic acid and N-acetyl-S-(2-hydroxyethyl)-cysteine.
Vinyl chloride may be quantified in expired air following acute moderate-to-high exposures (Azari et al.
2016). The expiration of vinyl chloride follows first-order kinetics; therefore, this parameter can be
directly correlated with exposure levels (Baretta et al. 1969). This measure provides the most direct
evidence for vinyl chloride exposure. However, measurement of exposure by this technique is limited by
the rapidity with which vinyl chloride is expired during breathing. The half-life of vinyl chloride in
expired air is between 20 and 30 minutes following an inhalation exposure and is approximately
60 minutes following oral dosing (Watanabe and Gehring 1976; Watanabe et al. 1976b, 1978a, 1978b).
Thus, testing must be initiated as soon as possible following termination of exposure. Measurement of
vinyl chloride in expired air has limited utility for low-level exposures (<50 ppm) because of competition
between absorptive uptake and rapid metabolism (Baretta et al. 1969). In addition, it provides no
information on the duration of exposure.
Thiodiglycolic acid is a major urinary metabolite of vinyl chloride. Measurement of thiodiglycolic acid in
urine can be used to monitor occupationally exposed workers (Cheng et al. 2001; Lee et al. 2020; Müller
et al. 1979) and children living in the vicinity of industrial vinyl chloride-using facilities (Huang et al.
2016; Wang et al. 2019b). The validity of this biomarker for community health studies has been
questioned in cases where exposure concentrations in air are generally low (<5 ppm) (Chen et al. 2018).
The amount of thiodiglycolic acid in the urine varies according to individual metabolic idiosyncrasies
because metabolism of vinyl chloride to thiodiglycolic acid is a saturable process. Therefore, when
VINYL CHLORIDE 130
3. TOXICOKINETICS, SUSCEPTIBLE POPULATIONS, BIOMARKERS, CHEMICAL INTERACTIONS
exposure exceeds a certain level, the excretion of vinyl chloride as thiodiglycolic acid will plateau
(Watanabe and Gehring 1976). In addition, the rate of metabolism of vinyl chloride to thiodiglycolic acid
can be influenced by the presence of liver disease and by ethanol consumption as well as intakes of other
substances such as barbiturates (Hefner et al. 1975b).
Similar to the measurement of vinyl chloride in expired air, the measurement of thiodiglycolic acid must
take place shortly after exposure because of its rapid excretion. The half-life for excretion of
thiodiglycolic acid following an acute-duration exposure is between 4 and 5 hours (Watanabe and
Gehring 1976; Watanabe et al. 1978a, 1978b). Cheng et al. (2001) suggested that urinary thiodiglycolic
acid levels should not be measured at the end of a work shift but are best detected at the beginning of the
following workday. Excretion of thiodiglycolic acid is not unique to vinyl chloride exposure. For
example, thiodiglycolic acid can be excreted in the urine as the result of exposure to vinylidene chloride,
ethylene oxide, or 2,2-dichloroethylether (Norpoth et al. 1986; Pettit 1986). Infants delivered prematurely
can have high levels of urinary thiodiglycolic acid. A correlation was observed between the
thiodiglycolic acid levels and the number of weeks that the infant was born prematurely. The origin of
this thiodiglycolic acid in neonates is unknown but is likely not associated with vinyl chloride exposure
(Pettit 1986).
Boyle et al. (2016) suggest that urinary levels of N-acetyl-S-(2-hydroxyethyl)-cysteine may be a useful
biomarker for combined exposure to vinyl chloride, ethylene oxide, and acrylonitrile. This compound is
measured as a urinary biomarker for the listed volatile compounds in the National Health and Nutrition
Examination Survey (NHANES) (Konkle et al. 2020).
3.3.2 Biomarkers of Effect
Biomarkers of effect for vinyl chloride include altered liver function, DNA adducts, and measures of
genotoxicity including chromosomal aberrations, micronuclei, and DNA damage (i.e., strand breaks).
Liver function tests are sensitive indicators of the hepatic damage resulting from vinyl chloride exposure.
These assays include the indocyanine clearance test, measurement of serum bile acids, and measurement
of serum hyaluronic acid concentration (Berk et al. 1975; Liss et al. 1985; McClain et al. 2002; Vihko et
al. 1984). In general, serum enzymes were found to be of limited value in monitoring the progression of
vinyl chloride-induced hepatic changes (Berk et al. 1975; Liss et al. 1985; Vihko et al. 1984). This is
likely due to the extent of hepatic damage produced by vinyl chloride and the late development of
VINYL CHLORIDE 131
3. TOXICOKINETICS, SUSCEPTIBLE POPULATIONS, BIOMARKERS, CHEMICAL INTERACTIONS
necrotic areas in the disease process (Popper et al. 1981). A study of hepatic ultrasound abnormalities
suggests that functional and imaging tests may be useful biomarkers of liver toxicity in workers exposed
to vinyl chloride (Wang et al. 2008). Cave et al. (2010) suggested that an elevation of total cytokeratin 18
levels in serum may be indicative of liver cell necrosis (a known vinyl chloride effect).
The intermediary metabolites, 2-chloroethylene oxide and 2-chloroacetaldehyde, bind to macromolecules
in the body. 2-Chloroethylene oxide is hypothesized to bind primarily to DNA and RNA, whereas
2-chloroacetaldehyde binds primarily to proteins (Bolt 1986; Guengerich and Watanabe 1979;
Guengerich et al. 1979, 1981; Kappus et al. 1976; Watanabe et al. 1978a, 1978b). Several DNA adducts
have been reported following vinyl chloride exposure (Mutlu et al. 2010, 2012; Pottenger et al. 2014;
Swenberg et al. 2011; Yun et al. 2020). 7-(2-Oxoethyl) guanine (7-OEG) is the primary DNA adduct;
however, it is not mutagenic (i.e., does not cause mispairing during replication) and would not be a
biomarker of effect (Mutlu et al. 2010). N
2
,3-Ethenoguanine is a mutagenic adduct and may be an
important effect biomarker of vinyl chloride (Mutlu et al. 2010). Liquid chromatography-mass
spectrometry (LCMS) and stable isotope methods have been used to detect DNA adducts in several
tissues, including white blood cells and oral cells in humans (Yun et al. 2020) and liver, lung, and kidney
in animals (Mutlu et al. 2010, 2012; Pottenger et al. 2014; Swenberg et al. 2011).
Ethenoguanine adducts may be quantified from urine following base excision repair and excretion where
they can be measured using an LCMS method (Gonzalez-Reche et al. 2002). This method would also
include the measurement of endogenously formed etheno-adducts; thus, it is critical to determine the
background level of urinary adducts in a control population.
Chromosomal aberrations found in lymphocytes can be indicative of the genotoxic effects of vinyl
chloride (Anderson 2000; Anderson et al. 1980; Ducatman et al. 1975; Fucic et al. 1990a, 1990b, 1992;
Funes-Cravioto et al. 1975; Garaj-Vrhovac et al. 1990; Hansteen et al. 1978; Hrivnak et al. 1990;
Kucerova et al. 1979; Purchase et al. 1978; Sinués et al. 1991). However, any of a number of genotoxic
substances can produce chromosomal aberrations. de Jong et al. (1988) found that variability in the
control population may obscure the observation of chromosomal aberrations in persons exposed to low
levels of vinyl chloride. G-banding analysis appeared to provide a more sensitive indication of
chromosomal alteration than sister chromatid exchanges (Zhao et al. 1996). DNA damage in
lymphocytes can be directly assessed using a single-cell gel electrophoresis technique. The severity of
the damage may correlate with the duration of exposure (Awara et al. 1998). The DNA adducts produced
by the reactive intermediary metabolites of vinyl chloride, including 1,N
6
-ethenoadenosine and
VINYL CHLORIDE 132
3. TOXICOKINETICS, SUSCEPTIBLE POPULATIONS, BIOMARKERS, CHEMICAL INTERACTIONS
3,N
4
-ethenocytidine, may be more specific indicators of vinyl chloride’s genotoxic potential (Bolt 1986;
Guengerich and Watanabe 1979; Guengerich et al. 1979, 1981; Kappus et al. 1976; Watanabe et al.
1978a, 1978b).
The micronucleus assay, performed using peripheral lymphocytes of 32 vinyl chloride workers, was used
to indicate the time elapsed since the last vinyl chloride exposure occurred (Fucic et al. 1994, 1997). The
study showed a decrease in the frequency of micronuclei and mitotic activity in proportion to the length
of the interval after the last vinyl chloride exposure. For the group with 10 years of employment, the
percentage of micronuclei decreased from 12.82 when exposure occurred on the day of blood sampling to
3.16 when the last exposure occurred 90 days before blood sampling (Fucic et al. 1994). Similar changes
were noted when the mean duration of employment was 5 years. However, this use of the micronucleus
assay must consider the total duration of exposure. Micronucleus frequency was shown to be several
times higher in binucleated lymphocytes as compared to mononuclear lymphocytes in 25 workers
exposed to vinyl chloride for an average of 10 years (Fučić et al. 2004). Zheng et al. (2019) suggested
that reduced relative telomere length and gene expression of telomere associated proteins (i.e., shelterin
complex) were associated with increased micronuclei and could be considered as potential biomarkers;
however, these effects may be caused by many genotoxic compounds and are not specific to vinyl
chloride.
3.4 INTERACTIONS WITH OTHER CHEMICALS
ATSDR (2007) prepared an interaction profile for chloroform, 1,1-dichloroethylene, trichloroethylene,
and vinyl chloride. This report indicated that no direct data are available to characterize health hazards
(and dose-response relationships) from mixtures containing all four components. In addition, PBPK/PD
models have not yet been developed that would predict pertinent target doses of the components under
mixture exposure scenarios. Toxicological data for the individual compounds suggest that sites of joint
toxic action may include hepatic, renal, immunological, neurological, developmental effects, and cancer;
however, no experimental data are available for mixtures (ATSDR 2007).
Studies have been performed to examine the effect of agents intended to alter the metabolism of vinyl
chloride on its toxicity. For example, the effects of phenobarbital pretreatment on vinyl chloride-induced
hepatotoxicity were examined by Jaeger et al. (1974, 1977), Jedrychowski et al. (1985), and Reynolds et
al. (1975a, 1975b). Pretreatment of rats with phenobarbital for 7 days prior to a 4-hour vinyl chloride
exposure produced an increase in microsomal CYP activity (Reynolds et al. 1975b) and enhanced
VINYL CHLORIDE 133
3. TOXICOKINETICS, SUSCEPTIBLE POPULATIONS, BIOMARKERS, CHEMICAL INTERACTIONS
hepatotoxicity (Jaeger et al. 1974, 1977; Jedrychowski et al. 1985; Reynolds et al. 1975a, 1975b). In the
absence of the phenobarbital pretreatment, a single exposure to approximately 50,000 ppm had no
detectable adverse effect on the livers of exposed rats. However, following phenobarbital pretreatment,
50,000 ppm of vinyl chloride produced increased serum activity of hepatic enzymes (Jaeger et al. 1977;
Jedrychowski et al. 1985), areas of hepatic necrosis (Reynolds et al. 1975a), or both (Jaeger et al. 1974;
Reynolds et al. 1975b). Activation of ALDH2 with an agonist (Alda-1) was shown to attenuate liver
injury and reduce oxidative stress in high-fat diet mice exposed to vinyl chloride (Chen et al. 2019).
Another agent known to increase CYP activity, Aroclor 1254, was also tested for its ability to enhance
vinyl chloride-induced hepatotoxicity (Conolly and Jaeger 1979; Conolly et al. 1978; Jaeger et al. 1977;
Reynolds et al. 1975b). Pretreatment of rats with Aroclor 1254 for several days prior to exposure to vinyl
chloride resulted in an increase in serum activity of hepatic enzymes (Conolly and Jaeger 1979; Conolly
et al. 1978; Jaeger et al. 1977; Reynolds et al. 1975b) and areas of hepatic necrosis (Conolly et al. 1978;
Reynolds et al. 1975b). Additional support for a role for CYP in the enhanced toxicity of vinyl chloride
was obtained using SKF525A, a CYP inhibitor. If SKF525A was administered following phenobarbital
pretreatment and before vinyl chloride exposure, it blocked the ability of phenobarbital pretreatment to
enhance vinyl chloride-induced hepatotoxicity (Jaeger et al. 1977).
The role of glutathione conjugation in vinyl chloride-induced toxicity was also examined (Conolly and
Jaeger 1979; Jaeger et al. 1977). The investigators hypothesized that depletion of glutathione might
enhance the toxicity of vinyl chloride by preventing the excretion of toxic intermediary metabolites.
However, diethylmaleate, an agent known to deplete hepatic glutathione levels, had no effect on the
toxicity produced by vinyl chloride following pretreatment with either phenobarbital (Jaeger et al. 1977)
or Aroclor 1254 (Conolly and Jaeger 1979). Trichloropropene oxide (TCPO), another agent known to
deplete hepatic glutathione, produced enhancement of the hepatic toxicity produced by Aroclor 1254
pretreatment and vinyl chloride exposure but only when the animals had been fasted prior to the vinyl
chloride exposure (Conolly and Jaeger 1979). In this study, the authors hypothesized that the
enhancement of vinyl chloride toxicity was a result of the ability of TCPO to inhibit epoxide hydrolase
rather than its ability to deplete glutathione levels.
Although the depletion of cellular glutathione levels did not appear to enhance vinyl chloride toxicity,
treatment with cysteine, the rate-limiting precursor in hepatic glutathione synthesis, increased hepatic
glutathione levels and provided partial protection against the toxic effects produced by Aroclor 1254 and
vinyl chloride (Conolly and Jaeger 1979).
VINYL CHLORIDE 134
3. TOXICOKINETICS, SUSCEPTIBLE POPULATIONS, BIOMARKERS, CHEMICAL INTERACTIONS
Mastrangelo et al. (2004) showed that alcohol increased the risk of hepatocellular carcinoma and liver
fibrosis in vinyl chloride workers. Possible mechanisms for this synergistic effect include alcohol
induction of CYP2E1 and decreased liver glutathione levels resulting in increased formation of mutagenic
metabolites (Voigt 2005). CYP2E1 induction may also increase hepatocellular proliferation and
formation of ROS. In the experiment by Radike et al. (1981), ethanol-consuming rats exposed to vinyl
chloride for a year had an enhanced incidence of hepatic angiosarcomas, hepatomas, and lymphosarcoma,
earlier onset of the tumors, and an enhanced death rate. The incidence of vinyl chloride-induced
angiosarcomas was potentiated by ethanol, whereas the increased incidences of hepatoma and
lymphosarcoma by ethanol were additive in nature.
The effects of smoking on chromosomal aberrations in vinyl chloride-exposed workers was examined by
Hrivnak et al. (1990), who found no effect of smoking in 43 workers exposed for an average of 11.2 years
to levels of vinyl chloride ranging from 0.8 to 16 ppm. Most cytogenetic studies of the effects of smoking
in humans have reported no effect on chromosomal aberrations, although the sister chromatid exchange
frequency is usually elevated (Wong et al. 1998).
A study that examined the interaction between vinyl chloride and trichloroethylene using both inhalation
exposures of rats and pharmacokinetic modeling found that trichloroethylene exposure inhibited vinyl
chloride in a competitive manner (Barton et al. 1995). This interaction was observed only at high
concentrations (both chemicals >10 ppm), and the study authors concluded that the interaction is not
likely to be important for environmental exposures.

VINYL CHLORIDE 135
CHAPTER 4. CHEMICAL AND PHYSICAL INFORMATION
4.1 CHEMICAL IDENTITY
Vinyl chloride is a manmade substance. Information regarding the chemical identity of vinyl chloride is
presented in Table 4-1. This information includes synonyms, chemical formula and structure, and
identification numbers.
Table 4-1. Chemical Identity of Vinyl Chloride
Characteristic
Information
Reference
Chemical name
Vinyl chloride
NLM 2023
Synonym(s) and registered
trade name(s)
Chloroethene; chloroethylene; 1-chloroethylene;
ethylene monochloride; monovinyl chloride;
monochloroethene; monochloroethylene; MVCs;
Trovidur; VC; VCM; vinyl chloride monomer
Fire 1986; NLM
2023
Chemical formula
C
2
H
3
Cl
NLM 2023
SMILES
C=CCl
NLM 2023
Chemical structure
C C
Cl
HH
H
NLM 2023
CAS Registry Number
75-01-4
NLM 2023
CAS = Chemical Abstracts Service; SMILES = simplified molecular-input line-entry system
4.2 PHYSICAL AND CHEMICAL PROPERTIES
Vinyl chloride is a colorless, flammable gas with a sweet odor. It is heavier than air and will tend to
accumulate at the bottom of vessels, rooms, or near ground levels. Information regarding the physical
and chemical properties of vinyl chloride is in Table 4-2.

VINYL CHLORIDE 136
4. CHEMICAL AND PHYSICAL INFORMATION
Table 4-2. Physical and Chemical Properties of Vinyl Chloride
Property
Information
Reference
Molecular weight
62.5
Lewis 1996
Color
Colorless
Budavari 1989
Physical state
Gas
Budavari 1989
Melting point
-153.8°C
Budavari 1989
Boiling point
-13.4°C
Cowfer and Gorensek 2006
Density:
at -14.2°C
0.969 g/cm
3
Cowfer and Gorensek 2006
at 15°C
0.9195 g/cm
3
Lewis 1996
at 20°C
0.9106 g/cm
3
NIOSH 1986
Vapor density
2.16
Fire 1986
Odor
Sweet
NLM 2023
Odor threshold:
Water
3.4 ppm
Amoore and Hautala 1983
Air
3,000 ppm
Amoore and Hautala 1983
Taste threshold
No data
Solubility:
Water at 25°C
2,763 mg/L
EPA 1985a
1,100 mg/L
Cowfer and Gorensek 2006
at 26°C
8,800 mg/L
Delassus and Schmidt 1981
Organic solvent(s)
Soluble in hydrocarbons, oil,
alcohol, chlorinated solvents, and
most common organic liquids
Cowfer and Gorensek 2006
Partition coefficients:
Log K
ow
1.38
NIOSH 1986
1.46
Sakuratani et al. 2007
Log K
oc
2.38–2.95
Lu et al. 2011
Vapor pressure:
at 20°C
2,530 mmHg
Budavari 1989
at 25°C
2,600 mmHg
Lewis 1996
Henry’s law constant:
10.3°C
0.0147 (atm-m
3
)/mol
Gossett 1987
17.5°C
0.0193 (atm-m
3
)/mol
Gossett 1987
24.8°C
0.0278 (atm-m
3
)/mol
Gossett 1987
34.6°C
0.0358 (atm-m
3
)/mol
Gossett 1987
Autoignition temperature
472°C
Lewis 1996
Flashpoint
-78°C (closed cup)
Budavari 1989
Flammability limits
3.6–33 volume %
NIOSH 1986
Conversion factors:
ppm to mg/m
3
in air
1 ppm=2.6 mg/m
3
NIOSH 1990
mg/m
3
to ppm in air
1 mg/m
3
=0.38 ppm
NIOSH 1990
Explosive limits
4–22 volume %
Lewis 1996
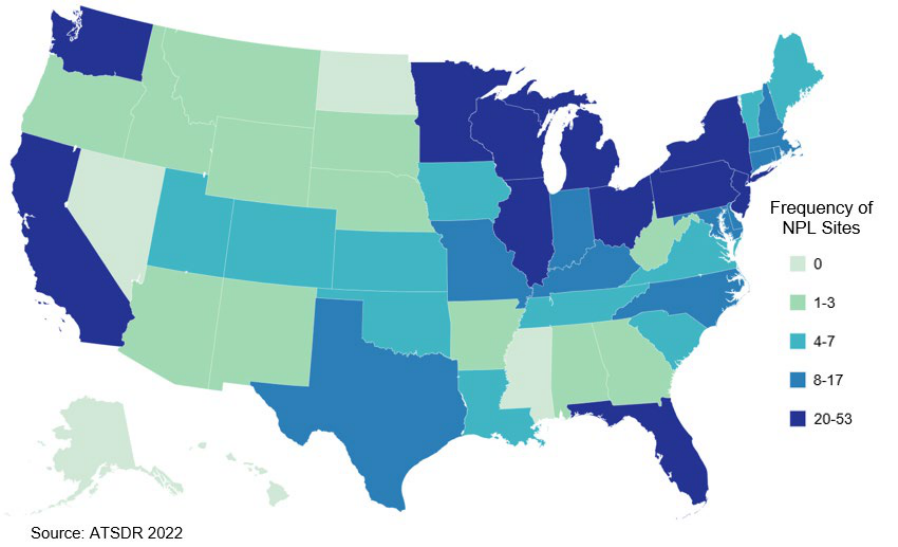
VINYL CHLORIDE 137
CHAPTER 5. POTENTIAL FOR HUMAN EXPOSURE
5.1
OVERVIEW
Vinyl chloride has been identified in at least 594 of the 1,868 hazardous waste sites that have been
proposed for inclusion on the EPA National Priorities List (NPL) (ATSDR 2022). However, the number
of sites in which vinyl chloride has been evaluated is not known. The number of sites in each state is
shown in Figure 5-1. Of these sites, 590 are located within the United States, 1 site is located in the
Virgin Islands, and 3 sites are located in Puerto Rico (not shown).
Figure 5-1. Number of NPL Sites with Vinyl Chloride Contamination
• T
he major route of exposure to vinyl chloride is through inhalation. This mostly occurs in the
occupational setting but can occur near manufacturing facilities, hazardous waste sites, and
natural gas extraction sites, where the air may be contaminated.
• Inhalation of cigarette and cigar smoke can also be an exposure route.
• In air, vinyl chloride will degrade photochemically with a half-life of 1–2 days.
• Vinyl chloride released to water is mostly expected to volatilize into the atmosphere. Small
amounts could degrade by photochemical reaction and biodegradation.
VINYL CHLORIDE 138
5. POTENTIAL FOR HUMAN EXPOSURE
• Vinyl chloride released to the soil is expected to volatilize or leach into groundwater.
• Aerobically, vinyl chloride is expected to degrade by 25% in a week and by >99% in 15.4 weeks.
The rate of anaerobic degradation is dependent on the components of the media (e.g., increased
iron).
Most vinyl chloride entering the environment is discharged to the air where it is degraded by reaction
with photochemically generated atmospheric oxidants with a typical half-life of a few days. The bacterial
degradation of chlorinated solvents such as trichloroethylene, tetrachloroethylene, 1,1,1-trichloroethane,
and cis-dichloroethene can also produce vinyl chloride as a degradation product. Most emissions of vinyl
chloride arise from its use in the production of PVC materials and copolymers. Over the past several
decades, significant reductions in vinyl chloride emissions have been achieved from improved
engineering controls in PVC manufacturing facilities. Moreover, optimization of the PVC production
process has lowered residual levels of vinyl chloride in finished products such as PVC pipe and food and
nonfood packaging material.
If released to water, vinyl chloride is expected to volatilize rapidly. Degradation processes such as
hydrolysis and biodegradation occur slowly in comparison to the rate of volatilization. Vinyl chloride is
not expected to bioconcentrate in aquatic organisms. When released to soil, volatilization is the most
important environmental fate process, although it possesses high mobility in soil.
General population exposure to vinyl chloride is typically low; however, some populations that are
exposed from an accidental release such as the Norfolk Southern train derailment that occurred on
February 3, 2023, near East Palestine, Ohio are at risk for higher exposures. Occupational exposure to
vinyl chloride is higher than exposures to the general population; however, since the mid-1970s
regulatory changes and voluntary improvements in the PVC manufacturing process have dramatically
lowered workplace exposure.
5.2 PRODUCTION, IMPORT/EXPORT, USE, AND DISPOSAL
5.2.1 Production
Table 5-1 summarizes information on companies that reported the production, import, or use of vinyl
chloride for the Toxics Release Inventory (TRI) in 2021 (TRI21 2023a). TRI data should be used with
caution since only certain types of industrial facilities are required to report. This is not an exhaustive list.
VINYL CHLORIDE 139
5. POTENTIAL FOR HUMAN EXPOSURE
Vinyl chloride was first produced commercially in the 1930s by reacting hydrogen chloride with
acetylene. Currently, vinyl chloride is produced commercially by the chlorination of ethylene through
one of two processes, direct chlorination or oxychlorination. Direct chlorination reacts ethylene with
chlorine to produce 1,2-dichloroethane. Similarly, oxychlorination produces 1,2-dichloroethane, but this
is accomplished by reacting ethylene with dry hydrogen chloride and oxygen.
After both processes, the 1,2-dichloroethane is subjected to high pressures (2.5–3.0 megapascals) and
temperatures (500–550°C). This causes the 1,2-dichloroethane to undergo pyrolysis, or thermal cracking,
which forms the vinyl chloride monomer and hydrogen chloride. The vinyl chloride monomer is then
isolated (Cowfer and Magistro 1985). The technical-grade product is available in 99.9% purity (NLM
2023). Efforts have been made to minimize by-product formation (hydrocarbons, chlorinated
hydrocarbons, and unreacted material) in 1,2-dichloroethane pyrolysis (Cowfer and Magistro 1985).
Table 5-1 summarizes the facilities in the United States that either manufacture or process vinyl chloride.
The Toxic Release Inventory (TRI21 2023a) provides the data for Table 5-1 including the maximum
amounts of vinyl chloride that are present at these sites and the end uses of vinyl chloride. Table 5-2 lists
the 12 reporting facilities that solely manufacture vinyl chloride for commercial purposes and their
production capacities (EPA 2021). Because of confidential business information, specific quantities are
not available (EPA 2021).
Table 5-1. Facilities that Produce, Process, or Use Vinyl Chloride
State
a
Number of
facilities
Minimum
amount on site
in pounds
b
Maximum
amount on site
in pounds
b
Activities and uses
c
AL
1
100,000
999,999
6
AR
1
1,000
9,999
1, 2, 3, 5, 9, 12
IL
1
1,000,000
9,999,999
6
KY
3
1,000,000
9,999,999
1, 4, 6
LA
8
10,000,000
49,999,999
1, 3, 4, 5, 6, 12, 13
MO
1
1,000
9,999
1, 5, 14
MS
1
10,000,000
49,999,999
6
NC
1
0
99
6, 7, 8, 11
NE
1
1,000
9,999
9, 12
NJ
2
1,000,000
49,999,999
6, 12

VINYL CHLORIDE 140
5. POTENTIAL FOR HUMAN EXPOSURE
Table 5-1. Facilities that Produce, Process, or Use Vinyl Chloride
State
a
Number of
facilities
Minimum
amount on site
in pounds
b
Maximum
amount on site
in pounds
b
Activities and uses
c
NY
1
0
99
12
OH
3
100
9,999
6, 12
TX
12
100
499,999,999
1, 3, 4, 5, 6, 9, 12, 13, 14
UT
1
1,000
9,999
9, 12
WA
1
Not available
Not available
Not available
a
Post office state abbreviations used.
b
Amounts on site reported by facilities in each state.
c
Activities/Uses:
1. Produce
2. Import
3. Used Processing
4. Sale/Distribution
5. Byproduct
6. Reactant
7. Formulation Component
8. Article Component
9. Repackaging
10. Chemical Processing Aid
11. Manufacture Aid
12. Ancillary
13. Manufacture Impurity
14. Process Impurity
Source: TRI21 2023a (Data are from 2021)
Table 5-2. U.S. Production Capacity of Vinyl Chloride
U.S. Producer
Location
Capacity (million pounds per year)
Axiall
Plaquemine, Louisiana
CBI
Axiall
Westlake, Louisiana
CBI
Axiall
Westlake, Calcasieu, Louisiana
1,026
C-K Tech
Plaquemine, Louisiana
CBI
Formosa Plastics
Baton Rouge, Louisiana
1,188
Formosa Plastics
Point Comfort, Texas
1,497
GEON Oxy Vinyl
Laporte, Texas
CBI
Olin Blue Cube
Freeport, Texas
CBI
Oxy Vinyls LP
Deer Park, Texas
CBI
Oxychem Ingleside
San Patricio, Texas
CBI
Westlake Vinyls
Geismar, Louisiana
540
Westlake Vinyls
Calvert City, Kentucky
1,316
U.S. total capacity: 10,000 - <20,000 million pounds
CBI = Confidential Business Information
Source: EPA 2021 (data are from 2015)
VINYL CHLORIDE 141
5. POTENTIAL FOR HUMAN EXPOSURE
5.2.2 Import/Export
One facility reported 37,000 pounds imported in 2015, down from 48,700 pounds in 2014 (EPA 2021); no
further import data were located. Export volumes for 2004 and 2005 were 2.367 and 1.88 billion pounds,
respectively (ICIS 2006). Current export volumes were not located.
5.2.3 Use
Vinyl chloride is an important industrial chemical because of its wide variety of end-use products and the
low cost of producing polymers from it. About 95–99% of the global vinyl chloride capacity is used for
the production of PVC and its copolymers; other uses include the production of chlorinated solvents such
as 1,1,1-trichloroethane (Dreher et al. 2014; Kielhorn et al. 2000).
Vinyl chloride has been used in the past as a refrigerant, as an extraction solvent for heat-sensitive
materials, and in the production of chloroacetaldehyde and methyl chloroform (IARC 2012). In the
United States, limited quantities of vinyl chloride were used as an aerosol propellant and as an ingredient
of drug and cosmetic products; however, these practices were banned by the EPA in 1974 (IARC 2012;
NLM 2023).
5.2.4 Disposal
Since vinyl chloride has been identified by EPA as a hazardous material, its disposal is regulated under
the Federal Resource Conservation and Recovery Act (RCRA) (EPA 1993). The Department of
Transportation monitors compliance with RCRA (and therefore disposal) (DOT 1993). The
recommended method of disposal is total destruction by incineration.
The temperature of the incinerator must be sufficient to ensure the complete combustion of the vinyl
chloride in order to prevent the formation of phosgene. The recommended temperature range for
incineration is 450–1,600°C, with residence times of seconds for gases and liquids, and hours for solids
(NLM 2023). If in solution, the vinyl chloride product may need to be adsorbed onto a combustible
material prior to incineration. Alternately, vinyl chloride can also be dissolved in a flammable solvent
prior to incineration. An acid scrubber should be used in conjunction with the incinerator in order to
remove any hydrogen chloride that is produced by the combustion process (NLM 2023).

VINYL CHLORIDE 142
5. POTENTIAL FOR HUMAN EXPOSURE
Vinyl chloride can also be chemically destroyed. This destruction method is used, especially with small
quantities. Generally, 1–2 days is sufficient for complete chemical destruction (NLM 2023).
Aqueous byproduct solutions from the production of vinyl chloride are usually steam-stripped. This step
removes volatile organic compounds. The remaining solution is then neutralized. Lastly, the solution is
treated in an activated sludge system to remove nonvolatile organic compounds (Cowfer and Gorensek
2006).
5.3 RELEASES TO THE ENVIRONMENT
The Toxics Release Inventory (TRI) data should be used with caution because only certain types of
facilities are required to report (EPA 2022). This is not an exhaustive list. Manufacturing and processing
facilities are required to report information to the TRI only if they employ ≥10 full-time employees; if
their facility's North American Industry Classification System (NAICS) codes is covered under EPCRA
Section 313 or is a federal facility; and if their facility manufactures (defined to include importing) or
processes any TRI chemical in excess of 25,000 pounds, or otherwise uses any TRI chemical in excess of
10,000 pounds, in a calendar year (EPA 2022).
5.3.1 Air
Estimated releases of 428,185 pounds (~194 metric tons) of vinyl chloride to the atmosphere from
38 domestic manufacturing and processing facilities in 2021, accounted for about 99.9% of the estimated
total environmental releases from facilities required to report to the TRI (TRI21 2023a). These releases
are summarized in Table 5-3.
Table 5-3. Releases to the Environment from Facilities that Produce, Process, or
Use Vinyl Chloride
a
Reported amounts released in pounds per year
b
State
c
RF
d
Air
e
Water
f
UI
g
Land
h
Other
i
Total release
On-site
j
Off-site
k
On- and off-site
AL
1
1,820
0
0
0
0
1,820
0
1,820
AR
1
6
0
0
108
0
6
108
114
IL
1
19,115
0
0
21
0
19,115
21
19,135
KY
3
117,526
1
0
0
7
117,526
8
117,534
LA
8
117,553
31
0
1
51
117,584
52
117,636

VINYL CHLORIDE 143
5. POTENTIAL FOR HUMAN EXPOSURE
Table 5-3. Releases to the Environment from Facilities that Produce, Process, or
Use Vinyl Chloride
a
Reported amounts released in pounds per year
b
State
c
RF
d
Air
e
Water
f
UI
g
Land
h
Other
i
Total release
On-site
j
Off-site
k
On- and off-site
MO
1
287
0
0
0
0
287
0
287
MS
1
4,779
0
0
0
0
4,779
0
4,779
NC
1
68
0
0
0
0
68
0
68
NE
1
23
0
0
0
2
23
2
25
NJ
2
24,407
10
0
10
0
24,417
10
24,427
NY
1
0
0
0
0
0
0
0
0
OH
3
10
0
0
0
0
10
0
10
TX
12
142,591
8
0
0
88
142,598
88
142,686
UT
1
0
0
0
0
0
0
0
0
WA
1
0
0
0
0
0
0
0
0
Total
38
428,185
49
0
140
148
428,233
289
428,522
a
The TRI data should be used with caution since only certain types of facilities are required to report. This is not an
exhaustive list. Data are rounded to nearest whole number.
b
Data in TRI are maximum amounts released by each facility.
c
Post office state abbreviations are used.
d
Number of reporting facilities.
e
The sum of fugitive and point source releases are included in releases to air by a given facility.
f
Surface water discharges, wastewater treatment (metals only), and publicly owned treatment works (POTWs) (metal
and metal compounds).
g
Class I wells, Class II-V wells, and underground injection.
h
Resource Conservation and Recovery Act (RCRA) subtitle C landfills; other onsite landfills, land treatment, surface
impoundments, other land disposal, other landfills.
i
Storage only, solidification/stabilization (metals only), other off-site management, transfers to waste broker for
disposal, unknown
j
The sum of all releases of the chemical to air, land, water, and underground injection wells.
k
Total amount of chemical transferred off-site, including to POTWs.
RF = reporting facilities; UI = underground injection
Source: TRI21 2023a (Data are from 2021)
The major sources of vinyl chloride releases to the environment are believed to be emissions and effluents
from plastic industries, primarily vinyl chloride and PVC manufacturers. Due to modifications in the
PVC manufacturing process, decreases in emissions of vinyl chloride have been achieved over the past
several decades. According to data from the TRI, total air emissions of vinyl chloride were reported as
885,387 pounds in 1998 but have declined to 428,184 pounds in 2021 (TRI21 2023a).
EPA’s National Emission Inventory (NEI) database contains detailed information about sources that emit
criteria air pollutants and their precursors, and hazardous air pollutants (HAPs) for the 50 United States,
VINYL CHLORIDE 144
5. POTENTIAL FOR HUMAN EXPOSURE
Washington DC, Puerto Rico, and the U.S. Virgin Islands. In 2011, there were 920,128 pounds of vinyl
chloride released to air from 15 different emissions categories, the most prominent being waste disposal
and industrial processes, accounting for roughly 30 and 60% of all of the emissions, respectively (EPA
2014). Over an 11-year emission study within the Greater Houston area, spanning from 2003 to 2013,
vinyl chloride was released in an emission event at a high of 6,520 kg in 2005 from Dow Texas
Operations Freeport (Luong and Zhang 2017). This event contributed 99% of the emissions for that year.
Vinyl chloride detected at hazardous waste sites may not necessarily arise from industrial sources. The
bacterial degradation of chlorinated solvents such as trichloroethylene, tetrachloroethylene,
1,1,1-trichloroethane, and cis-dichloroethene can produce vinyl chloride as a degradation product, and
this may be the origin of vinyl chloride at these sites (Smith and Dragun 1984; Xiao et al. 2020).
Ahn et al. (2020) estimated that concentrations of 45.5 µg/m
3
(17.8 ppbv) vinyl chloride could have been
released into the air from soil at nighttime as a result of the release of 43,780 kg of volatile organic
compounds (VOCs) from an oil refinery in Texas after Hurricane Harvey in Houston in August 2017,
with daytime emissions estimated to be 10 times lower. This value was based on modeling data of
mineral-type soils under water-saturated conditions (67% soil-water content at 25°C), and an estimated
soil-air partition coefficient (K
SA
4.60) and an octanol-air partition coefficient (log K
oa
0.92) for vinyl
chloride.
Five vinyl chloride monomer tank cars carrying 115,580 gallons of vinyl chloride were derailed in the
Norfolk Southern Railway Train Derailment on February 3, 2023, in East Palestine, Ohio (National
Transport Safety Board 2023). To avoid an explosion hazard of the tank cars, controlled venting was
performed to release and burn the vinyl chloride. Venting took place for several hours beginning on
February 6, 2023. Released liquid vinyl chloride was contained in ditches dug by responders while it
vaporized and burned.
5.3.2 Water
Estimated releases of 49 pounds (~0.02 metric tons) of vinyl chloride to surface water from 38 domestic
manufacturing and processing facilities in 2021, accounted for about 0.01% of the estimated total
environmental releases from facilities required to report to the TRI21 (TRI21 2023a). This estimate
includes releases to wastewater treatment and publicly owned treatment works (POTWs) (TRI21 2023a).
These releases are summarized in Table 5-3.
VINYL CHLORIDE 145
5. POTENTIAL FOR HUMAN EXPOSURE
Vinyl chloride released in wastewater from the plastics industries is expected to volatilize fairly rapidly
(on the order of hours to days) into the atmosphere. Anaerobic reductive dehalogenation of trichloro-
ethylene, tetrachloroethylene, and 1,1,1-trichloroethane also releases vinyl chloride into groundwater at
hazardous waste sites (Smith and Dragun 1984) or other locations where the proper conditions are found
in the subterranean strata. Since vinyl chloride possesses high mobility in soils, it leaches into
groundwater from spills, landfills, and industrial sources that may release it to soil (TRI21 2023a).
According to data collected from the analysis of leachates and monitoring wells at sites where
groundwater was contaminated by municipal solid waste landfill leachate, vinyl chloride was present in
both the leachates and the groundwater samples (Sabel and Clark 1984).
5.3.3 Soil
Estimated releases of 140 pounds (~0.06 metric tons) of vinyl chloride to soil from 38 domestic
manufacturing and processing facilities in 2021, accounted for about 0.03% of the estimated total
environmental releases from facilities required to report to the TRI21 (TRI21 2023a). These releases are
summarized in Table 5-3.
The bacterial degradation of chlorinated solvents such as trichloroethylene, tetrachloroethylene,
1,1,1-trichloroethane, and cis-dichloroethene can produce vinyl chloride as a degradation product, and
this may be a significant source of vinyl chloride at contaminated and hazardous waste sites (Smith and
Dragun 1984; Xiao et al. 2020).
5.4 ENVIRONMENTAL FATE
5.4.1 Transport and Partitioning
Air. Based on a vapor pressure of 2,660 mmHg at 25°C, essentially all vinyl chloride in the atmosphere
is expected to exist solely as a gas (Eisenreich et al. 1981; Verschueren 1983). Consequently, removal
from the atmosphere by dry deposition is not expected to be an important fate process.
Water. The primary transport process for vinyl chloride from natural water systems is volatilization into
the atmosphere. The Henry’s law constant of vinyl chloride has been measured as 0.0278 atm-m
3
/mol at
24.8°C (Gossett 1987), which suggests that vinyl chloride should partition rapidly to the atmosphere. The
half-life for vinyl chloride volatilization from a typical pond, river, and lake has been estimated to be
VINYL CHLORIDE 146
5. POTENTIAL FOR HUMAN EXPOSURE
43.3, 8.7, and 34.7 hours, respectively. These values are based on an experimentally determined
reaeration rate ratio of approximately 2 and assumed oxygen reaeration rates of 0.008, 0.04, and 0.01 per
hour for a typical pond, river, and lake, respectively (EPA 1982a).
Predicted half-lives should be considered rough estimates since the presence of various salts in natural
water systems may affect the volatility of vinyl chloride significantly (EPA 1979). Many salts can form
complexes with vinyl chloride and increase its water solubility; therefore, the presence of salts in natural
waters may significantly influence the amount of vinyl chloride remaining in the water (EPA 1975). The
half-life of vinyl chloride in bodies of water is also affected by depth and turbidity.
The uptake of vinyl chloride by trees from groundwater was examined by sampling and analyzing tree
trunk matrices for the uptake of vinyl chloride in four sampling events at two sites with known
contamination in groundwaters, the “Caretti site” (Ferrara, Emilia-Romagna Region) and the “Bussi site”
(Bussi sul Tirino, Pescara, Abruzzo Region) to assess the potential for vapor intrusion (Filippini et al.
2022). In May 2019, vinyl chloride was detected in groundwater at concentrations of 3,550.0–
7,181.0 µg/L and in trunk cores at below the detection limit to 33.0 µg/kg; in October 2019, vinyl
chloride was detected in groundwater at concentrations of 110.0–1649.0 µg/L and in trunk cores at 3.0–
13.0 µg/kg; in June 2020, vinyl chloride was detected in groundwater at concentrations of 164.0–
2,230.0 µg/L and in trunk cores at 1.6 to 19.7 µg/kg; and in September 2020, vinyl chloride was detected
in groundwater at concentrations of 119.0–1,529.0 µg/L and in trunk cores in concentrations that were
below the limit of detection (LOD).
Sediment and Soil. The relatively high vapor pressure of vinyl chloride indicates that this compound
volatilizes rapidly from dry soil surfaces (Verschueren 1983). Volatilization from soil is affected by
several factors, including temperature and soil characteristics (Ahn et al. 2020; Rossabi et al. 2018). The
effective half-life (due to volatilization and degradation) of vinyl chloride incorporated 10 cm deep in dry
soil is predicted to be 12 hours (Jury et al. 1984). Vinyl chloride is soluble in water and can therefore
leach through the soil and enter groundwater before evaporation can occur (Cowfer and Gorensek 2006).
The soil organic carbon adsorption coefficient (K
oc
) for vinyl chloride was determined to range from
240 to 890 in seven natural clayey till samples (Lu et al. 2011). These K
oc
values suggest a low sorption
tendency, meaning that this compound would be highly mobile in soil. Thus, vinyl chloride has the
potential to leach into groundwater.
VINYL CHLORIDE 147
5. POTENTIAL FOR HUMAN EXPOSURE
Other Media. Vinyl chloride is soluble in most common organic solvents (Cowfer and Gorensek 2006).
In situations where organic solvents exist in relatively high concentrations (e.g., landfills, hazardous
waste sites), co-solvation of vinyl chloride could reduce its volatility, thus causing it to have greater
mobility than indicated by measured K
oc
values.
Vinyl chloride’s low octanol/water partition coefficient (log K
ow
of 1.23) indicates that the potential for
bioconcentration in aquatic organisms is low (EPA 1982a). Using a log K
ow
of 1.23 and a regression-
derived equation (Meylan et al. 1999), the bioconcentration factor (BCF) for vinyl chloride is estimated
as 3. Freitag et al. (1985) measured BCFs for vinyl chloride in algae, fish, and activated sludge. The
vinyl chloride BCFs for algae, fish, and activated sludge were 40, <10, and 1,100, respectively. The very
low value for fish, in comparison to the algae and activated sludge, may suggest a detoxification process
in more highly developed organisms such as fish. Lu et al. (1977) examined the bioaccumulation of
14
C-vinyl chloride in a closed model aquatic ecosystem over a 3-day period. The high volatility of vinyl
chloride minimized any potential bioaccumulation. The relatively low tissue concentrations found in fish
suggest that vinyl chloride is not biomagnified in aquatic food chains to any substantial degree.
5.4.2 Transformation and Degradation
Air. Reaction of gaseous vinyl chloride with photochemically generated hydroxyl radicals is predicted to
be the primary degradation mechanism for this compound in the atmosphere (Cox et al. 1974; Howard
1976; Perry et al. 1977). The rate constant for this reaction was measured as 6.96x10
-12
cm
3
/molecule-
second (Kwok and Atkinson 1995). This rate constant corresponds to an atmospheric half-life of about
18 hours assuming a hydroxyl radical concentration of 1.5x10
6
molecules/cm
3
. Products of this reaction
are hydrochloric acid, formaldehyde, formyl chloride, carbon monoxide, carbon dioxide, chloro-
acetaldehyde, acetylene, chloroethylene epoxide, chloroacetylchloranil, and water (Müller and Korte
1977; Woldbaek and Klaboe 1978). Under conditions of photochemical smog and increased nitric oxide
(NO) concentrations, the half-life of vinyl chloride has been shown to be reduced to 1.2–4.2 hours,
depending on both vinyl chloride and NO concentrations (Carassiti et al. 1977). Reaction of vinyl
chloride with ozone and nitrate radicals in the atmosphere is expected to be slow; half-lives of ca. 45 and
37 days can be expected based on the ozone reaction rate constant of 2.45±0.45x10
-19
cm
3
/molecule-
second and the nitrate radical reaction rate constant of 4.3x10
-16
cm
3
/molecule-second, respectively
(Atkinson 1991; EPA 1976, 1985b; Zhang et al. 1983). Direct photolysis is not expected to be an
important degradation mechanism in the atmosphere because vinyl chloride in the gas phase does not
absorb light of wavelengths above 220 nm (EPA 1976). Since atmospheric ozone blocks almost all
VINYL CHLORIDE 148
5. POTENTIAL FOR HUMAN EXPOSURE
sunlight with wavelengths <295 nm, direct photolysis is likely to occur very slowly, if at all, in the
atmosphere (EPA 1976).
Water. The primary removal process for vinyl chloride from surface waters is volatilization into the
atmosphere. Vinyl chloride in water does not absorb ultraviolet radiation above 218 nm; therefore, direct
photolysis in the aquatic environment is expected to occur very slowly, if at all (EPA 1976). In sunlit
surface waters containing photosensitizers, such as humic materials, photodegradation may be more rapid.
If so, in some waters, sensitized photodegradation may be an important removal mechanism (EPA 1976).
Vinyl chloride decomposed rapidly when irradiated with ultraviolet light in the presence of acetone, a
high energy triplet sensitizer, or hydrogen peroxide, a free radical source (EPA 1976).
The hydrolytic half-life of vinyl chloride is estimated to be <10 years at 25°C (EPA 1976). Since the
volatilization rate of vinyl chloride is much more rapid than the predicted rate of hydrolysis, hydrolysis is
not a significant aquatic fate (EPA 1976, 1979). Vinyl chloride is not oxidized chemically by reaction
with photochemically generated molecular oxygen in natural water systems (EPA 1976). Experiments
carried out at 20 mg/L vinyl chloride in water saturated with molecular oxygen at elevated temperatures
showed that, after 12 hours at 85°C, no degradation of vinyl chloride was observed. At temperatures and
oxygen concentrations in natural waters, therefore, vinyl chloride is not expected to degrade by molecular
oxygen at a significant rate (EPA 1976).
Biodegradation of vinyl chloride in water typically occurs via three important pathways: (1) anaerobic
reductive dichlorination producing ethene; (2) anaerobic oxidation to carbon dioxide under iron or
manganese reducing conditions; and (3) aerobic ultimate biodegradation to carbon dioxide
(SERDP/ESTCP 2012). The degradation of vinyl chloride under anaerobic conditions was studied using
iron-enriched aquifer microcosms obtained from a site contaminated with various chlorinated compounds
(Smits et al. 2011). Two separate microcosm columns were prepared in which one column was fed solely
vinyl chloride while the second column had both vinyl chloride and acetate in the influent. Degradation
of vinyl chloride and formation of ethene was noticeable in the vinyl chloride and acetate influent
column. This suggests a reductive dichlorination pathway for vinyl chloride degradation; however,
ethene was not produced in the column where vinyl chloride was the only substance in the influent,
suggesting that oxidation to carbon dioxide was the important degradation pathway in this column.
Vinyl chloride (1 ppm) was rapidly degraded under aerobic conditions in a laboratory study that used soil-
water microcosms from aquifer material without the addition of other nutrients, such as nitrogen or
VINYL CHLORIDE 149
5. POTENTIAL FOR HUMAN EXPOSURE
phosphorus (Davis and Carpenter 1990). About 25% of the vinyl chloride was degraded after 1 week and
>99% was degraded after 108 days. Sixty-five percent of labeled vinyl chloride was recovered as
14
CO
2
after 108 days, demonstrating the extent of the mineralization.
Multiple vinyl chloride degrading bacteria have been isolated and demonstrate capacity to degrade vinyl
chloride under aerobic conditions (Coleman and Spain 2003; Coleman et al. 2002; Danko et al. 2006;
Zalesak et al. 2021). Rhodococcus sp. Strain SM-1, a member of the order Actinomycetales, obtained
from a trichloroethylene-degrading consortium was found to degrade vinyl chloride to CO
2
by using
propane as an energy source during growth experiments or cell suspension experiments (Phelps et al.
1991). Vinyl chloride concentrations decreased by more than 90%; growth cultures and cell suspensions
incorporated about 10% of the transformed vinyl chloride into biomass (Phelps et al. 1991).
Mycobacterium vaccae JOB5 degraded 100% of vinyl chloride in a 2-hour incubation (Wackett et al.
1989). In sediment and groundwater microcosms constructed from a contaminated site containing
ethenotrophic bacteria, biodegradation of vinyl chloride was observed under fully aerobic (dissolved
oxygen >8 mg/L), limited oxygen, and low oxygen conditions (dissolved oxygen of <1 mg/L) with 22–
24% removal after 110 days, 74% removal after 110 days, and 100% removal after 62 days, respectively
(Richards et al. 2022).
Degradation of vinyl chloride generally occurs slowly in anaerobic groundwater and sediment; however,
under methanogenic or Fe(III)-reducing conditions, anaerobic degradation occurs more rapidly. Vinyl
chloride was mineralized approximately 34% in 84 hours in anaerobic aquifer microcosms supplemented
with Fe(III) and held under Fe(III)-reducing conditions (Bradley and Chapelle 1996). Reductive
dechlorination of vinyl chloride to ethene was observed in enrichment cultures containing
Dehalococcoides as the dominant species, along with Acetobacterium and Sporomusa (Puentes Jacome et
al. 2019). Dechlorination rates of 2.0–8.8 µmol Cl
-
released/L/day were reported at pH values ranging
from 5.5 to 7.0 after an incubation period of ca. 400 days, with slower rates observed at the lowest pH of
5.5.
Sediment and Soil. Most vinyl chloride present on soil surfaces will volatilize to the atmosphere.
Vinyl chloride is also mobile in soil and susceptible to leaching (Lyman et al. 1982). Because vinyl
chloride is soluble in organic solvents (Cowfer and Gorensek 2006), the presence of other organic
solvents, such as those found at hazardous waste sites, may affect the mobility of the substance in the soil.
Photodegradation on the surface of soils is possible since sensitized photodegradation in water occurs;
VINYL CHLORIDE 150
5. POTENTIAL FOR HUMAN EXPOSURE
however, this is not expected to be an important environmental fate process for vinyl chloride in most
soils and sediment.
Several laboratory studies indicated that both aerobic and anaerobic biodegradation of vinyl chloride can
occur in soils and aquifer materials via a number of mechanisms (Barrio-Lage et al. 1990; Castro et al.
1992a, 1992b; Davis and Carpenter 1990), although these degradation processes were generally slow.
Nelson and Jewell (1993) investigated methanotrophic degradation of vinyl chloride using a laboratory-
scale, methanotrophic, attached-film, expanded-bed bioreactor. The study authors found that this
technique is an efficient way to degrade vinyl chloride, with the removal efficiency >90%. Under
methanotrophic conditions, vinyl chloride was mineralized between 5 and 44% over 37 days using creek
bed sediment microcosms obtained from a naval station near Jacksonville, Florida (Bradley and Chapelle
1997). Slightly higher mineralization rates were observed under Fe(III)-reducing conditions. With vinyl
chloride-oxidizing transfer cultures and microcosms derived from authentic site materials, vinyl chloride
oxidation was sustained at what can be considered anaerobic conditions with dissolved oxygen
concentrations below 0.02 and 0.1 mg/L, respectively (Gossett 2010). Vinyl chloride was degraded
approximately 50 and 100% in 25 and 19 days under these respective conditions (Gossett 2010).
5.5 LEVELS IN THE ENVIRONMENT
Reliable evaluation of the potential for human exposure to vinyl chloride depends, in part, on the
reliability of supporting analytical data from environmental samples and biological specimens.
Concentrations of vinyl chloride in unpolluted atmospheres and in pristine surface waters are often so low
as to be near the limits of current analytical methods. In reviewing data on vinyl chloride levels
monitored or estimated in the environment, it should also be noted that the amount of chemical identified
analytically is not necessarily equivalent to the amount that is bioavailable.
The EPA maintains a Water Quality Portal (WQP) database that aggregates environmental monitoring
data from the National Water Information System (NWIS) and STORage and RETrieval (STORET)
system. Summaries of the data for ambient surface and groundwater from recent years are presented in
Tables 5-4 and 5-5. Based on limited sampling, vinyl chloride has been detected most frequently in
groundwater, with limited detections in sediments and soil. Detections were generally at or below
reporting limits, and the highest concentrations were found in groundwater samples (WQP 2021, 2023).

VINYL CHLORIDE 151
5. POTENTIAL FOR HUMAN EXPOSURE
Table 5-4. Vinyl Chloride Detected in Samples Collected Throughout the United
States from 2011 to 2021
Type
Number of
samples
Number of
positive
Concentration range
Ambient air
58
0
0.039–0.052 ppb (detection limit)
Indoor air
4
0
0.039–0.052 ppb (detection limit)
Groundwater
a
6,838
254
0.2–7,380 ppb; 0.1–20 ppb (lower
quantification limit)
Surface water
a
1,358
0
<0.02–5.0 ppb (lower quantification limit)
Wastewater
2
0
0.1 ppb (lower quantification limit)
Leachate
48
0
0.5–1.0 ppb (lower quantification limit)
Sediment
306
1
1,300 ppb; 0.5–1,000 ppb (lower quantification
limit)
Soil
45
4
2.4–9.6 ppb (values are below reporting limit)
a
Samples reported are from 2017 to 2021.
Source: WQP 2021
Table 5-5. Vinyl Chloride Detected in Samples Collected Throughout the United
States in 2022 and 2023
a
Type
Number of
samples
Positive
detections (%)
Average concentration (maximum)
Ambient air
0
0
Not applicable
Ambient air at
Superfund site
10
0
Not detected (method detection limit 0.039–
0.059 ppb [0.1–0.15 μg/m
3
])
Ambient
groundwater
a
2,674
1.0
2022: 12.4 µg/L or ppb (61.3 µg/L or ppb)
2023: 6.79 µg/L or ppb (7.71 µg/L or ppb)
Ambient groundwater
at Superfund site
226
0
Not detected (method detection limit 0.06–
2,000 µg/L or ppb)
Surface water
69
1.4
2022: 0.5 µg/L or ppb (0.5 µg/L or ppb)
2023: not detected (method detection limit
0.06–1 µg/L)
Surface water at
Superfund site
4
0
Not detected (method detection limit 0.2 µg/L
or ppb)
Sediment
0
0
Not applicable
Sediment at
Superfund site
0
0
Not applicable

VINYL CHLORIDE 152
5. POTENTIAL FOR HUMAN EXPOSURE
Table 5-5. Vinyl Chloride Detected in Samples Collected Throughout the United
States in 2022 and 2023
a
Type
Number of
samples
Positive
detections (%)
Average concentration (maximum)
Soil
0
0
Not applicable
Soil at Superfund site
28
0
Not detected (method detection limit 0.00045–
0.0011 mg/kg)
a
Samples reported are from January 2022 through June 2023.
Source: WQP 2023
Detections of vinyl chloride in air, water, and soil at NPL sites are summarized in Table 5-6.
Table 5-6. Vinyl Chloride Levels in Water, Soil, and Air of National Priorities List
(NPL) Sites
Medium
Median
a
Geometric mean
a
Geometric
standard
deviation
a
Number of
quantitative
measurements
NPL sites
Water (ppb)
34
55.9
17.1
505
266
Soil (ppb)
733
962
34.1
45
38
Air (ppbv)
1.6 (4.09 μg/m
3
)
3.25 (8.31 μg/m
3
)
26.4 (67.5 μg/m
3
)
56
37
a
Concentrations found in ATSDR site documents from 1981 to 2022 for 1,868 NPL sites (ATSDR 2022). Maximum
concentrations were abstracted for types of environmental media for which exposure is likely. Pathways do not
necessarily involve exposure or levels of concern.
5.5.1 Air
Air in rural/remote and urban/suburban areas of the United States typically contains very low or no
detectable amounts of vinyl chloride (EPA 1982b; Grimsrud and Rasmussen 1975a, 1975b; Harkov et al.
1984; Pratt et al. 2000; Stephens et al. 1986; Wallace et al. 1984). In a background air toxics
concentration study for North America conducted in 2001–2002, vinyl chloride concentrations were
estimated to be <0.02 μg/m
3
(<0.0075 ppbv) (McCarthy et al. 2006). In a residential region of Southwest
Memphis surrounded by fossil fuel burning, steel, refining, and food processing industries, vinyl chloride
was found in 38% of 103 samples at a mean concentration of 0.02 μg/m
3
(Jia and Foran 2013).
Concentrations in air samples collected in 2000–2017 at Denton Airport South, Texas (in close proximity
to the Barnett Shale location) were reported as 0.02–0.08 parts per billion carbon, which is equivalent to

VINYL CHLORIDE 153
5. POTENTIAL FOR HUMAN EXPOSURE
0.01–0.04 ppbv and in 19 positive detections out of 1,085 samples (Lim and John 2020). Vinyl chloride
was not detected (detection limit 0.1–0.14 μg/m
3
) in 58 ambient air or 4 indoor air data points compiled
for 2011–2021 from Palermo Wellfield Superfund Site, as reported in the EPA STORET database (WQP
2021). Based on limited sampling, vinyl chloride was not detected in ambient air samples collected in
2022 through June of 2023 from the WQP database (WQP 2023).
Vinyl chloride levels in atmospheric samples collected across the United States are available from the Air
Quality System (AQS), which is the EPA’s repository of ambient air quality data that has >5,000 active
monitors nationwide (EPA 2023c). The vinyl chloride levels have remained fairly consistent over the
period of 2020–2022 as indicated by data summarized in Table 5-7.
Table 5-7. Summary of Annual Concentrations of Vinyl Chloride in ppbv
Measured in Ambient Air at Locations Across the United States
a
Year
Number of monitoring
locations
Number of
observations
Range of arithmetic
mean
Maximum
2022
94
10,339
<LOD–0.68
6.05
2021
97
10,925
<LOD–0.68
6.05
2020
92
8,123
<LOD–0.88
5.40
a
Values initially reported in ppbC but converted to ppbv.
LOD = limit of detection
Vinyl chloride concentrations were reported at 0.12–12 μg/m
3
(0.047–4.56 ppbv) for flowback pits used
to store natural gas drilling hydraulic fracturing waste (Bloomdahl et al. 2014).
Monitoring of vinyl chloride levels in ambient air has been conducted in response to the East Palestine,
Ohio Train Derailment, which occurred on February 3, 2023. Real-time air monitoring began on
February 4, 2023, in 12 locations surrounding the fire and in neighboring communities (EPA 2023a,
2023e). All final samples were analyzed according to the EPA Toxic Organics-15 (TO-15) selected ion-
monitoring mode (SIM) method. TO-15 has reporting limits ranging from 0.029 to 0.074 µg/m
3
(EPA
1999). Of the 644 validated samples collected through June 7, 2023, in which vinyl chloride was
detected, there were 427 detections between February 6 and May 16, 2023 that were above the method
reporting limit, with concentrations ranging from 0.035 to 16 µg/m
3
(0.013–6.08 ppbv), an average of
0.69 µg/m
3
, and a median value of 0.32 µg/m
3
(EPA 2023b, EPA 2023d).
VINYL CHLORIDE 154
5. POTENTIAL FOR HUMAN EXPOSURE
5.5.2 Water
Vinyl chloride has been detected at varying concentrations in surface water, groundwater, and drinking
water throughout the United States (Tables 5-4 and 5-5). Vinyl chloride was not reported above the lower
quantification limit of 0.02–5.0 μg/L (ppb) in approximately 1,360 ambient surface water samples as
reflected in data points compiled for 2017–2021 from EPA’s STORET and NWIS databases (WQP
2021). Vinyl chloride was detected in approximately 2.3% of 43 surface water samples in 2022 at
average concentrations of ca. 0.5 ppb (maximum of 0.5 ppb) and was not detected in 26 surface water
samples collected through April 2023 (WQP 2023). Vinyl chloride was not detected in four samples of
surface water collected at Palermo Wellfield Superfund Site in 2022 (WQP 2023). Vinyl chloride was
detected in approximately 1% of 2,385 groundwater samples in 2022 and approximately 1% of
289 groundwater samples collected through April 2023 at average concentrations of ca. 12.4 ppb
(maximum of 61.3 ppb) and ca. 6.8 ppb (maximum of 7.7), respectively (WQP 2023). Vinyl chloride was
not detected in 226 samples of groundwater collected at Palermo Wellfield Superfund Site in 2022 (WQP
2023).
During an assessment of groundwater in the United States from 1985 to 2001, vinyl chloride was detected
at a median concentration of 1.1 µg/L (positive detections only) in samples obtained from >50 of the
nation’s most important river basins and aquifers (USGS 2006). It was also detected in 0.083% of
2,401 samples of domestic wells at a level of 0.20 µg/L and in 0.042% of samples at a level of 1 µg/L.
Vinyl chloride was not detected in any samples at assessment levels >5 µg/L. The median level of vinyl
chloride in these domestic wells (positive detections only) was 0.74 µg/L (USGS 2006). Bexfield et al.
(2022) summarized groundwater monitoring results from 1,531 wells and 6 springs sampled between
2013 to 2019 and found that vinyl chloride was detected most commonly in anoxic groundwater. Based
on the maximum contaminant level (MCL) of 2.0 µg/L, two wells had a benchmark quotient (BQ) of
>0.1 (maximum of 0.19), which accounted for an area proportion of <0.01% and none of the wells had
concentrations of vinyl chloride that resulted in a BQ of >1, indicating that vinyl chloride was not
detected above 2.0 µg/L in any samples.
Vinyl chloride was detected in 6 out of 518 monitoring wells sampled in 19 urban land-use watersheds in
the United States during a U.S. Geological Survey (USGS) analysis of groundwater contaminants
conducted from 1996 to 2002 (Squillace et al. 2004). The median level was reported as 0.2 µg/L and the
maximum concentration was 8.1 µg/L. Vinyl chloride was found in 1.12% of 448 groundwater supply
wells monitored from 2002 to 2009 across the United States at an assessment level of 0.05 µg/L and in
VINYL CHLORIDE 155
5. POTENTIAL FOR HUMAN EXPOSURE
0.89% of samples at an assessment level of 0.10 µg/L (USGS 2014). Vinyl chloride was detected in
254 of 6,838 (3.7%) groundwater data points compiled for 2017 to 2021 from EPA STORET and NWIS
databases at concentrations of 0.2 to 7,380 μg/L (WQP 2021). This includes data from hazardous waste
sites.
Vinyl chloride was detected at levels ranging from 11 to 23 ng/L in water samples collected from 15 PVC
or chlorinated polyvinyl chloride (CPVC)-utilizing homes located in Ithaca, New York (Walter et al.
2011). Most of the samples obtained from the homes tested negative for vinyl chloride, but each of the
positive detections occurred from homes using municipal (chlorinated) water and CPVC type pipe. A
report compiled by NSF International on the amount of vinyl chloride monomer measured during
certification testing of drinking water systems from January 1998 through October 2000 indicated that
there were no detectable levels (≥0.1 mg/kg) of vinyl chloride in 445 of 519 PVC pipe samples or 157 of
178 PVC fittings (Borrelli et al. 2005). The average residual vinyl chloride monomer found was
0.07 mg/kg in pipes tested and 0.03 mg/kg in the fittings tested when considering non-detects as zero.
During an assessment of drinking water sources from 2002 to 2010, vinyl chloride was not detected in
300 samples from 20 surface water sites across the United States (USGS 2014).
In a study of three landfills located in Orange County, Florida, vinyl chloride was detected in water
samples obtained at four of nine wells with average concentrations ranging from 2.0 to 26.5 μg/L
(Hallbourg et al. 1992). Vinyl chloride levels in 50 domestic wells located distal and proximal to shale-
gas wells in upland areas of Marcellus Shale region of New York and Pennsylvania were <0.06 μg/L
(ppb) (McMahon et al. 2019).
A groundwater monitoring study assessing correlation of household water from wells and springs near
active unconventional oil and gas (UOG) wells was conducted in the Appalachian Basin where active
UOG wells were present between July and September 2018 (Bradford County, Pennsylvania) or May to
August 2019 (Belmont and Monroe Counties, Ohio). Water samples were collected upstream from home
water treatment/filtration systems (Clark et al. 2022). The study authors reported that vinyl chloride was
detected above the LOD in 26% of Pennsylvania samples, with the median concentration below the LOD
and the interquartile range of <LOD–0.0004 µg/L (limit of quantification [LOQ] defined as 0.047 µg/L;
EPA method 624); in Ohio samples, 57% of detections were greater than the LOD; the median
concentration was 0.003 µg/L with the interquartile range of <LOD–0.023 µg/L (LOQ defined as
0.046 µg/L; EPA method 624). Based on spatial surrogate analysis there was no correlation observed
with proximity or distance to the nearest UOG well.

VINYL CHLORIDE 156
5. POTENTIAL FOR HUMAN EXPOSURE
Monitoring of vinyl chloride levels in water has been conducted in response to the East Palestine, Ohio
Train Derailment, which occurred on February 3, 2023. Surface water monitoring was conducted in
nearby creeks including Sulphur Run, Leslie Run, and waterways downstream of the Ohio River. All
final samples have been analyzed according to EPA method 8260D (EPA 2023a, 2023e), with a reporting
limit of 1 or 100 µg/L, or 0.27 mg/kg (EPA 2018a). Of the 14 validated samples collected through June
7, 2023, in which vinyl chloride was detected on February 8 and 10, 2023, there were six detections above
the method reporting limit with concentrations ranging from 1.1 to 2400 µg/L and one detection listed as
the free product in surface water at a concentration of 0.65 mg/kg (EPA 2023d, 2023f). Treated drinking
water samples tested did not show detections of vinyl chloride.
The majority of drinking water supplies in the United States do not contain detectable levels of vinyl
chloride (Borrelli et al. 2005; EPA 1982b; Westrick et al. 1984). As part of the Safe Drinking Water Act
(SDWA), EPA reviews each national primary drinking water regulation at least once every 6 years and
make revisions if necessary. Vinyl chloride monitoring data from public water supplies in the United
States as part of the 6-year reviews (1998–2005 and 2006–2011) is shown in Table 5-8 (EPA 2016).
Table 5-8. Safe Drinking Water Act (SDWA) 6-Year Reviews (1998–2005 and
2006–2011)
1998–
2005
Percent of
totals for
1998–2005
2006–2011
Percent of
totals for
2006–2011
Total number of samples
373,161
100
368,740
100
Total number of samples with detection >0.5 µg/L
550
0.15
725
0.20
Total number of samples with detection >2 µg/L
107
0.03
125
0.03
Average of samples with detection >0.5 µg/L
1.8
2.3
Average of samples with detection >2 µg/L
6.2
8.9
Source: EPA 2016
A U.S. survey that combined drinking water contaminant occurrence for all 50 states (2014–2019) and
5-year (2015–2019) population estimates from the U.S. Census Bureau’s American Community Survey
detected vinyl chloride in 122 out of 47,820 community water systems, with an average concentration of
0.5 ng/L. The U.S. population served by these systems is 2.1 million, which corresponds to a population-
weighted average contaminant concentration of 1.8 ng/L or 0.0018 µg/L (Uche et al. 2021).
VINYL CHLORIDE 157
5. POTENTIAL FOR HUMAN EXPOSURE
5.5.3 Sediment and Soil
Data (Table 5-4) from the EPA Great Lakes National Program reported vinyl chloride in one (1,300 ppb)
of 212 sediment samples collected in 2011–2021 at a level above the quantification or reporting limit of
1.2–7,300 ppb (WQP 2021). Vinyl chloride was not detected (detection limit not reported) in sediment
samples at any other sites reported in the EPA STORET database. Vinyl chloride was detected in 4 of
45 soil data points reported for 2011–2021 and included in the EPA STORET database, but not at levels
above the lower reporting level of 9.4–38 ppb (WQP 2021).
Monitoring of vinyl chloride levels in soil and sediment has been conducted in response to the East
Palestine, Ohio Train Derailment, which occurred on February 3, 2023. Soil and sediment samples were
collected at the derailment site. Soils were sampled at four locations near derailed train cars, which
contained hazardous materials, and sediments were sampled in two locations near the Sulphur Run
stream. All final samples were analyzed according to the EPA method 8260D (EPA 2023a, 2023e), with
reporting limits ranging from 0.0064 to 9 mg/kg (EPA 2018a). Of the nine validated samples collected
through June 7, 2023, in which vinyl chloride was detected, there were four detections on February 8
th
and 10
th
, 2023 (soil n=3; sediment n=1) above the method reporting limit with concentrations ranging
from 3.9 mg/kg (soil) to 11 mg/kg (sediment)
(average of 6.1 mg/kg, median of 4.8 mg/kg) (EPA 2023d,
2023g). Vinyl chloride was not detected above the method limits in sediment or soil samples collected in
2022 or 2023 at the Palermo Wellfield Superfund Site (WQP 2023).
5.5.4 Other Media
In the past, vinyl chloride was detected in various foods and bottled drinking water samples as a result of
migration from PVC food wrappings and containers (Benfenati et al. 1991; Gilbert et al. 1980). The Food
and Drug Administration (FDA) regulates the use of PVC polymers in food packaging materials and the
amount of residual monomer in polymers and as a result, there has been a significant reduction in the
reported levels of vinyl chloride in food samples based on data collected since the early 1970s (WHO
1999). Since the late 1970s, modifications to the vinyl chloride and PVC manufacturing and production
processes have greatly reduced the amount of residual vinyl chloride monomer in food packaging and
other PVC-related items.
To determine whether the residual vinyl chloride levels in PVC containing food packages in current use
are <10 ppb, a survey and analysis of PVC-containing food packages were conducted (McNeal et al.
VINYL CHLORIDE 158
5. POTENTIAL FOR HUMAN EXPOSURE
2003). The results showed that vinyl chloride levels found in the packages ranged from none detected
(<1 ppb) to about 275 ppb. The package containing 275 ppb residual vinyl chloride was not a food
contact material (McNeal et al. 2003). The Vinyl Institute presented results from an effort to assess
residual vinyl chloride monomer in food and non-food packaging materials that demonstrated that all but
one of the materials had residual levels <5 ppb; one sample from a plastic bottle of Turkish olive oil was
found to contain 28.3 ppb residual vinyl chloride monomer of which 0.6 ppb was identified in the oil
contained therein (Borrelli et al. 2005).
Dietary exposure to vinyl chloride from PVC packages used for food was estimated by several agencies
and based upon estimated average intakes in the United Kingdom and the United States, an exposure of
<0.0004 μg/kg/day was estimated for the late 1970s and early 1980s (WHO 1999). Because vinyl
chloride levels in food and drinking water are now well below detection limits, exposure levels from
ingestion are expected to be even lower.
Vinyl chloride at concentrations of 0.55–3.32 ppb (1.4–8.49 µg/m
3
) have been identified in the VOC
profile of surgical smoke samples collected from breast surgeries with the highest level of 3.32 ppb
(8.49 µg/m
3
) observed when using conventional electrosurgical knives and levels ranging from 0.6 to
1.62 ppb (1.5–4.14 µg/m
3
) when using other electrosurgical units (Cheng et al. 2021).
Vinyl chloride was identified as a constituent of chicken manure wastewater emissions at concentrations
of 3.8±0.20 ppm (9.7 mg/m
3
); the samples were collected from the influent of an anaerobic lagoon at a
chicken farm wastewater treatment plant in northern Thailand (Fakkaew et al. 2022).
Vinyl chloride has been detected in tobacco smoke. Cigarette smoke and smoke from small cigars was
found to contain 5.6–27.3 ng vinyl chloride per cigarette (Hoffmann et al. 1976). The study authors
suggested that the inorganic chloride concentrations in the tobacco determine the amount of vinyl chloride
formed upon combustion of tobacco and released with the smoke (Hoffmann et al. 1976). Vinyl chloride
was detected in cigarette smoke at levels ranging from 6.31 to 8.04 ng per cigarette for Electrically
Heated Cigarette Smoking Systems (EHCSS) and <12.4–18.0 ng per cigarette for conventional lit-end
cigarettes in a test using three versions of an EHCSS and four different brands of conventional cigarettes
under International Organization for Standardization smoking conditions (Zenzen et al. 2012). When
additional smoking regiments were utilized, smoke from conventional cigarettes was found to contain
vinyl chloride up to 34.8 ng per cigarette.
VINYL CHLORIDE 159
5. POTENTIAL FOR HUMAN EXPOSURE
Gas emissions from household aerosol products purchased from retail stores in Japan in 2017 (n=38 spray
paints) and 2021 (n=1 coating agent) were evaluated for the presence of vinyl chloride (Sugaya et al.
2023). Vinyl chloride was identified in the emissions of 3 out of 39 products tested at concentrations of
0.095, 0.098, and 0.28 µg/L (method LOQ = 0.095 µg/L).
5.6 GENERAL POPULATION EXPOSURE
A review of vapor intrusion data from 148 ATSDR public health assessments completed between 1994
and 2009 identified 42 sites with detected concentrations of vinyl chloride in groundwater, soil gas, or air
(Burk and Zarus 2013). Indoor air was sampled at 13 of the sites, with vinyl chloride detected at levels
ranging from 0.021 to 35 µg/m
3
, which are all below ATSDR’s inhalation MRLs (50–1,300 mg/m
3
).
Vinyl chloride was detected in groundwater at 31 of the sites ranging from 0.148 to 27,000 µg/L, and
14 of the sites had vinyl chloride groundwater concentrations at levels of concern from noncancer effects
from vapor intrusion. Twelve of the 14 sites with groundwater concentrations at levels of concern from
noncancer effects from vapor intrusion did not report measured indoor air concentrations for vinyl
chloride.
Vinyl chloride in water is expected to rapidly volatilize; thus, there is potential for inhalation exposure
during showering and bathing, and during other household water uses. ATSDR’s three-compartment
Shower and Household-Use Exposure (SHOWER) model predicts air concentrations in the shower stall,
bathroom, and main house throughout the day by estimating the contribution from showering or bathing
and the contribution from other water sources in the house, such as the dishwasher, clothes washer, and
sink faucets. This information along with human activity patterns are used to calculate a daily time-
weighted average exposure concentration via inhalation exposure and from dermal uptake from skin
contact. ATSDR’s SHOWER model is available by sending a request to showermode[email protected].
Table 5-9 displays the calculated reasonable maximum exposure (RME) levels for groups exposed to
vinyl chloride using the most conservative representative treated water levels (0.0018 µg/L; Uche et al.
2021) from Section 5.5.2 and representative outdoor air levels (0.68 ppb; EPA 2023c) in Section 5.5.1.
For a 15-minute exposure time, the SHOWER model predicts that the average human exposure
concentration from showering is 1.8 µg/m
3
accounting for 1.1% exposure and that the average human
exposure concentration of 1.7 µg/m
3
from bathroom use after showering and from usage of the main
house accounts for 0.35 and 99% exposure, respectively (ATSDR 2023).

VINYL CHLORIDE 160
5. POTENTIAL FOR HUMAN EXPOSURE
Table 5-9. Reasonable Maximum Exposure Daily Inhalation Dose in µg/kg/day
and Administered Dermal Dose of Chloroethane for the Target Person
Exposure group
Inhalation
Dermal
Birth–<1 year
1.2
6.2x10
-6
1–<2 years
1.3
5.7 x10
-6
2–<6 years
1.0
4.9 x10
-6
6–<11 years
0.66
4.0 x10
-6
11–<16 years
0.47
3.2 x10
-6
16–<21 years
0.40
3.0 x10
-6
Adults
0.33
2.9 x10
-6
Pregnant and breastfeeding women
0.53
2.9 x10
-6
Source: ATSDR 2023
A study investigated the potential correlations and associations of vinyl chloride concentrations and
detections in household water from wells and springs in Ohio and near (<10 km) previously active UOG
wells (Clark et al. 2022). There was no correlation (rho=0.04, p>0.05) with vinyl chloride concentration
and distance to the nearest UOG well in Ohio (Clark et al. 2022). The median concentration of 0.003 µg
vinyl chloride/L was reported in 57% of the wells or springs that supplied water to 161 Ohio homes
(Clark et al. 2022). However, Clark et al. (2022) did not find associations in odds ratios for detecting
vinyl chloride with distance in either Pennsylvania (0.71, 95% confidence interval [CI]: 0.33, 1.53) or
Ohio (1.08, 95% CI 0.85, 1.37) homes.
Inhalation of ambient or workplace air containing vinyl chloride is the most likely route of exposure for
the general population. Typical values for the average daily intake of vinyl chloride by inhalation in
urban/suburban and rural/remote areas not near emission sources are very small, since only trace levels of
vinyl chloride are usually found in ambient air. While industry emissions of vinyl chloride have generally
decreased (Ernes and Griffin 1996; TRI21 2023b), workers involved in the production or use of vinyl
chloride are likely to be exposed to levels of vinyl chloride greater than the levels that the general public
may be exposed to (TRI21 2023b).
Individuals located near or downwind of production facilities, hazardous waste disposal sites, and
landfills may be exposed to atmospheric levels of vinyl chloride higher than ambient background levels.
Concentrations around <5–30.7 μg/m
3
(<0.002–0.012 ppm) were measured in the air above some landfills
(Baker and MacKay 1985; Stephens et al. 1986). Homes near one hazardous waste site in southern
California were found to contain levels as high as 1,040 μg/m
3
(0.4 ppm) of vinyl chloride (Stephens et al.
VINYL CHLORIDE 161
5. POTENTIAL FOR HUMAN EXPOSURE
1986) and homes near another site contained levels between 2.6 and 23.4 μg/m
3
(0.001–0.009 ppm)
(Miller and Beizer 1985). These concentrations are several times greater than ambient air levels that are
generally <0.02 μg/m
3
(McCarthy et al. 2006).
Cigarette smoke and smoke from small cigars have been found to contain vinyl chloride at levels of 5.6–
27 ng per cigarette (Hoffmann et al. 1976) and as high as 34.8 ng per cigarette from conventional
cigarettes utilizing human puffing behavior (Zenzen et al. 2012). Therefore, people who smoke may be
potentially exposed to higher levels of vinyl chloride than nonsmokers.
Children are likely to be exposed to vinyl chloride via the same pathways that affect non-occupationally
exposed adults; namely inhalation of ambient air and ingestion of food items or drinking water that may
contain low levels of vinyl chloride. According to the information from Chemical Data Reporting (CDR)
for 2020, there are no reported consumer or commercial uses of vinyl chloride in products intended for
children from reporting facilities in the United States; data for 2012 and 2016 include one facility in the
United States where the use intended for children’s products is unknown or not reasonably ascertainable
(ChemView 2023; EPA 2021). No body burden studies that quantitatively or qualitatively identified
vinyl chloride in children were located.
5.7 POPULATIONS WITH POTENTIALLY HIGH EXPOSURES
In the past, airborne levels of vinyl chloride in occupational settings were often >200 ppmv; however,
beginning in 1974, Occupational Safety and Health Administration (OSHA) regulations resulted in
increased engineering controls in the PVC manufacturing process, which have reduced airborne levels to
1–2 ppmv (Borrelli et al. 2005).
Workers who are involved in welding applications that use PVC pipes or other PVC materials may
potentially be exposed to higher levels of vinyl chloride than the general population. Welding PVC
containing material produces fumes that may contain vinyl chloride; however, exposure is expected to be
limited and minimized by process control methods and good practice. In an older PVC thermal welding
study that varied welding temperature and environmental conditions, vinyl chloride levels in air were
below the detection limit (0.05–0.2 ppm [0.13–0.51 mg/m
3
]) (Williamson and Kavanagh 1987). The
highest levels were observed under normal welding with reduced ventilation (0.2 ppm) and during severe
heating without ventilation (<0.5–0.1ppm). Ernes and Griffin (1996) evaluated VOC emissions from
VINYL CHLORIDE 162
5. POTENTIAL FOR HUMAN EXPOSURE
PVC extrusion processes using a resin mixture continuously fed into the extruder under normal operating
conditions. The study authors found no evidence of vinyl chloride.
The exposure concentration of vinyl chloride for employees working near flowback pits in the Marcellus
Shale natural gas drilling sites was determined to be 0.028–2.8 μg/m
3
(0.011–1.096 ppb) (Bloomdahl et
al. 2014).
In the United States, vinyl chloride is an OSHA regulated substance. Current OSHA regulations impose a
permissible exposure limit (PEL) of 1 ppm (2.6 mg/m
3
) averaged over an 8-hour period or a short-term
exposure of no more than 5 ppm over a 15-minute period (Cowfer and Gorensek 2006). Where
concentrations cannot be lowered below the PEL of 1 ppm, employers must create an area with controlled
access and a respirator program conforming to OSHA standards.
VINYL CHLORIDE
CHAPTER 6. ADEQUACY OF THE DATABASE
Section 104(i)(5) of CERCLA, as amended, directs the Administrator of ATSDR (in consultation with the
Administrator of EPA and agencies and programs of the Public Health Service) to assess whether
adequate information on the health effects of vinyl chloride is available. Where adequate information is
not available, ATSDR, in conjunction with NTP, is required to assure the initiation of a program of
research designed to determine the adverse health effects (and techniques for developing methods to
determine such health effects) of vinyl chloride.
Data need
s are defined as substance-specific informational needs that, if met, would reduce the
uncertainties of human health risk assessment. This definition should not be interpreted to mean that all
data needs discussed in this section must be filled. In the future, the identified data needs will be
evaluated and prioritized, and a substance-specific research agenda will be proposed.
6.1 EXI
STING INFORMATION ON HEALTH EFFECTS
Studi
es evaluating the health effects of inhalation, oral, and dermal exposure of humans and animals to
vinyl chloride that are discussed in Chapter 2 are summarized in Figure 6-1. The purpose of this figure is
to illustrate the information concerning the health effects of vinyl chloride. The number of human and
animal studies examining each endpoint is indicated regardless of whether an effect was found and the
quality of the study or studies.
6.2 IDENTIFICATION OF DATA NEEDS
Mis
sing information in Figure 6-1 should not be interpreted as a “data need.” A data need, as defined in
ATSDR’s Decision Guide for Identifying Substance-Specific Data Needs Related to Toxicological
Profiles (ATSDR 1989), is substance-specific information necessary to conduct comprehensive public
health assessments. Generally, ATSDR defines a data gap more broadly as any substance-specific
information missing from the scientific literature.
163

VINYL CHLORIDE 164
6. ADEQUACY OF THE DATABASE
Figure 6-1. Summary of Existing Health Effects Studies on Vinyl Chloride by
Route and Endpoint*
Cancer, hepatic, and neurological effects were the most studied endpoints
The majority of the studies examined inhalation exposure in humans (versus animals)
*Includes studies discussed in Chapter 2; the number of studies include those
finding no effect. No dermal studies in humans or animals were located. Most
studies examined multiple endpoints.
VINYL CHLORIDE 165
6. ADEQUACY OF THE DATABASE
Acute-Duration MRLs. The inhalation database is adequate to derive an acute-duration inhalation
MRL. The oral database is inadequate to derive an acute-duration oral MRL (no acute-duration oral
studies are available). Acute-duration oral studies providing data at low doses are needed.
Intermediate-Duration MRLs. The inhalation database is adequate to derive an intermediate-duration
inhalation MRL. The oral database is inadequate to derive an intermediate-duration oral MRL (no
intermediate-duration oral studies were available). Intermediate-duration oral studies providing data at
low doses are needed.
Chronic-Duration MRLs. The inhalation database is inadequate to derive a chronic-duration
inhalation MRL as data for the most likely sensitive effect (hepatic) was not reported for noncancer
effects in chronic-duration studies. Chronic-duration inhalation studies providing data on noncancer liver
effects at low doses are needed. The oral database is adequate to derive a chronic-duration MRL.
Health Effects. Identification of data needs for health effects in animal studies is limited to targets
included in the systematic review.
Hepatic Toxicity. Hepatic effects are fairly well studied in humans. Liver effects in animals
have been characterized in acute- and intermediate-duration inhalation studies and chronic-
duration oral studies. Data on potential noncancer hepatic effects following chronic-duration
inhalation exposure and acute- and intermediate-duration oral exposure may be helpful.
Immunotoxicity. Studies of workers occupationally exposed to vinyl chloride suggest that an
autoimmune-like syndrome may develop. Immunotoxicity studies in animals that are known to
have a propensity for developing autoimmune diseases may be useful in further evaluating this
syndrome.
Neurotoxicity. Vinyl chloride is a central nervous system depressant following brief high-level
inhalation exposures in humans. Limited animal studies found degenerative effects in central
nervous system tissue following chronic-duration inhalation exposure to high levels of vinyl
chloride. A study examining the effects of a range of lower doses would be informative. In
addition, studies present suggestive evidence that vinyl chloride may also produce peripheral
nerve damage in humans exposed chronically via inhalation. Animal studies examining
histopathological and electrophysiological endpoints in peripheral nerves would be helpful for
VINYL CHLORIDE 166
6. ADEQUACY OF THE DATABASE
assessing what doses may be associated with this effect. Epidemiological studies examining
exposed populations for subclinical peripheral nerve damage would also be helpful.
Developmental Toxicity. Older epidemiological studies that addressed developmental
toxicity in offspring of vinyl chloride workers have limitations. A few recent case-control studies
evaluated the association between potential developmental effects and exposure to multiple
compounds in air and drinking water during pregnancy; these found no effects. Additional,
multiple- and low-dose concentration exposures in animal studies may help to further elucidate
potential developmental effects and whether a dose-response exists. There are no developmental
studies for oral exposures. Because of this deficiency, oral studies examining a range of
developmental end points would be useful in assessing the possibility of these effects in humans.
Epidemiology and Human Dosimetry Studies. Virtually all of the data on effects in humans
following inhalation exposure to vinyl chloride come from epidemiological studies of workers exposed
during the production of PVC. Many of these studies are limited by the absence of information on
individual exposure levels. In North America and Western Europe, only limited numbers of females have
been studied. For the most part, studies examining the carcinogenic potential of vinyl chloride are
adequate to distinguish an increased incidence of the rare cancer, angiosarcoma. However, many studies
used cohorts that were too small to detect increases in other types of cancer (respiratory, central nervous
system, lymphatic, or hematopoietic). Epidemiological studies designed to investigate reproductive and
developmental effects of vinyl chloride have not been useful, in part because of a poor choice of statistical
analysis, inadequate controls, lack of effects due to current low levels of exposure, or failure to account
for nutritional status and exposures to other chemicals. Additional cohort studies of these end points
would be useful for examining these effects in humans.
Biomarkers of Exposure and Effect. Vinyl chloride measured in expired air is an adequate
indicator of recent, moderate-to-high-level exposures. However, for low-level exposures or exposures
that occur over 1–2 hours prior to the time of measurement, this biomarker is not useful. Thiodiglycolic
acid, a major urinary metabolite of vinyl chloride, has been used to monitor workers occupationally
exposed to vinyl chloride; however, this biomarker is rapidly excreted and not specific for vinyl chloride;
because it may also be produced as a result of the metabolism of 1,1-dichloroethene, ethylene oxide, or
2,2-dichloroethylether. The DNA adducts 1,N
6
-ethenoadenosine and 3,N
4
-ethenocytidine, remain in the
body longer than free vinyl chloride or thiodiglycolic acid; however, these adducts may also result from
exposure to other compounds (e.g., vinyl bromide, ethyl carbamate, acrylonitrile, 2-cyanoethylene,
VINYL CHLORIDE 167
6. ADEQUACY OF THE DATABASE
1,2-dichloroethane). Studies attempting to identify a metabolite more specific to vinyl chloride may be
helpful in developing a biomarker suitable for improved medical surveillance, thereby useful for early
detection and initiation of possible treatment.
Vinyl chloride-induced genetic alterations have been identified in the Ki-ras oncogene and the p53 tumor
suppressor gene. Oncoproteins and p53 antibodies have been detected in the serum of cancer patients
with angiosarcoma. Statistical analyses suggest a relationship between vinyl chloride exposure and the
presence of these serum biomarkers. Further investigation into the ability of these assays to predict
individuals at increased risk for developing angiosarcoma of the liver would be useful. Further work
identifying the correlation between specific DNA adducts and genotoxicity would also be useful.
Absorption, Distribution, Metabolism, and Excretion. There are few data on humans for all
toxicokinetic parameters across all exposure routes. There are a number of animal studies describing the
absorption, distribution, metabolism, and excretion of vinyl chloride administered via the oral route and
the inhalation route but few describing the toxicokinetics of vinyl chloride administered via the dermal
route. No information is available regarding dermal absorption of vinyl chloride from liquid or solid
media (i.e., water, soil). Dermal exposure from these media is expected to be minimal; however, a study
confirming this assumption would be useful. Furthermore, the intermediary metabolites of vinyl chloride
appear to be responsible for many of the toxic effects observed. Therefore, information regarding
differences in the metabolic pattern according to sex, age, nutritional status, and species and correlations
to differences in health effects would also be useful.
Comparative Toxicokinetics. The absorption, distribution, metabolism, and excretion of vinyl
chloride have been studied in animals but information on toxicokinetics in humans is extremely limited.
Human and animal data indicate that similar target organs (liver, central nervous system) for the toxic
effects of vinyl chloride exist, suggesting some similarities of kinetics. Limited information is available
regarding interspecies differences in kinetics. Most toxicokinetic studies have been conducted using rats,
but one study in primates indicates that metabolism may saturate at lower concentrations in primates than
rats. This could suggest a lower saturation point in humans also. Modeling studies might continue to
provide information on the toxicokinetics of vinyl chloride in humans.
Children’s Susceptibility. Data needs relating to prenatal exposure and developmental effects are
discussed in the Developmental Toxicity subsection above. Carcinogenicity studies with animals suggest
that younger animals may be more sensitive to the toxicity and carcinogenicity of vinyl chloride than
VINYL CHLORIDE 168
6. ADEQUACY OF THE DATABASE
mature animals. Further mechanistic research may be useful in establishing the mechanism of early life
stage sensitivity in laboratory animals and determining whether it is anticipated to be relevant to humans.
For example, the human embryonic liver does not express CYP2E1, but its expression rapidly increases
during the first 24 hours after birth. Between the developmental ages of 1 and 10 years, children’s
CYP2E1 protein levels and enzyme activity are comparable to adults (EPA 2000). No studies were
located that specifically address the toxicokinetics of vinyl chloride in children; however, the
toxicokinetic behavior of vinyl chloride in children is expected to be similar to that in adults. Further
information on the toxicokinetics and toxicodynamics of vinyl chloride and metabolites during
pregnancy, lactation, and early childhood would be valuable.
Physical and Chemical Properties. The physical and chemical properties of vinyl chloride are
sufficiently well characterized to permit estimation of its environmental fate (Amoore and Hautala 1983;
Cowfer and Gorensek 2006; EPA 1985a; Fire 1986; IARC 2012; Lewis 1996; Lyman et al. 1982; NLM
2023).
Production, Import/Export, Use, Release, and Disposal. Vinyl chloride is released primarily to
the atmosphere via emissions from vinyl chloride and PVC manufacturing facilities (Hartmans et al.
1985; SRI 1990a, 1990b, 1993, 1994; TRI21 2023a). The risk of exposure to vinyl chloride is highest for
workers in the plastics industry and populations living near industrial areas or hazardous waste sites.
Production, use, and manufacturing methods are well described in the literature (Cowfer and Magistro
1985; NLM 2023; IARC 2012; SRI 1990a, 1990b, 1993, 1994; TRI21 2023a; USITC 1994). More
current information on releases and disposal methods might assist in estimating potential exposures to
vinyl chloride, particularly for populations living near hazardous waste sites.
Environmental Fate. Vinyl chloride primarily partitions to the air where it is degraded relatively
quickly by photochemically produced hydroxyl radicals (Kwok and Atkinson 1995). It is removed from
surface water and soils mainly by volatilization and photodegradation (EPA 1976). Biodegradation and
hydrolysis also occur (Barrio-Lage et al. 1990; Castro et al. 1992a, 1992b; Davis and Carpenter 1990;
EPA 1976; Gossett 2010), but these reactions are generally slow as compared to the volatilization rate.
Bacterial communities capable of degrading vinyl chloride in aquatic environments under both aerobic
and anaerobic conditions have been identified (Coleman and Spain 2003; Coleman et al. 2002; Danko et
al. 2006; Puentes Jacome et al. 2019; Richards et al. 2022; Zalesak et al. 2021). More information
regarding the transformation and degradation in soil and water would be helpful for defining the potential
pathways for human exposure.
VINYL CHLORIDE 169
6.
ADEQUACY OF THE DATABASE
B
ioavailability from Environmental Media. Vinyl chloride can be absorbed following inhalation
(Bolt et al. 1977; Krajewski et al. 1980; Withey 1976), oral (Feron et al. 1981; Watanabe et al. 1976a;
Withey 1976), and to a much lesser extent, dermal exposure (Hefner et al. 1975a). These routes of
exposure may be of concern to humans because of the potential for vinyl chloride to contaminate air
(Bloomdahl et al. 2014; Gordon and Meeks 1977; Jia and Foran 2013; Lim and John 2020; McCarthy et
al. 2006; Stephens et al. 1986), water (McMahon et al. 2019; Squillace et al. 2004; USGS 2006, 2014;
Walter et al. 2011), and food (Gilbert et al. 1980; McNeal et al. 2003). Information regarding the
bioavailability from ingestion and dermal contact with contaminated soils would be helpful, particularly
for populations living near hazardous waste sites, although vinyl chloride is not believed to be
considerably absorbed through skin.
Food C
hain Bioaccumulation. Vinyl chloride can bioconcentrate to a limited extent in aquatic
organisms (EPA 1982a; Freitag et al. 1985). Biomagnification of vinyl chloride in terrestrial and aquatic
food chains does not appear to be important because of its high volatility and the fact that it is readily
metabolized by higher-trophic-level organisms (Freitag et al. 1985; Lu et al. 1977). No data were located
regarding biomagnification in terrestrial food chains.
E
xposure Levels in Environmental Media. Vinyl chloride has been detected in air, water,
sediment, soil, cigarette smoke, and food (references in Section 5.5). Site-specific data on concentrations
of vinyl chloride in air, soil, and water would be helpful in estimating the risk of exposure for populations
living in the vicinity of hazardous waste sites. Data on the extent of release of vinyl chloride from PVC
pipes has been reported (Borrelli et al. 2005). Data on the potential release of vinyl chloride from car
interiors would assist the estimation of the risk of exposure of the general population.
E
xposure Levels in Humans. Vinyl chloride has been detected in exhaled breath of humans (Baretta
et al. 1969; Conkle et al. 1975), but no other body burden studies are available. More information on
exposure levels for populations living in the vicinity of hazardous waste sites would be helpful. This
information is necessary for assessing the need to conduct health studies on these populations. It is noted
that it is difficult to directly analyze for vinyl chloride in humans, which may limit the practicality of
conducting these tests.
Exposures of Children. No data exist regarding the levels of vinyl chloride in children. Children are
exposed to vinyl chloride by the same pathways that affect adults; inhalation of ambient air and the

VINYL CHLORIDE 170
6. ADEQUACY OF THE DATABASE
ingestion of foods or drinking water. Data regarding the use of PVC in children’s products is limited; as
of 2012, no determinative use of PVC in products intended for children has been reported in the United
States. According to information from CDR for 2020, there are no reported consumer or commercial uses
of vinyl chloride in products intended for children from reporting facilities in the United States. Data for
2012 and 2016 include one facility in the United States where the use intended for children’s products is
unknown or not reasonably ascertainable (ChemView 2023; EPA 2021). Data regarding global product
of products intended for children would be useful. Quantitative determination of residual vinyl chloride
monomer that can be extracted or emitted from children’s products produced with PVC would assist in
estimating potential exposure to children.
6.3 ONGOING STUDIES
There are several ongoing studies evaluating the potential adverse effects of vinyl chloride exposure in
humans and laboratory animals, as well as underlying mechanisms of toxicity (Table 6-1).
Table 6-1. Ongoing Studies on Vinyl Chloride
Investigator
Affiliation
Research description
Sponsor
Human, animal, and mechanistic research
Matthew C. Cave
University of Louisville
Collaborative research program, the
Environmental Liver Disease Revolutionizing
Innovative, Visionary Environmental Health
Research Program (ELD-RIVER)
NIEHS
Animal toxicity studies (some with associated mechanistic studies)
Christopher J.
States
University of Louisville
Multidisciplinary research on multi-organ
toxicology, cancer, and neurodevelopmental
effects of industrial chemicals.
NIEHS
Arun Kumar
Pandiri
NIEHS
Evaluation of the genomic and epigenomic
alterations in chemical carcinogenesis
studies using in vitro and in vivo models
NIEHS
Juliane Beier
University of Pittsburgh
Study mitochondrial dysfunction,
endoplasmic reticulum stress and autophagy
as mechanisms of nonalcoholic fatty liver
disease modified by vinyl chloride
NIDDK

VINYL CHLORIDE 171
6.
ADEQUACY OF THE DATABASE
Table 6-1. Ongoing Studies on Vinyl Chloride
Investigator
Affiliation
Research description
Sponsor
Mechanistic studies
Deyu Li
University of Rhode
Island
Investigate key mechanisms and critical
differences that influence repair of the
etheno DNA adducts and how cells minimize
the harmful consequences of these lesions
NIGMS
DNA = deoxyribonucleic acid; NIDDK = National Institute of Diabetes and Digestive and Kidney Diseases;
NIEHS = National Institute of Environmental Health Sciences; NIGMS = National Institute of General Medical
Sciences
Source: National Institute of Health (NIH) RePORTER 2023

VINYL CHLORIDE 172
CHAPTER 7. REGULATIONS AND GUIDELINES
Pertinent international and national regulations, advisories, and guidelines regarding vinyl chloride in air,
water, and other media are summarized in Table 7-1. This table is not an exhaustive list, and current
regulations should be verified by the appropriate regulatory agency.
ATSDR develops MRLs, which are substance-specific guidelines intended to serve as screening levels by
ATSDR health assessors and other responders to identify contaminants and potential health effects that
may be of concern at hazardous waste sites. See Section 1-3 and Appendix A for detailed information on
the MRLs for vinyl chloride.
Table 7-1. Regulations and Guidelines Applicable to Vinyl Chloride
Agency
Description
Information
Reference
Air
EPA
RfC
1x10
-1
mg/m
3
(0.04 ppm)
EPA 2000
WHO
Indoor air quality guidelines
No data
WHO 2010
Ambient air quality guidelines
WHO 2000
10
-6
Cancer risk
1 μg/m
3
Water & Food
EPA
Drinking water standards and health
advisories
EPA 2018b
1-Day health advisory (10-kg child)
3 mg/L
10-Day health advisory (10-kg child)
3 mg/L
DWEL
0.1 mg/L
10
-4
Cancer risk
0.002 mg/L
National primary drinking water regulations
EPA 2009
MCL
0.002 mg/L
PHG
0 mg/L
RfD
3x10
-3
mg/kg/day
EPA 2000
WHO
Drinking water quality guidelines
0.0003 mg/L
WHO 2022
FDA
Substances Added to Food
a
Vinyl chloride
monomer not listed
FDA 2022
Allowable level in bottled water
0.002 mg/L
FDA 2017
Cancer
HHS
Carcinogenicity classification
Known to be a human
carcinogen
NTP 2021

VINYL CHLORIDE 173
7. REGULATIONS AND GUIDELINES
Table 7-1. Regulations and Guidelines Applicable to Vinyl Chloride
Agency
Description
Information
Reference
EPA
Carcinogenicity classification
Known human
carcinogen by inhalation
and oral exposure
routes; highly likely
human carcinogen by
dermal exposure route
EPA 2000
Oral slope factor (continuous lifetime exposure
during adulthood)
7.2x10
-1
per mg/kg/day
Oral slope factor (continuous lifetime exposure
from birth)
1.4 per mg/kg/day
IARC
Carcinogenicity classification
Group 1
b
IARC 2012
Occupational
OSHA
PEL (8-hour TWA) for general industry,
shipyards, and construction
1 ppm
OSHA 2021a,
2021b, 2021c
Ceiling PEL (15-minute TWA) for general
industry, shipyards, and construction
5 ppm
NIOSH
REL (up to 10-hour TWA)
No data
c
NIOSH 2019
Emergency Criteria
EPA
AEGLs-air
EPA 2018c
AEGL 1
d
10-minute
450 ppm
30-minute
310 ppm
60-minute
250 ppm
4-hour
140 ppm
8-hour
70 ppm
AEGL 2
d
10-minute
2,800 ppm
30-minute
1,600 ppm
60-minute
1,200 ppm
4-hour
820 ppm
8-hour
820 ppm
AEGL 3
d
10-minute
12,000 ppm
e
30-minute
6,800 ppm
e
60-minute
4,800 ppm
e
4-hour
3,400 ppm
8-hour
3,400 ppm
VINYL CHLORIDE 174
7. REGULATIONS AND GUIDELINES
Table 7-1. Regulations and Guidelines Applicable to Vinyl Chloride
Agency
Description
Information
Reference
DOE
PACs-air
DOE 2015
PAC-1
f
250 ppm
PAC-2
f
1,200 ppm
PAC-3
f
4,800 ppm
e
a
The Substances Added to Food inventory replaces EAFUS and contains the following types of ingredients: food and
color additives listed in FDA regulations, flavoring substances evaluated by FEMA or JECFA, GRAS substances
listed in FDA regulations, substances approved for specific uses in food prior to September 6, 1958, substances that
are listed in FDA regulations as prohibited from use in food, delisted color additives, and some substances "no longer
FEMA GRAS."
b
Group 1: carcinogenic to humans.
c
Potential occupational carcinogen.
d
Definitions of AEGL terminology are available from EPA (2018d).
e
Greater than or equal to 10% of the Lower Explosion Limit. Safety considerations against the hazard of explosion
must be taken into account.
f
Definitions of PAC terminology are available from DOE (2023).
AEGL = acute exposure guideline levels; DOE = Department of Energy; DWEL = drinking water equivalent level;
EAFUS = Everything Added to Food in the United States; EPA = Environmental Protection Agency; FDA = Food and
Drug Administration; FEMA = Flavor and Extract Manufacturers Association of the United States; GRAS = generally
recognized as safe; HHS = Department of Health and Human Services; IARC = International Agency for Research on
Cancer; JECFA = Joint Food and Agriculture Organization/WHO Expert Committee on Food Additives;
MCL = maximum contaminant level; NIOSH = National Institute for Occupational Safety and Health; NTP = National
Toxicology Program; OSHA = Occupational Safety and Health Administration; PAC = protective action criteria; PEL =
permissible exposure limit; PHG = public health goal; REL = recommended exposure limit; RfC = inhalation reference
concentration; RfD = oral reference dose; TWA = time-weighted average; WHO = World Health Organization
VINYL CHLORIDE 175
CHAPTER 8. REFERENCES
Adkins B, Van Stee EW, Simmons JE, et al. 1986. Oncogenic response of strain A/J mice to inhaled
chemicals. J Toxicol Environ Health 17(2-3):311-322.
https://doi.org/10.1080/15287398609530825.
Ahn J, Rao G, Mamun M, et al. 2020. Soil–air partitioning of volatile organic compounds into soils with
high water content. Environ Chem 17(8):545-557. https://doi.org/10.1071/en20032.
Akasaka S, Langouet S, Fink S, et al. 1997. Site-specific mutagenesis with two vinyl chloride-derived
DNA adducts and comparison to homologs. Proc Am Assoc Cancer Res 38:124.
Albertini R, Clewell H, Himmelstein MW, et al. 2003. The use of non-tumor data in cancer risk
assessment: reflections on butadiene, vinyl chloride, and benzene. Regul Toxicol Pharmacol
37(1):105-132. https://doi.org/10.1016/s0273-2300(02)00019-3.
Alkhouri N, Mansoor S, Giammaria P, et al. 2014. The development of the pediatric NAFLD fibrosis
score (PNFS) to predict the presence of advanced fibrosis in children with nonalcoholic fatty liver
disease. PLoS ONE 9(8):e104558. https://doi.org/10.1371/journal.pone.0104558.
Amoore JE, Hautala E. 1983. Odor as an aid to chemical safety: Odor thresholds compared with
threshold limit values and volatilities for 214 industrial chemicals in air and water dilution. J Appl
Toxicol 3:272-290. https://doi.org/10.1002/jat.2550030603.
Anders LC, Lang AL, Anwar-Mohamed A, et al. 2016a. Vinyl chloride metabolites potentiate
inflammatory liver injury caused by LPS in mice. Toxicol Sci 151(2):312-323.
https://doi.org/10.1093/toxsci/kfw045.
Anders LC, Yeo H, Kaelin BR, et al. 2016b. Role of dietary fatty acids in liver injury caused by vinyl
chloride metabolites in mice. Toxicol Appl Pharmacol 311:34-41.
https://doi.org/10.1016/j.taap.2016.09.026.
Anderson D. 1999. Factors contributing to biomarker responses in exposed workers. Mutat Res 428(1-
2):197-202. https://doi.org/10.1016/s1383-5742(99)00047-2.
Anderson D. 2000. Examination of various biomarkers after occupational exposure to different
chemicals. NATO ASI Ser A Life Sci 313:94-100.
Anderson D, Richardson CR. 1981. Issues relevant to the assessment of chemically induced
chromosome damage in vivo and their relationship to chemical mutagenesis. Mutat Res 90:261-272.
https://doi.org/10.1016/0165-1218(81)90006-9.
Anderson D, Hodge MC, Purchase IF. 1976. Vinyl chloride: dominant lethal studies in male CD-1 mice.
Mutat Res 40(4):359-370. https://doi.org/10.1016/0165-1218(76)90034-3.
Anderson D, Richardson CR, Weight TM, et al. 1980. Chromosomal analyses in vinyl chloride exposed
workers. Results from analysis 18 and 42 months after an initial sampling. Mutat Res 79(2):151-
162. https://doi.org/10.1016/0165-1218(80)90083-x.
Anderson D, Richardson CR, Purchase IF, et al. 1981. Chromosomal analysis in vinyl chloride exposed
workers: comparison of the standard technique with the sister-chromatid exchange technique. Mutat
Res 83(1):137-144. https://doi.org/10.1016/0027-5107(81)90078-6.
Andrews AW, Zawistowski ES, Valentine CR. 1976. A comparison of the mutagenic properties of vinyl
chloride and methyl chloride. Mutat Res 40(3):273-276. https://doi.org/10.1016/0165-
1218(76)90054-9.
Asakura M, Sasaki T, Sugiyama T, et al. 2008. An improved system for exposure of cultured
mammalian cells to gaseous compounds in the chromosomal aberration assay. Mutat Res
652(2):122-130. https://doi.org/10.1016/j.mrgentox.2008.01.004.
Atkinson R. 1991. Kinetics and mechanisms of the gas-phase reactions of the NO3 radical with organic
compounds. J Phys Chem Ref Data 20(3):459-507. https://doi.org/10.1063/1.555887.
ATSDR. 1989. Decision guide for identifying substance-specific data needs related to toxicological
profiles; Notice. Fed Regist 54(174):37618-37634.
VINYL CHLORIDE 176
8. REFERENCES
Andersen ME, Krishnan K. 1994. Relating in vitro to in vivo exposures with physiologically based
tissue dosimetry and tissue response models. In: Salem H, ed. Animal test alternatives:
Refinement, reduction, replacement. New York, NY: Marcel Dekker, Inc., 9-25.
ATSDR. 2007. Interaction profile for: Chloroform, 1,1-dichloroethylene, trichloroethylene, and vinyl
chloride. Agency for Toxic Substances and Disease Registry.
https://www.atsdr.cdc.gov/interactionprofiles/ip-13/ip13.pdf. August 26, 2021.
ATSDR. 2022. Vinyl chloride. Full SPL data. Substance priority list (SPL) resource page. Agency for
Toxic Substances and Disease Registry. https://www.atsdr.cdc.gov/spl/resources/index.html. July
19, 2023.
ATSDR. 2023. Sanders2015 HLCs used in SHOWER Model v3 and PHAST. Atlanta, GA: Agency for
Toxic Substances and Disease Registry. https://www.atsdr.cdc.gov/pha-
guidance/toolbox/ATSDR_SHOWER_Model_v3_0_0.zip. July 13, 2023.
Attarchi MS, Aminian O, Dolati M, et al. 2007. Evaluation of liver enzyme levels in workers exposed to
vinyl chloride vapors in a petrochemical complex: a cross-sectional study. J Occup Med Toxicol
2:6. https://doi.org/10.1186/1745-6673-2-6.
Awara WM, El-Nabi SH, El-Gohary M. 1998. Assessment of vinyl chloride-induced DNA damage in
lymphocytes of plastic industry workers using a single-cell gel electrophoresis technique.
Toxicology 128:9-16. https://doi.org/10.1016/S0300-483X(98)00008-0.
Azari MR, Tayefeh-Rahimian R, Jafari MJ, et al. 2016. Exploring a new method for the biological
monitoring of plastic workers exposed to the vinyl chloride monomer. Toxicol Ind Health
32(12):1921-1926. https://doi.org/10.1177/0748233715596663.
Baker LW, MacKay KP. 1985. Hazardous waste management. Screening models for estimating toxic
air pollution near a hazardous waste landfill. J Air Pollut Control Assoc 35:1190-1195.
https://doi.org/10.1080/00022470.1985.10466023.
Ballering LA, Nivard MJ, Vogel EW. 1996. Characterization by two-endpoint comparisons of the
genetic toxicity profiles of vinyl chloride and related etheno-adduct forming carcinogens in
Drosophila. Carcinogenesis 17(5):1083-1092. https://doi.org/10.1093/carcin/17.5.1083.
Bao YS, Jiang H, Liu J. 1988. [The effects of vinyl chloride on pregnancy, parturition, and fetal
development among female workers]. Zhonghua Yu Fang Yi Xue Za Zhi 22(6):343-346. (Chinese)
Barbin A. 1998. Formation of DNA etheno adducts in rodents and humans and their role in
carcinogenesis. Acta Biochim Pol 45(1):145-161. https://doi.org/10.18388/abp.1998_4329.
Barbin A. 1999. Role of etheno DNA adducts in carcinogenesis induced by vinyl chloride in rats. IARC
Sci Publ 150(150):303-313.
Barbin A. 2000. Etheno-adduct-forming chemicals: from mutagenicity testing to tumor mutation spectra.
Mutat Res 462(2-3):55-69. https://doi.org/10.1016/s1383-5742(00)00014-4.
Barbin A, Froment O, Boivin S, et al. 1997. p53 Gene mutation pattern in rat livers tumors induced by
vinyl chloride. Cancer Res 57:1695-1698.
Baretta ED, Stewart RD, Mutchler JE. 1969. Monitoring exposures to vinyl chloride vapor: breath
analysis and continuous air sampling. Am Ind Hyg Assoc J 30(6):537-544.
https://doi.org/10.1080/00028896909343171.
Barnes DG, Dourson M. 1988. Reference dose (RfD): Description and use in health risk assessments.
Regul Toxicol Pharmacol 8:471-486. https://doi.org/10.1016/0273-2300(88)90047-5.
Barrio-Lage GA, Parsons FZ, Narbaitz RM, et al. 1990. Enhanced anaerobic biodegradation of vinyl
chloride in groundwater. Environ Toxicol Chem 9(4):403-415.
https://doi.org/10.1002/etc.5620090402.
Barton HA, Creech JR, Godin CS, et al. 1995. Chloroethylene mixtures: pharmacokinetic modeling and
in vitro metabolism of vinyl chloride, trichloroethylene, and trans-1,2-dichloroethylene in rat.
Toxicol Appl Pharmacol 130(2):237-247. https://doi.org/10.1006/taap.1995.1029.
Bartsch H. 1976. Predictive value of mutagenicity test in chemical carcinogenesis. Mutat Res 38:177
190. https://doi.org/10.1016/0165-1161(76)90189-8.
VINYL CHLORIDE 177
8. REFERENCES
Bartsch H, Nair J. 2000. Ultrasensitive and specific detection methods for exocyclic DNA adducts:
Markers for lipid peroxidation and oxidative stress. Toxicology 153(1-3):105-114.
https://doi.org/10.1016/S0300-483X(00)00307-3.
Bartsch H, Malaveille C, Montesano R. 1975. Human, rat and mouse liver-mediated mutagenicity of
vinyl chloride in S. typhimurium strains. Int J Cancer 15(3):429-437.
https://doi.org/10.1002/ijc.2910150309.
Bartsch H, Malaveille C, Montesano R. 1976. The predictive value of tissue-mediated mutagenicity
assays to assess the carcinogenic risk of chemicals. IARC Sci Publ 12:467-491.
Bartsch H, Malaveille C, Barbin A, et al. 1979. Mutagenic and alkylating metabolites of halo-ethylenes,
chlorobutadienes and dichlorobutenes produced by rodent or human liver tissues. Evidence for
oxirane formation by P450-linked microsomal mono-oxygenases. Arch Toxicol 41(4):249-277.
https://doi.org/10.1007/BF00296896.
Becker R, Nikolova T, Wolff I, et al. 2001. Frequency of HPRT mutants in humans exposed to vinyl
chloride via an environmental accident. Mutat Res 494(1-2):87-96. https://doi.org/10.1016/s1383-
5718(01)00182-6.
Belli S, Bertazzi PA, Comba P, et al. 1987. A cohort study on vinyl chloride manufacturers in Italy:
study design and preliminary results. Cancer Lett 35(3):253-261. https://doi.org/10.1016/0304-
3835(87)90127-3.
Bencko V, Wagner V, Wagnerova M, et al. 1988. Immunobiochemical profiles of workers differing in
the degree of occupational exposure to vinyl chloride. J Hyg Epidemiol Microbiol Immunol
32(4):375-384.
Benfenati E, Natangelo M, Davoli E, et al. 1991. Migration of vinyl chloride into PVC-bottled drinking-
water assessed by gas chromatography-mass spectrometry. Food Chem Toxicol 29(2):131-134.
https://doi.org/10.1016/0278-6915(91)90168-7.
Berk PD, Martin JF, Waggoner JG. 1975. Persistence of vinyl chloride-induced liver injury after
cessation of exposure. Ann N Y Acad Sci 246:70-77. https://doi.org/10.1111/j.1749-
6632.1975.tb51081.x.
Bexfield LM, Belitz K, Fram MS, et al. 2022. Volatile organic compounds in groundwater used for
public supply across the United States: Occurrence, explanatory factors, and human-health context.
Sci Total Environ 827:154313. https://doi.org/10.1016/j.scitotenv.2022.154313.
Bi WF, Wang YS, Huang MY, et al. 1985. Effect of vinyl chloride on testis in rats. Ecotoxicol Environ
Saf 10(3):281-289. https://doi.org/10.1016/0147-6513(85)90074-0.
Black CM, Welsh KI, Walker AE, et al. 1983. Genetic susceptibility to scleroderma-like syndrome
induced by vinyl chloride. Lancet 1(8314-5):53-55. https://doi.org/10.1016/s0140-6736(83)91578-
7.
Black C, Pereira S, McWhirter A, et al. 1986. Genetic susceptibility to scleroderma-like syndrome in
symptomatic and asymptomatic workers exposed to vinyl chloride. J Rheumatol 13(6):1059-1062.
Bloomdahl R, Abualfaraj N, Olson M, et al. 2014. Assessing worker exposure to inhaled volatile organic
compounds from Marcellus Shale flowback pits. J Nat Gas Sci Eng 21:348-356.
https://doi.org/10.1016/j.jngse.2014.08.018.
Boffetta P, Matisane L, Mundt KA, et al. 2003. Meta-analysis of studies of occupational exposure to
vinyl chloride in relation to cancer mortality. Scand J Work Environ Health 29(3):220-229.
https://doi.org/10.5271/sjweh.725.
Bogdanikowa B, Zawilska J. 1984. Immune complexes in the serum of patients occupationally exposed
to vinyl chloride. Przegl Lek 41:253-257.
Boivin S, Pedron S, Bertrand S, et al. 1997. Myofibroblast-like cells exhibiting a mutated p53 gene
isolated from a liver human tumor of a worker exposed to vinyl chloride. In: Wisse E, Knook DL,
Balabaud C, eds. Cells of the hepatic sinusoid. Vol. 6. The Netherlands: Kupffer Cell Foundation,
449-451.
VINYL CHLORIDE 178
8. REFERENCES
Boivin-Angele S, Lefrancois L, Froment O, et al. 2000. Ras gene mutations in vinyl chloride-induced
liver tumours are carcinogen-specific but vary with cell type and species. Int J Cancer 85(2):223-
227. https://doi.org/10.1002/(sici)1097-0215(20000115)85:2<223::aid-ijc12>3.0.co;2-h.
Bolt HM. 1986. Metabolic activation of vinyl chloride, formation of nucleic acid adducts and relevance
to carcinogenesis. IARC Sci Publ 70(70):261-268.
Bolt HM, Kappus H, Buchter A, et al. 1976a. Disposition of (1,2-14C) vinyl chloride in the rat. Arch
Toxicol 35(3):153-162. https://doi.org/10.1007/BF00293562.
Bolt HM, Kappus H, Kaufmann R, et al. 1976b. Metabolism of 14C-vinyl chloride in vitro and in vivo.
IARC Sci Publ 13:151-163.
Bolt HM, Laib RJ, Kappus H, et al. 1977. Pharmacokinetics of vinyl chloride in the rat. Toxicology
7(2):179-188. https://doi.org/10.1016/0300-483x(77)90063-4.
Bolt HM, Filser JG, Laib RJ, et al. 1980. Binding kinetics of vinyl chloride and vinyl bromide at very
low doses. Arch Toxicol Suppl 3:129-142. https://doi.org/10.1007/978-3-642-67389-4_10.
Bolt HM, Laib RJ, Peter H, et al. 1986. DNA adducts of halogenated hydrocarbons. J Cancer Res Clin
Oncol 112(2):92-96. https://doi.org/10.1007/BF00404388.
Borrelli FE, de la Cruz PL, Paradis RA. 2005. Residual vinyl chloride levels in U.S. PVC resins and
products: Historical perspective and update. J Vinyl Addit Technol 11(2):65-69.
https://doi.org/10.1002/vnl.20040.
Bosetti C, La Vecchia C, Lipworth L, et al. 2003. Occupational exposure to vinyl chloride and cancer
risk: a review of the epidemiologic literature. Eur J Cancer Prev 12(5):427-430.
https://doi.org/10.1097/00008469-200310000-00012.
Bove FJ, Ruckart PZ, Maslia M, et al. 2014. Evaluation of mortality among marines and navy personnel
exposed to contaminated drinking water at USMC base Camp Lejeune: a retrospective cohort study.
Environ Health 13(1):10. https://doi.org/10.1186/1476-069x-13-10.
Boyle EB, Viet SM, Wright DJ, et al. 2016. Assessment of exposure to VOCs among pregnant women
in the National Children's Study. Int J Environ Res Publ Health 13(4):376.
https://doi.org/10.3390/ijerph13040376.
Bradley PM, Chapelle FH. 1996. Anaerobic mineralization of vinyl chloride in Fe(III)-reducing aquifer
sediments. Environ Sci Technol 30:2084-2086. https://doi.org/10.1021/es950926k.
Bradley PM, Chapelle FH. 1997. Kinetics of DCE and VC mineralization under methanogenic and
Fe(III)-reducing conditions. Environ Sci Technol 31(9):2692-2696.
https://doi.org/10.1021/es970110e.
Brandt-Rauf PW, Marion MJ, DeVito I. 1995. Mutant p21 protein as a biomarker of chemical
carcinogenesis in humans. In: Mendelsohn ML, Peeters JP, Normandy MJ, eds. Biomarkers and
occupational health. Progress and perspectives. Washington, DC: Joseph Henry Press, 163-173.
Brandt-Rauf PW, Luo JC, Cheng TJ, et al. 2000a. Mutant oncoprotein biomarkers of vinyl chloride
exposure: Applications to risk assessment. NATO ASI Ser A Life Sci 313:233-248.
Brandt-Rauf PW, Do TN, Weinberg MD, et al. 2000b. Growth signal transduction proteins as
biomarkers of occupational exposures. NATO ASI Ser A Life Sci 313:208-212.
Brinker K, Lumia M, Markiewicz KV, et al. 2015. Assessment of emergency responders after a vinyl
chloride release from a train derailment - New Jersey, 2012. MMWR Morb Mortal Wkly Rep
63(53):1233-1237.
Brock WJ, Rusch GM, Trochimowicz HJ. 2003. Cardiac sensitization: methodology and interpretation
in risk assessment. Regul Toxicol Pharmacol 38(1):78-90. https://doi.org/10.1016/s0273-
2300(03)00072-2.
Buchter A, Bolt HM, Kappus H, et al. 1977. [Tissue distribution of 1,2-
14
C–vinyl chloride in the rat].
Int Arch Occup Environ Health 39:27-32. (German)
Buchter A, Filser JG, Peter H, et al. 1980. Pharmacokinetics of vinyl chloride in the Rhesus monkey.
Toxicol Lett 6(1):33-36. https://doi.org/10.1016/0378-4274(80)90099-5.
Budavari S. 1989. An encyclopedia of chemicals, drugs, and biologicals. In: The Merck index. 11
th
ed.
Rahway, NJ: Merck & Co., Inc., 1572.
VINYL CHLORIDE 179
8. REFERENCES
Buffler PA, Wood S, Eifler C, et al. 1979. Mortality experience of workers in a vinyl chloride monomer
production plant. J Occup Med 21(3):195-203.
Burk T, Zarus G. 2013. Community exposures to chemicals through vapor intrusion: A review of past
ATSDR public health evaluations. J Environ Health 75(9):36-41.
Byren D, Engholm G, Englund A, et al. 1976. Mortality and cancer morbidity in a group of Swedish
VCM and PCV production workers. Environ Health Perspect 17:167-170.
https://doi.org/10.1289/ehp.17-1475258.
Carassiti V, Chiorboli C, Bignozzi CA, et al. 1977. Atmospheric photo-oxidation of vinyl chloride: A
generalized treatment of the relative rates of product formation. Ann Chim 67:499-512.
Carr CJ, Burgison RM, Vitcha JF, et al. 1949. Anesthesia; chemical constitution of hydrocarbons and
cardiac automaticity. J Pharmacol Exp Ther 97(1):1-3.
Carreón T, Hein MJ, Hanley KW, et al. 2014. Coronary artery disease and cancer mortality in a cohort
of workers exposed to vinyl chloride, carbon disulfide, rotating shift work, and o-toluidine at a
chemical manufacturing plant. Am J Ind Med 57(4):398-411. https://doi.org/10.1002/ajim.22299.
Castro CE, Riebeth DM, Belser NO. 1992a. Biodehalogenation: The metabolism of vinyl chloride by
Methylosinus trichosporium OB-3b. A sequential oxidative and reductive pathway through
chloroethylene oxide. Environ Toxicol Chem 11(6):749-755.
https://doi.org/10.1002/etc.5620110604.
Castro CE, Wade RS, Riebeth DM, et al. 1992b. Biodehalogenation: Rapid metabolism of vinyl
chloride by a soil Pseudomonas sp. direct hydrolysis of a vinyl C-Cl bond. Environ Toxicol Chem
11(6):757-764. https://doi.org/10.1002/etc.5620110605.
Cave M, Falkner KC, Ray M, et al. 2010. Toxicant-associated steatohepatitis in vinyl chloride workers.
Hepatology 51(2):474-481. https://doi.org/10.1002/hep.23321.
Chappell G, Pogribny IP, Guyton KZ, et al. 2016. Epigenetic alterations induced by genotoxic
occupational and environmental human chemical carcinogens: A systematic literature review. Mutat
Res 768:27-45. https://doi.org/10.1016/j.mrrev.2016.03.004.
ChemView. 2023. Vinyl chloride. Chemical data reporting details. U.S. Environmental Protection
Agency. https://chemview.epa.gov/chemview/. July 13, 2023.
Chen HM, Wu MT. 2017. Residential exposure to chlorinated hydrocarbons from groundwater
contamination and the impairment of renal function-An ecological study. Sci Rep 7:40283.
https://doi.org/10.1038/srep40283.
Chen CW, Hsieh D, Sung FC, et al. 2018. Feasibility of using urinary TDGA as a biomarker for VCM
exposures. Regul Toxicol Pharmacol 97:82-87. https://doi.org/10.1016/j.yrtph.2018.06.008.
Chen L, Lang AL, Poff GD, et al. 2019. Vinyl chloride-induced interaction of nonalcoholic and toxicant-
associated steatohepatitis: Protection by the ALDH2 activator Alda-1. Redox Biol 24:101205.
https://doi.org/10.1016/j.redox.2019.101205.
Cheng TJ, Wong RH, Du CL, et al. 1999a. A mortality study of vinyl chloride workers in Taiwan. Proc
Am Assoc Cancer Res 40:41.
Cheng TJ, Huang ML, You NC, et al. 1999b. Abnormal liver function in workers exposed to low levels
of ethylene dichloride and vinyl chloride monomer. J Occup Environ Med 41(12):1128-1133.
https://doi.org/10.1097/00043764-199912000-00018.
Cheng TJ, Huang YF, Ma YC. 2001. Urinary thiodiglycolic acid levels for vinyl chloride monomer-
exposed polyvinyl chloride workers. J Occup Environ Med 43(11):934-938.
https://doi.org/10.1097/00043764-200111000-00002.
Cheng MH, Chiu CH, Chen CT, et al. 2021. Sources and components of volatile organic compounds in
breast surgery operating rooms. Ecotoxicol Environ Saf 209:111855.
https://doi.org/10.1016/j.ecoenv.2020.111855.
Chiang SY, Swenberg JA, Weisman WH, et al. 1997. Mutagenicity of vinyl chloride and its reactive
metabolites, chloroethylene oxide and chloroacetaldehyde in a metabolically competent human B-
lymphoblastoid line. Carcinogenesis 18(1):31-36. https://doi.org/10.1093/carcin/18.1.31.
VINYL CHLORIDE 180
8. RE
FERENCES
Chiu WA, White P. 2006. Steady-state solutions to PBPK models and their applications to risk
assessment I: Route-to-route extrapolation of volatile chemicals. Risk Anal 26(3):769-780.
https://doi.org/10.1111/j.1539-6924.2006.00762.x.
Christner PJ, Artlett CM, Conway RF, et al. 2000. Increased numbers of microchimeric cells of fetal
origin are associated with dermal fibrosis in mice following injection of vinyl chloride. Arthritis
Rheum 43(11):2598-2605. https://doi.org/10.1002/1529-0131(200011)43:11<2598::AID-
ANR30>3.0.CO;2-8.
Cicalese L, Raun L, Shirafkan A, et al. 2017. An ecological study of the association between air
pollution and hepatocellular carcinoma incidence in Texas. Liver cancer 6(4):287-296.
https://doi.org/10.1159/000475776.
Ciroussel F, Barbin A, Eberle G, et al. 1990. Investigations on the relationship between DNA
ethenobase adduct levels in several organs of vinyl chloride-exposed rats and cancer susceptibility.
Biochem Pharmacol 39:1109-1113. https://doi.org/10.1016/0006-2952(90)90291-R.
Clark DG, Tinston DJ. 1973. Correlation of the cardiac sensitizing potential of halogenated
hydrocarbons with their physicochemical properties. Br J Pharmacol 49:355-357.
https://doi.org/10.1111/j.1476-5381.1973.tb08382.x.
Clark CJ, Xiong B, Soriano MA, et al. 2022. Assessing unconventional oil and gas exposure in
Appalachian basin: Comparison of exposure surrogates and residential drinking water measurements.
Environ Sci Technol 56(2):1091-1103. https://doi.org/10.1021/acs.est.1c05081.
Clewell HJ. 1995. The application of physiologically based pharmacokinetic modeling in human health
risk assessment of hazardous substances. Toxicol Lett 79(1-3):207-217.
https://doi.org/10.1016/0378-4274(95)03372-r.
Clewell HJ, Andersen ME. 1985. Risk assessment extrapolations and physiological modeling. Toxicol
Ind Health 1(4):111-131. https://doi.org/10.1177/074823378500100408.
Clewell HJ, Gentry PR, Gearhart JM, et al. 1995. Considering pharmacokinetic and mechanistic
information in cancer risk assessments for environmental contaminants: examples with vinyl
chloride and trichloroethylene. Chemosphere 31(1):2561-2578. https://doi.org/10.1016/0045-
6535(95)00124-q.
Clewell HJ, Gentry PR, Gearhart JM, et al. 2001. Comparison of cancer risk estimates for vinyl chloride
using animal and human data with a PBPK model. Sci Total Environ 274:37-66.
https://doi.org/10.1016/S0048-9697(01)00730-6.
Clewell HJ, Gentry PR, Covington TR, et al. 2004. Evaluation of the potential impact of age- and
gender-specific pharmacokinetic differences on tissue dosimetry. Toxicol Sci 79(2):381-393.
https://doi.org/10.1093/toxsci/kfh109.
Cogliano VJ, Parker JC. 1992. Some implications of toxicology and pharmacokinetics for exposure
assessment. J Expo Anal Environ Epidemiol 2(Supp 1):189-207.
Cogliano VJ, Hiatt GF, Den A. 1996. Quantitative cancer assessment for vinyl chloride: indications of
early-life sensitivity. Toxicology 111(1-3):21-28. https://doi.org/10.1016/0300-483x(96)03390-2.
Coleman NV, Spain JC. 2003. Epoxyalkane: coenzyme M transferase in the ethene and vinyl chloride
biodegradation pathways of mycobacterium strain JS60. J Bacteriol 185(18):5536-5545.
https://doi.org/10.1128/JB.185.18.5536-5545.2003.
Coleman NV, Mattes TE, Gossett JM, et al. 2002. Phylogenetic and kinetic diversity of aerobic vinyl
chloride-assimilating bacteria from contaminated sites. Appl Environ Microbiol 68(12):6162-6171.
https://doi.org/10.1128/AEM.68.12.6162-6171.2002.
Collins JJ, Jammer B, Sladeczek FM, et al. 2014. Surveillance for angiosarcoma of the liver among
vinyl chloride workers. J Occup Environ Med 56(11):1207-1209.
https://doi.org/10.1097/jom.0000000000000247.
Conkle JP, Camp BJ, Welch BE. 1975. Trace composition of human respiratory gas. Arch Environ
Health 30(6):290-295. https://doi.org/10.1080/00039896.1975.10666702.
VINYL CHLORIDE 181
8. REFERENCES
Conolly RB, Jaeger RJ. 1979. Acute hepatotoxicity of vinyl chloride and ethylene: Modification by
trichloropropane oxide, diethylmaleate, and cysteine. Toxicol Appl Pharmacol 50:523-531.
https://doi.org/10.1016/0041-008X(79)90407-1.
Conolly RB, Jaeger RJ, Szabo S. 1978. Acute hepatotoxicity of ethylene, vinyl fluoride, vinyl chloride,
and vinyl bromide after Aroclor 1254 pretreatment. Exp Mol Pathol 28(1):25-33.
https://doi.org/10.1016/0014-4800(78)90060-6.
Cooper WC. 1981. Epidemiologic study of vinyl chloride workers: Mortality through December 31,
1972. Environ Health Perspect 41:101-106. https://doi.org/10.1289/ehp.8141101.
Cowfer JA, Magistro AJ. 1985. Vinyl polymers: Vinyl chloride. In: Kirk-Othmer’s concise
encyclopedia of chemical technology. New York, NY: John Wiley and Sons, Inc, 1229-1233.
Cowfer JA, Gorensek MB. 2006. Vinyl chloride. In: Kirk-Othmer’s encyclopedia of chemical
technology. New York, NY: John Wiley & Sons, online.
https://doi.org/10.1002/0471238961.2209142503152306.a01.pub2.
Cox RA, Enggleton AEJ, Sandalls FJ. 1974. Photochemical reactivity of vinyl chloride. Oxfordshire,
England: AERE Harwell. AERE-R-7820.
Criscuolo M, Valerio J, Gianicolo ME, et al. 2014. A vinyl chloride-exposed worker with an adrenal
gland angiosarcoma: a case report. Ind Health 52(1):66-70. https://doi.org/10.2486/indhealth.2013-
0044.
Cullinan D, Johnson F, Grollman AP, et al. 1997. Solution structure of a DNA duplex containing the
exocyclic lesion 3,N4-etheno-2’-deoxycytidine opposite 2’-deoxyguanosine. Biochemistry
36(39):11933 11943. https://doi.org/10.1021/bi9705725.
Danko AS, Saski CA, Tomkins JP, et al. 2006. Involvement of coenzyme M during aerobic
biodegradation of vinyl chloride and ethene by Pseudomonas putida strain AJ and Ochrobactrum sp.
strain TD. Appl Environ Microbiol 72(5):3756-3758. https://doi.org/10.1128/AEM.72.5.3756-
3758.2006.
Danziger H. 1960. Accidental poisoning by vinyl chloride: report of two cases. Can Med Assoc J
82:828-830.
Davis JW, Carpenter CL. 1990. Aerobic biodegradation of vinyl chloride in groundwater samples. Appl
Environ Microbiol 56(12):3878-3880. https://doi.org/10.1128/AEM.56.12.3878-3880.1990.
de Jong G, van Sittert NJ, Natarajan AT. 1988. Cytogenetic monitoring of industrial populations
potentially exposed to genotoxic chemicals and of control populations. Mutat Res 204(3):451-464.
https://doi.org/10.1016/0165-1218(88)90041-9.
de Meester C, Duverger-van Bogaert M, Lambotte-Vandepaer M, et al. 1980. Mutagenicity of vinyl
chloride in the Ames test: possible artifacts related to experimental conditions. Mutat Res 77(2):175-
179. https://doi.org/10.1016/0165-1218(80)90135-4.
DeLassus PT, Schmidt DD. 1981. Solubilities of vinyl chloride and vinylidene chloride in water. J
Chem Eng Data 26(3):274-276. https://doi.org/10.1021/je00025a015.
DeVivo I, Marion MJ, Smith SJ, et al. 1994. Mutant c-Ki-ras p21 protein in chemical carcinogenesis in
humans exposed to vinyl chloride. Cancer Causes Control 5:273-278.
https://doi.org/10.1007/BF01830248.
Dinman BD, Cook WA, Whitehouse WM, et al. 1971. Occupational acroosteolysis. I. An
epidemiological study. Arch Environ Health 22(1):61-73.
https://doi.org/10.1080/00039896.1971.10665816.
DOE. 2015. Vinyl chloride. PAC Database. U.S. Department of Energy. https://pacteels.pnnl.gov/.
July 20, 2023.
DOE. 2023. Definition of PACs. U.S. Department of Energy. https://pacteels.pnnl.gov/#/definitions.
July 20, 2023.
Dogliotti E. 2006. Molecular mechanisms of carcinogenesis by vinyl chloride. Ann Ist Super Sanita
42(2):163-169.
VINYL CHLORIDE 182
8. REFERENCES
Dosanjh MK, Chenna A, Kim E, et al. 1994. All four known cyclic adducts formed in DNA by the vinyl
chloride metabolite chloroacetaldehyde are released by a human DNA glycosylase. Proc Natl Acad
Sci U S A 91:1024-1028. https://doi.org/10.1073/pnas.91.3.1024.
DOT. 1993. Hazardous materials regulations. U.S. Department of Transportation. Code of Federal
Regulations. 49 CFR 171.2. https://www.ecfr.gov/current/title-49/subtitle-B/chapter-I/subchapter-
C/part-171/subpart-A/section-171.2. August 26, 2022.
Dreher E, Beutel KK, Myers JD, et al. 2014. Chloroethanes and chloroethylenes. In: Ullmann's
encyclopedia of industrial chemistry. New York, NY: John Wiley & Sons, online.
https://doi.org/10.1002/14356007.o06_o01.pub2.
Drevon C, Kuroki T, Montesano R. 1978. Microsome-mediated mutagenesis of a Chinese hamster cell
line by various chemicals. Mutat Res Lett 53:181-182.
Drew RT, Boorman GA, Haseman JK, et al. 1983. The effect of age and exposure duration on cancer
induction by a known carcinogen in rats, mice, and hamsters. Toxicol Appl Pharmacol 68(1):120-
130. https://doi.org/10.1016/0041-008x(83)90361-7.
D'Souza RW, Andersen ME. 1988. Physiologically based pharmacokinetic model for vinylidene
chloride. Toxicol Appl Pharmacol 95(2):230-240. https://doi.org/10.1016/0041-008x(88)90159-7.
Du CL, Wang JD. 1998. Increased morbidity odds ratio of primary liver cancer and cirrhosis of the liver
among vinyl chloride monomer workers. Occup Environ Med 55:528-532.
https://doi.org/10.1136/oem.55.8.528.
Du JT, Sandoz JP, Tseng MT, et al. 1979. Biochemical alterations in livers of rats exposed to vinyl
chloride. J Toxicol Environ Health 5(6):1119-1132. https://doi.org/10.1080/15287397909529818.
Du CL, Kuo ML, Chang HL, et al. 1995. Changes in lymphocyte single strand breakage and liver
function of workers exposed to vinyl chloride monomer. Toxicol Lett 77(1-3):379-385.
https://doi.org/10.1016/0378-4274(95)03321-1.
du Pont. 1992a. Initial submission: In vitro microbial mutagenicity studies of chloroethylene with cover
letter dated 050892 and attachments. E.I. du Pont de Nemours & Company. Submitted to the U.S.
Environmental Protection Agency under Section 8E. OTS0539817. 88920002857.
8EHQ05924215.
https://ntrl.ntis.gov/NTRL/dashboard/searchResults/titleDetail/OTS0539817.xhtml. October 5,
2021.
du Pont. 1992b. Initial submission: Mutagenicity activity of ethylene, chloro- in the
Salmonella/microsome assay with cover letter dated 050892 & attachments. E.I. du Pont de
Nemours & Company. Submitted to the U.S. Environmental Protection Agency under TSCA
Section 8E. OTS0539547. 88920002788. 8EHQ05924146.
https://ntrl.ntis.gov/NTRL/dashboard/searchResults/titleDetail/OTS0539547.xhtml. October 5,
2021.
du Pont. 1992c. Initial submission: Ethylene, chloro-: Chinese hamster ovary cell assay for mutagenicity
with cover letter dated 050892 and attachments. E.I. du Pont de Nemours & Company. Submitted
to the U.S. Environmental Protection Agency under TSCA Section 8E. OTS0539535.
88920002776. 8EHQ05924134
https://ntrl.ntis.gov/NTRL/dashboard/searchResults/titleDetail/OTS0539535.xhtml. October 5,
2021.
Ducatman A, Hirschhorn K, Selikoff IJ. 1975. Vinyl chloride exposure and human chromosome
aberrations. Mutat Res 31(3):163-168. https://doi.org/10.1016/0165-1161(75)90085-0.
Eberle G, Barbin A, Laib RJ, et al. 1989. 1,N
6
-etheno-2'-deoxyadenosine and 3,N
4
-etheno-2'-
deoxycytidine detected by monoclonal antibodies in lung and liver DNA of rats exposed to vinyl
chloride. Carcinogenesis 10:209-212. https://doi.org/10.1093/carcin/10.1.209.
Eckardt F, Muliawan H, de Ruiter N, et al. 1981. Rat hepatic vinyl chloride metabolites induce gene
conversion in the yeast strain D7RAD in vitro and in vivo. Mutat Res Lett 91(4-5):381-390.
https://doi.org/10.1016/0165-7992(81)90019-1.
VINYL CHLORIDE 183
8. REFERENCES
Edmonds LD, Falk H, Nissim JE. 1975. Letter: Congenital malformations and vinyl chloride. Lancet
2(7944):1098. https://doi.org/10.1016/s0140-6736(75)90479-1.
Edmonds LD, Anderson CE, Flynt JW, et al. 1978. Congenital central nervous system malformations
and vinyl chloride monomer exposure: a community study. Teratology 17(2):137-142.
https://doi.org/10.1002/tera.1420170205.
Edwards D, Voronina A, Attwood K, et al. 2021. Association between occupational exposures and
sarcoma incidence and mortality: systematic review and meta-analysis. Syst Rev 10(1):231.
https://doi.org/10.1186/s13643-021-01769-4.
Eisenreich SJ, Looney BB, Thornton JD. 1981. Airborne organic contaminants in the Great Lakes
ecosystem. Environ Sci Technol 15:30-38. https://doi.org/10.1021/es00083a002.
el Ghissassi F, Barbin A, Bartsch H. 1998. Metabolic activation of vinyl chloride by rat liver
microsomes: low-dose kinetics and involvement of cytochrome P450 2E1. Biochem Pharmacol
55(9):1445-1452. https://doi.org/10.1016/s0006-2952(97)00645-x.
Elliott P, Kleinschmidt I. 1997. Angiosarcoma of the liver in Great Britain in proximity to vinyl chloride
sites. Occup Environ Med 54(1):14-18. https://doi.org/10.1136/oem.54.1.14.
El-Masri HA, Mumtaz MM, Yushak ML. 2004. Application of physiologically-based pharmacokinetic
modeling to investigate the toxicological interaction between chlorpyrifos and parathion in the rat.
Environ Toxicol Pharmacol 16(1-2):57-71. https://doi.org/10.1016/j.etap.2003.10.002.
El-Metwally D, Chain K, Stefanak MP, et al. 2018. Urinary metabolites of volatile organic compounds
of infants in the neonatal intensive care unit. Pediatr Res 83(6):1158-1164.
https://doi.org/10.1038/pr.2018.52.
Elmore JD, Wong JL, Laumbach AD, et al. 1976. Vinyl chloride mutagenicity via the metabolites
chlorooxirane and chloroacetaldehyde monomer hydrate. Biochim Biophys Acta 442(3):405-419.
https://doi.org/10.1016/0005-2787(76)90314-2.
EPA. 1975. Scientific and technical assessment report on vinyl chloride and polylinyl chloride.
Washington, DC: U.S. Environmental Protection Agency. EPA600675004.
https://nepis.epa.gov/Exe/ZyPURL.cgi?Dockey=20013GER.txt. August 25, 2021.
EPA. 1976. Dynamic behavior of vinyl chloride in aquatic ecosystems. Atlanta, GA: U.S.
Environmental Protection Agency. PB249302. EPA600376001.
https://ntrl.ntis.gov/NTRL/dashboard/searchResults/titleDetail/PB249302.xhtml. August 26, 2021.
EPA. 1979. Water-related environmental fate of 129 priority pollutants. Volume II. Washington, DC:
U.S. Environmental Protection Agency. PB80204381. EPA4404790298.
https://ntrl.ntis.gov/NTRL/dashboard/searchResults/titleDetail/PB80204381.xhtml. August 27,
2021.
EPA. 1982a. Aquatic fate process data for organic priority pollutants. Washington, DC: U.S.
Environmental Protection Agency. PB87169090. EPA440481014.
https://ntrl.ntis.gov/NTRL/dashboard/searchResults/titleDetail/PB87169090.xhtml. July 10, 2021.
EPA. 1982b. Vinyl chloride occurrence in drinking water, food, and air. Washington, DC: U.S.
Environmental Protection Agency. PB95183158. EPA68016388.
https://ntrl.ntis.gov/NTRL/dashboard/searchResults/titleDetail/PB95183158.xhtml. April 7, 2021.
EPA. 1985a. Drinking water criteria document for vinyl chloride. Washington, DC: U.S.
Environmental Protection Agency. PB86118320.
https://ntrl.ntis.gov/NTRL/dashboard/searchResults/titleDetail/PB86118320.xhtml. August 13,
2021.
EPA. 1985b. Health and environmental effects profile for chloroethene. Cincinnati, OH: U.S.
Environmental Protection Agency. PB88174529. ECAOCINP155.
https://ntrl.ntis.gov/NTRL/dashboard/searchResults/titleDetail/PB88174529.xhtml. August 20,
2021.
EPA. 1987. Incorporation of biological information in cancer risk assessment: Example - vinyl chloride.
Washington, DC: U.S. Environmental Protection Agency. PB87198982.
VINYL CHLORIDE 184
8. REFERENCES
https://ntrl.ntis.gov/NTRL/dashboard/searchResults/titleDetail/PB87198982.xhtml. August 13,
2021.
EPA. 1993. Hazardous constituents. U.S. Environmental Protection Agency. Code of Federal
Regulations. 40 CFR 261 Appendix VIII. https://www.ecfr.gov/current/title-40/chapter-
I/subchapter-I/part-261/appendix-Appendix%20VIII%20to%20Part%20261. October 5, 2022.
EPA. 1994. Methods of derivation of inhalation reference concentrations and application of inhalation
dosimetry. Research Triangle Park, NC: U.S. Environmental Protection Agency. EPA600890066F.
https://cfpub.epa.gov/ncea/risk/recordisplay.cfm?deid=71993. October 25, 2021.
EPA. 1999. Compendium method TO-15: Determination of volatile organic compounds (VOCs) in air
collected in specially-prepared canisters and analyzed by gas chromatography/ mass spectrometry
(GC/MS). Compendium of methods for the determination of toxic organic compounds in ambient
air: Second edition. Cincinnati, OH: U.S. Environmental Protection Agency. EPA625R96010b.
https://www3.epa.gov/ttnamti1/files/ambient/airtox/to-15r.pdf. August 1, 2023.
EPA. 2000. Toxicological review of vinyl chloride. Washington, DC: U.S. Environmental Protection
Agency. PB2001108765. EPA635R00004.
https://ntrl.ntis.gov/NTRL/dashboard/searchResults/titleDetail/PB2001108765.xhtml. August 1,
2021.
EPA. 2009. National primary drinking water regulations. U.S. Environmental Protection Agency.
EPA816F090004. https://www.epa.gov/sites/production/files/2016-
06/documents/npwdr_complete_table.pdf. March 25, 2021.
EPA. 2012. Benchmark dose technical guidance. Washington, DC: U.S. Environmental Protection
Agency. EPA100R12001. https://www.epa.gov/sites/default/files/2015-
01/documents/benchmark_dose_guidance.pdf. October 3, 2023.
EPA. 2014. 2011 National Emissions Inventory (NEI) from the Technology Transfer Network
Clearinghouse for inventories and emissions factors. U.S. Environmental Protection Agency.
https://www.epa.gov/air-emissions-inventories/2011-national-emissions-inventory-nei-data#dataq.
March 24, 2021.
EPA. 2016. Six-year review 3 compliance monitoring data (2006-2011). U.S. Environmental Protection
Agency. https://www.epa.gov/dwsixyearreview/six-year-review-3-compliance-monitoring-data-
2006-2011. October 4, 2023.
EPA. 2018a. Method 8260D: Volatile organic compounds by gas chromatography/mass spectrometry.
U.S. Environmental Protection Agency. SW-846 Update VI.
https://www.epa.gov/sites/default/files/2018-06/documents/method_8260d_update_vi_final_06-11-
2018.pdf. July 31, 2023.
EPA. 2018b. 2018 Edition of the drinking water standards and health advisories. Washington, DC: U.S.
Environmental Protection Agency. EPA822S12001.
https://www.epa.gov/system/files/documents/2022-01/dwtable2018.pdf. July 16, 2023.
EPA. 2018c. Acute exposure guideline levels (AEGLs) values. U.S. Environmental Protection Agency.
https://www.epa.gov/sites/production/files/2018-
08/documents/compiled_aegls_update_27jul2018.pdf. April 12, 2020.
EPA. 2018d. About acute exposure guideline levels (AEGLs). U.S. Environmental Protection Agency.
https://www.epa.gov/aegl/about-acute-exposure-guideline-levels-aegls. July 26, 2018.
EPA. 2021. Chemical data reporting (CDR). Non-confidential 2016 chemical data reporting information
on chemical production and use in the United States. U.S. Environmental Protection Agency.
https://www.epa.gov/chemical-data-reporting/access-cdr-data. August 26, 2021.
EPA. 2022. Toxic chemical release inventory reporting forms and instructions: Revised 2021 version.
U.S. Environmental Protection Agency. EPA740B22002.
https://ordspub.epa.gov/ords/guideme_ext/guideme_ext/guideme/file/ry_2021_rfi.pdf. August 22,
2023.
VINYL CHLORIDE 185
8. REFERENCES
EPA. 2023a. Updates from the East Palestine train derailment emergency response. U.S. Environmental
Protection Agency. https://www.epa.gov/east-palestine-oh-train-derailment/daily-updates#feb423.
July 7, 2023.
EPA. 2023b. Air sampling data: East Palestine, Ohio Train Derailment. U.S. Environmental Protection
Agency. https://www.epa.gov/east-palestine-oh-train-derailment/air-sampling-data. June 12, 2023.
EPA. 2023c. Annual summary data: Vinyl chloride. Air quality system: Concentration by monitor.
U.S. Environmental Protection Agency. https://www.epa.gov/aqs. July 11, 2023.
EPA. 2023d. EPA Lab Results: through 09/27/23 - East Palestine, OH Response U.S. Environmental
Protection Agency. https://www.epa.gov/system/files/other-files/2023-02/Lab_Results_Air.csv.
September 28, 2023.
EPA. 2023e. East Palestine, Ohio train derailment. U.S. Environmental Protection Agency.
https://www.epa.gov/east-palestine-oh-train-derailment. October 4, 2023.
EPA. 2023f. East Palestine, Ohio train derailment: Water sampling data. U.S. Environmental Protection
Agency. https://www.epa.gov/east-palestine-oh-train-derailment/water-sampling-data. October 4,
2023.
EPA. 2023g. East Palestine, Ohio train derailment: Soil and sediment sampling data. U.S.
Environmental Protection Agency. https://www.epa.gov/east-palestine-oh-train-derailment/soil-and-
sediment-sampling-data. October 4, 2023.
Ernes DA, Griffin JP. 1996. Process emissions for vinyl pipe industry. J Vinyl Addit Technol 2(3):180-
183. https://doi.org/10.1002/vnl.10119.
Fakkaew K, Kongkratoke S, Tantrakarnapa K, et al. 2022. Characteristics of gases emitted from chicken
manure wastewater and potential effects on human health. Environ Sci Pollut Res Int 29(42):63227-
63232. https://doi.org/10.1007/s11356-022-20357-0.
Falk H, Creech JL, Heath CW, et al. 1974. Hepatic disease among workers at a vinyl chloride
polymerization plant. JAMA 230:59-63. https://doi.org/10.1001/jama.1974.03240010027023.
FDA. 2017. Subpart B - Requirements for specific standardized beverages. Bottled water. U.S. Food
and Drug Administration. Code of Federal Regulations. 21 CFR 165.110.
https://www.gpo.gov/fdsys/pkg/CFR-2017-title21-vol2/pdf/CFR-2017-title21-vol2-sec165-110.pdf.
September 7, 2017.
FDA. 2022. Substances added to food. U.S. Food and Drug Administration.
https://www.cfsanappsexternal.fda.gov/scripts/fdcc/?set=FoodSubstances. January 24, 2022.
Fedeli U, Girardi P, Mastrangelo G. 2019b. Occupational exposure to vinyl chloride and liver diseases.
World J Gastroenterol 25(33):4885-4891. https://doi.org/10.3748/wjg.v25.i33.4885.
Fedeli U, Girardi P, Gardiman G, et al. 2019a. Mortality from liver angiosarcoma, hepatocellular
carcinoma, and cirrhosis among vinyl chloride workers. Am J Ind Med 62(1):14-20.
https://doi.org/10.1002/ajim.22922.
Fedtke N, Boucheron JA, Walker VE, et al. 1990. Vinyl chloride-induced DNA adducts. II: Formation
and persistence of 7-(2-oxoethyl)guanine and N2,3-ethenoguanine in rat tissue DNA.
Carcinogenesis 11(8):1287-1292. https://doi.org/10.1093/carcin/11.8.1287.
Feng N, Li Y, Long C, et al. 2014. Effects of DNA repair gene polymorphisms on DNA damage in
human lymphocytes induced by a vinyl chloride metabolite in vitro. Biomarkers 19(4):281-286.
https://doi.org/10.3109/1354750x.2014.907345.
Feng N, Zheng G, Hao Y, et al. 2017. Mutations in apoptotic genes and micronucleus occurrence in
vinyl chloride-exposed workers in China. Environ Mol Mutagen 58(1):39-45.
https://doi.org/10.1002/em.22046.
Fernandes PH, Kanuri M, Nechev LV, et al. 2005. Mammalian cell mutagenesis of the DNA adducts of
vinyl chloride and crotonaldehyde. Environ Mol Mutagen 45(5):455-459.
https://doi.org/10.1002/em.20117.
Feron VJ, Kroes R. 1979. One-year time-sequence inhalation toxicity study of vinyl chloride in rats. II.
Morphological changes in the respiratory tract, ceruminous glands, brain, kidneys, heart and spleen.
Toxicology 13(2):131-141. https://doi.org/10.1016/S0300-483X(79)80017-7.
VINYL CHLORIDE 186
8. REFERENCES
Feron VJ, Kruysse A, Til HP. 1979a. One-year time sequence inhalation toxicity study of vinyl chloride
in rats. I. Growth, mortality, haematology, clinical chemistry and organ weights. Toxicology
13:25-28. https://doi.org/10.1016/S0300-483X(79)80006-2.
Feron VJ, Speek AJ, Willems MI, et al. 1975. Observations on the oral administration and toxicity of
vinyl chloride in rats. Food Cosmet Toxicol 13(6):633-638. https://doi.org/10.1016/0015-
6264(75)90153-4.
Feron VJ, Hendriksen CF, Speek AJ, et al. 1981. Lifespan oral toxicity study of vinyl chloride in rats.
Food Cosmet Toxicol 19(3):317-333. https://doi.org/10.1016/0015-6264(81)90391-6.
Filippini M, Leoncini C, Luchetti L, et al. 2022. Detecting vinyl chloride by phytoscreening in the
shallow critical zone at sites with potential human exposure. J Environ Manage 319:115776.
https://doi.org/10.1016/j.jenvman.2022.115776.
Filser JG, Bolt HM. 1979. Pharmacokinetics of halogenated ethylenes in rats. Arch Toxicol 42(2):123-
136. https://doi.org/10.1007/BF00316492.
Fire FL. 1986. Vinyl chloride. Fire Eng 139:39-43.
Fleig I, Thiess AM. 1978. Mutagenicity study of workers employed in the styrene and polystyrene
processing and manufacturing industry. Scand J Work Environ Health 4(2):254-258.
https://doi.org/10.5271/sjweh.2740.
Fontana L, Marion MJ, Ughetto S, et al. 2006. Glutathione S-transferase M1 and GST T1 genetic
polymorphisms and Raynaud's phenomenon in French vinyl chloride monomer-exposed workers. J
Hum Genet 51(10):879-886. https://doi.org/10.1007/s10038-006-0038-9.
Forman D, Bennett B, Stafford J, et al. 1985. Exposure to vinyl chloride and angiosarcoma of the liver: a
report of the register of cases. Br J Ind Med 42(11):750-753.
https://doi.org/10.1136/oem.42.11.750.
Fortwengler P, Lewis RD, Reynolds L, et al. 1999. Empirical evidence that angiosarcoma of the liver
caused by vinyl chloride exposure has a relatively high threshold dose in humans. Hepatology 30(4
Pt 2):504A.
Fox AJ, Collier PF. 1977. Mortality experience of workers exposed to vinyl chloride monomer in the
manufacture of polyvinyl chloride in Great Britain. Br J Ind Med 34(1):1-10.
https://doi.org/10.1136/oem.34.1.1.
Freitag D, Ballhorn L, Geyer H, et al. 1985. Environmental hazard profile of organic chemicals: An
experimental method for the assessment of the behavior of organic chemicals in the ecosphere by
means of simple laboratory tests with 14C labelled chemicals. Chemosphere 14:1589-1616.
https://doi.org/10.1016/0045-6535(85)90014-1.
Freudiger H, Bounameaux H, Garcia J. 1988. Acroosteolysis and Raynaud's phenomenon after vinyl
chloride exposure. Vasa 17(3):216-218.
Froment O, Boivin S, Barbin A, et al. 1994. Mutagenesis of ras proto-oncogenes in rat liver tumors
induced by vinyl chloride. Cancer Res 54(20):5340-5345.
Frullanti E, La Vecchia C, Boffetta P, et al. 2012. Vinyl chloride exposure and cirrhosis: a systematic
review and meta-analysis. Dig Liver Dis 44(9):775-779. https://doi.org/10.1016/j.dld.2012.02.007.
Fucic A, Horvat D, Dimitrović B. 1990a. Mutagenicity of vinyl chloride in man: comparison of
chromosome aberrations with micronucleus and sister-chromatid exchange frequencies. Mutat Res
242(4):265-270. https://doi.org/10.1016/0165-1218(90)90044-3.
Fucic A, Horvat D, Dimitrovic B. 1990b. Localization of breaks induced by vinyl chloride in the human
chromosomes of lymphocytes. Mutat Res 243(2):95-99. https://doi.org/10.1016/0165-
7992(90)90029-j.
Fucic A, Garaj-Vrhovac V, Dimitrovic B, et al. 1992. The persistence of sister-chromatid exchange
frequencies in men occupationally exposed to vinyl chloride monomer. Mutat Res 281(2):129-132.
https://doi.org/10.1016/0165-7992(92)90047-l.
Fucic A, Garaj-Vrhovac V, Barkovic D, et al. 1994. The sensitivity of the micronucleus assay for the
detection of occupational exposure to vinyl chloride monomer. Mutat Res 325(2-3):53-56.
https://doi.org/10.1016/0165-7992(94)90001-9.
VINYL CHLORIDE 187
8. REFERENCES
Fucic A, Hitrec V, Garaj-Vrhovac V, et al. 1995. Relationship between locations of chromosome breaks
induced by vinyl chloride monomer and lymphocytosis. Am J Ind Med 27(4):565-571.
https://doi.org/10.1002/ajim.4700270409.
Fucic A, Barkovic D, Garaj-Vrhovac V, et al. 1996a. An eight-year follow-up study of a population
occupationally exposed to vinyl chloride monomer. Mutat Res 360:237-238.
Fucic A, Barkovic D, Garaj-Vrhovac V, et al. 1996b. A nine-year follow up study of a population
occupationally exposed to vinyl chloride monomer. Mutat Res 361(1):49-53.
https://doi.org/10.1016/s0165-1161(96)90229-0.
Fucic A, Hitrec V, Garaj-Vrhovac V, et al. 1997. Clinical cytogenetics and toxogenetics with the
common aim-risk assessment of chemical mutagens. Cytogenet Cell Genet 77(1-2):69.
https://doi.org/10.1159/000317460.
Fucic A, Spacir Z, Barkovic D, et al. 1998. Relative lymphocytosis-disorder caused by occupational
exposure to vinyl chloride monomer. Bull Environ Contam Toxicol 61(5):583-590.
https://doi.org/10.1007/s001289900801.
Fučić A, Lasan R, Mijić A, et al. 2004. The frequency of micronuclei in mononucleated and binucleated
lymphocytes as an indicator of acute or chronic occupational exposure. Period Biol 106(3):279-282.
Fujiwara R. 2018. Exposure to sub-parts per million levels of vinyl chloride can increase the risk of
developing liver injury. Hepatol Commun 2(3):227-229. https://doi.org/10.1002/hep4.1169.
Funes-Cravioto F, Lambert B, Lindsten J, et al. 1975. Letter: chromosome aberrations in workers
exposed to vinyl chloride. Lancet 1(7904):459. https://doi.org/10.1016/s0140-6736(75)91534-2.
Gamble J, Liu S, McMichael AJ, et al. 1976. Effect of occupational and nonoccupational factors on the
respiratory system of vinyl chloride and other workers. J Occup Med 18(10):659-670.
https://doi.org/10.1097/00043764-197610000-00006.
Garaj-Vrhovac V, Fuãciäc A, Horvat D. 1990. Comparison of chromosome aberration and micronucleus
induction in human lymphocytes after occupational exposure to vinyl chloride monomer and
microwave radiation. Period Biol 92(4):411-416.
Garcia E, Hurley S, Nelson DO, et al. 2015. Hazardous air pollutants and breast cancer risk in California
teachers: a cohort study. Environ Health 14:14. https://doi.org/10.1186/1476-069x-14-14.
Gedigke P, Muller R, Bechtelsheimer H. 1975. Morphology of liver damage among polyvinyl chloride
production workers. A report on 51 cases. Ann N Y Acad Sci 246:278-285.
https://doi.org/10.1111/j.1749-6632.1975.tb51103.x.
Gehring PJ, Watanabe PG, Park CN. 1978. Resolution of dose-response toxicity data for chemicals
requiring metabolic activation: example-vinyl chloride. Toxicol Appl Pharmacol 44(3):581-591.
https://doi.org/10.1016/0041-008x(78)90266-1.
Gelin M, Van de Stadt J, Rickaert F, et al. 1989. Epithelioid hemangioendothelioma of the liver
following contact with vinyl chloride. Recurrence after orthotopic liver transplantation. J Hepatol
8(1):99-106. https://doi.org/10.1016/0168-8278(89)90168-2.
Gennaro V, Ceppi M, Crosignani P, et al. 2008. Reanalysis of updated mortality among vinyl and
polyvinyl chloride workers: Confirmation of historical evidence and new findings. BMC Public
Health 8:21. https://doi.org/10.1186/1471-2458-8-21.
Gennissen A, Boeckx M, Sevenster A. 2018. European Council of Vinyl Manufacturers comments on
Cicalese et al.: "An ecological study of the association between air pollution and hepatocellular
carcinoma incidence in Texas". Liver Cancer 7(3):297-298. https://doi.org/10.1159/000486528.
Gentry PR, Covington TR, Clewell HJ. 2003. Evaluation of the potential impact of pharmacokinetic
differences on tissue dosimetry in offspring during pregnancy and lactation. Regul Toxicol
Pharmacol 38(1):1-16. https://doi.org/10.1016/s0273-2300(03)00047-3.
Gilbert SG, Miltz J, Giacin JR. 1980. Transport considerations of potential migrants from food
packaging materials. J Food Process Preserv 4:27-49. https://doi.org/10.1111/j.1745-
4549.1980.tb00594.x.
Ginsberg GL. 2003. Assessing cancer risks from short-term exposures in children. Risk Anal 23(1):19-
34. https://doi.org/10.1111/1539-6924.00287.
VINYL CHLORIDE 188
8. REFERENCES
Girardi P, Barbiero F, Baccini M, et al. 2022. Mortality for lung cancer among PVC baggers employed
in the vinyl chloride industry. Int J Environ Res Public Health 19(10):6246.
https://doi.org/10.3390/ijerph19106246.
Giri AK. 1995. Genetic toxicology of vinyl chloride-a review. Mutat Res 339(1):1-14.
https://doi.org/10.1016/0165-1110(94)00011-z.
Gonzalez-Reche LM, Koch HM, Weib T, et al. 2002. Analysis of ethenoguanine adducts in human urine
using high performance liquid chromatography- tandem mass spectrometry. Toxicol Lett 134(1 to
3):71-77. https://doi.org/10.1016/S0378-4274(02)00165-0.
Gordon SJ, Meeks SA. 1977. A study of gaseous pollutants in the Houston, Texas area. AIChE
Symposium Series 73(165):84-94.
Gossett JM. 1987. Measurement of Henry’s law constants for C1 and C2 chlorinated hydrocarbons.
Environ Sci Technol 21:202-208. https://doi.org/10.1021/es00156a012.
Gossett JM. 2010. Sustained aerobic oxidation of vinyl chloride at low oxygen concentrations. Environ
Sci Technol 44(4):1405-1411. https://doi.org/10.1021/es9033974.
Grainger RG, Walker AE, Ward AM. 1980. Vinyl chloride monomer–induced disease: Clinical,
radiological and immunological aspects. In: Preger L, ed. Induced disease, drug, irradiation,
occupation. New York, NY: Grune and Stratton, 191-214.
Green T, Hathway DE. 1975. The biological fate in rats of vinyl chloride in relation to its oncogenicity.
Chem Biol Interact 11(6):545-562. https://doi.org/10.1016/0009-2797(75)90030-7.
Green T, Hathway DE. 1977. The chemistry and biogenesis of the S-containing metabolites of vinyl
chloride in rats. Chem Biol Interact 17(2):137-150. https://doi.org/10.1016/0009-2797(77)90080-1.
Green T, Hathway DE. 1978. Interactions of vinyl chloride with rat-liver DNA in vivo. Chem Biol
Interact 22(2-3):211-224. https://doi.org/10.1016/0009-2797(78)90126-6.
Grimsrud EP, Rasmussen RA. 1975a. The analysis of chlorofluorocarbons in the troposphere by gas
chromatography-mass spectrometry. Atmos Environ 9:1010-1013. https://doi.org/10.1016/0004-
6981(75)90021-9.
Grimsrud EP, Rasmussen RA. 1975b. Survey and analysis of halocarbons in the atmosphere by gas
chromatography-mass spectrometry. Atmos Environ 9:1014-1017. https://doi.org/10.1016/0004-
6981(75)90022-0.
Gros L, Ishchenko AA, Saparbaev M. 2003. Enzymology of repair of etheno-adducts. Mutat Res 531(1-
2):219-229. https://doi.org/10.1016/j.mrfmmm.2003.07.008.
Guardiola JJ, Beier JI, Falkner KC, et al. 2016. Occupational exposures at a polyvinyl chloride
production facility are associated with significant changes to the plasma metabolome. Toxicol Appl
Pharmacol 313:47-56. https://doi.org/10.1016/j.taap.2016.10.001.
Guardiola JJ, Hardesty JE, Beier JI, et al. 2021. Plasma metabolomics analysis of polyvinyl chloride
workers identifies altered processes and candidate biomarkers for hepatic hemangiosarcoma and its
development. Int J Mol Sci 22(10) https://doi.org/10.3390/ijms22105093.
Guengerich FP, Watanabe PG. 1979. Metabolism of [14C]- and [36Cl]-labeled vinyl chloride in vivo
and in vitro. Biochem Pharmacol 28:589-596. https://doi.org/10.1016/0006-2952(79)90140-0.
Guengerich FP, Ghodke PP. 2021. Etheno adducts: from tRNA modifications to DNA adducts and back
to miscoding ribonucleotides. Genes Environ 43(1):24. https://doi.org/10.1186/s41021-021-00199-
x.
Guengerich FP, Crawford WM, Watanabe PG. 1979. Activation of vinyl chloride to covalently bound
metabolites: roles of 2-chloroethylene oxide and 2-chloroacetaldehyde. Biochemistry 18(23):5177-
5182. https://doi.org/10.1021/bi00590a023.
Guengerich FP, Mason PS, Stott WJ, et al. 1981. Roles of 2-haloethylene oxide and 2-haloacetaldehydes
derived from vinyl bromide and vinyl chloride in irreversible binding to protein and DNA. Cancer
Res 41:4391-4398.
Guichard Y, el Ghissassi F, Nair J, et al. 1996. Formation and accumulation of DNA ethenobases in
adult Sprague-Dawley rats exposed to vinyl chloride. Carcinogenesis 17(8):1553-1559.
https://doi.org/10.1093/carcin/17.8.1553.
VINYL CHLORIDE 189
8. REFERENCES
Guido M, Sarcognato S, Pelletti G, et al. 2016. Sequential development of hepatocellular carcinoma and
liver angiosarcoma in a vinyl chloride-exposed worker. Hum Pathol 57:193-196.
https://doi.org/10.1016/j.humpath.2016.07.021.
Gwinner LM, Laib RJ, Filser JG, et al. 1983. Evidence of chloroethylene oxide being the reactive
metabolite of vinyl chloride towards DNA: Comparative studies with 2,2'-dichlorodiethyl ether.
Carcinogenesis 4:1483-1486. https://doi.org/10.1093/carcin/4.11.1483.
Hagmar L, Akesson B, Nielson J, et al. 1990. Mortality and cancer morbidity in workers exposed to low
levels of vinyl chloride monomer at a polyvinyl chloride processing plant. Am J Ind Med 17:553-
565. https://doi.org/10.1002/ajim.4700170502.
Hallbourg RR, Delfino JJ, Miller WL. 1992. Organic priority pollutants in groundwater and surface
water at three landfills in north central Florida. Water Air Soil Pollut 65:307-332.
https://doi.org/10.1007/BF00479894.
Hansteen IL, Hillestad L, Thiis-Evensen E, et al. 1978. Effects of vinyl chloride in man: a cytogenetic
follow-up study. Mutat Res 51(2):271-278. https://doi.org/10.1016/s0027-5107(78)80022-0.
Harkov R, Kebbekus B, Bozzelli JW, et al. 1984. Comparison of selected volatile organic compounds
during the summer and winter at urban sites in New Jersey. Sci Total Environ 38:259-274.
https://doi.org/10.1016/0048-9697(84)90220-1.
Harris DK. 1953. Health problems in the manufacture and use of plastics. Br J Ind Med 10:255-268.
https://doi.org/10.1136/oem.10.4.255.
Harris DK, Adams WG. 1967. Acro-osteolysis occurring in men engaged in the polymerization of vinyl
chloride. Br Med J 3(5567):712-714. https://doi.org/10.1136/bmj.3.5567.712.
Hartmans S, deBont JAM, Tramper J, et al. 1985. Bacterial degradation of vinyl chloride. Biotech
Letters 7:383-388. https://doi.org/10.1007/BF01166208.
Hatch M, Kline J, Stein Z. 1981. Power considerations in studies of reproductive effects of vinyl
chloride and some structural analogs. Environ Health Perspect 41:195-201.
https://doi.org/10.1289/ehp.8141195.
Hathway DE. 1977. Comparative mammalian metabolism of vinyl chloride and vinylidene chloride in
relation to oncogenic potential. Environ Health Perspect 21:55-59.
https://doi.org/10.1289/ehp.772155.
Heath CW, Fable H, Creech JL. 1975. Characteristics of cases of angiosarcoma of the liver among vinyl
chloride workers in the United States. Ann N Y Acad Sci 246:231-236.
https://doi.org/10.1111/j.1749-6632.1975.tb51097.x
Heath CW, Dumont CR, Gamble J, et al. 1977. Chromosomal damage in men occupationally exposed to
vinyl chloride monomer and other chemicals. Environ Res 14(1):68-72.
https://doi.org/10.1016/0013-9351(77)90067-6.
Hefner RE, Watanabe PG, Gehring PJ. 1975a. Percutaneous absorption of vinyl chloride. Toxicol Appl
Pharmacol 34(3):529-532. https://doi.org/10.1016/0041-008x(75)90149-0.
Hefner RE, Watanabe PG, Gehring PJ. 1975b. Preliminary studies on the fate of inhaled vinyl chloride
monomer (VCM) in rats. Environ Health Perspect 11:85-95. https://doi.org/10.1289/ehp.751185.
Hehir RM, McNamara BP, McLaughlin J, et al. 1981. Cancer induction following single and multiple
exposures to a constant amount of vinyl chloride monomer. Environ Health Perspect 41:63-72.
https://doi.org/10.1289/ehp.814163.
Heldaas SS, Langard SL, Andersen A. 1984. Incidence of cancer among vinyl chloride and polyvinyl
chloride workers. Br J Ind Med 41:25-30. https://doi.org/10.1136/oem.41.1.25.
Heldaas SS, Andersen AA, Langard S. 1987. Incidence of cancer among vinyl chloride and polyvinyl
chloride workers: Further evidence for an association with malignant melanoma. Br J Ind Med
44:278-280. https://doi.org/10.1136/oem.44.4.278.
Ho SF, Phoon WH, Gan SL, et al. 1991. Persistent liver dysfunction among workers at a vinyl chloride
monomer polymerization plant. J Soc Occup Med 41(1):10-16.
https://doi.org/10.1093/occmed/41.1.10.
VINYL CHLORIDE 190
8. RE
FERENCES
Hoffmann D, Patrianakos C, Brunnemann KD, et al. 1976. Chromatographic determination of vinyl
chloride in tobacco smoke. Anal Chem 48(1):47-50. https://doi.org/10.1021/ac60365a063.
Hollstein M, Marion MJ, Lehman T, et al. 1994. P53 mutations at A:T base pairs in angiosarcomas of
vinyl chloride-exposed factory workers. Carcinogenesis 15(1):1-3.
https://doi.org/10.1093/carcin/15.1.1.
Holmberg B, Kronevi T, Winell M. 1976. The pathology of vinyl chloride exposed mice. Acta Vet
Scand 17(3):328-342. https://doi.org/10.1186/BF03547913.
Holt S, Roy G, Mitra S, et al. 2000. Deficiency of N-methylpurine-DNA-glycosylase expression in
nonparenchymal cells, the target cell for vinyl chloride and vinyl fluoride. Mutat Res 460(2):105-
115. https://doi.org/10.1016/s0921-8777(00)00019-7
.
Hong CB, Winston JM, Thornburg LP, et al. 1981. Follow-up study on the carcinogenicity of vinyl
chloride and vinylidene chloride in rats and mice: tumor incidence and mortality subsequent to
exposure. J Toxicol Environ Health 7(6):909-924. https://doi.org/10.1080/15287398109530034.
Howard CJ. 1976. Rate constants for the gas-phase reactions of hydroxyl radicals with ethylene and
halogenated ethylene compounds. J Chem Phys 65:4771-4777. https://doi.org/10.1063/1.432932.
Hozo I, Andelinovic S, Ljutic D, et al. 1997. Two new classes of liver angiosarcoma: History and
perspectives of liver angiosarcoma among plastic industry workers. Toxicol Ind Health 13(5):639-
648. https://doi.org/10.1177/074823379701300505.
Hozo I, Miric D, Bojic L, et al. 2000. Liver angiosarcoma and hemangiopericytoma after occupational
exposure to vinyl chloride monomer. Environ Health Perspect 108(8):793-795.
https://doi.org/10.1289/ehp.00108793.
Hrivnak L, Rozinova Z, Korony S, et al. 1990. Cytogenetic analysis of peripheral blood lymphocytes in
workers exposed to vinyl chloride. Mutat Res 240(2):83-85. https://doi.org/10.1016/0165-
1218(90)90010-y.
Hsiao TJ, Wang JD, Yang PM, et al. 2004. Liver fibrosis in asymptomatic polyvinyl chloride workers. J
Occup Environ Med 46(9):962-966. https://doi.org/10.1097/01.jom.0000137722.66767.38.
Hsieh HI, Chen PC, Wong RH, et al. 2007. Effect of the CYP2E1 genotype on vinyl chloride monomer-
induced liver fibrosis among polyvinyl chloride workers. Toxicology 239(1-2):34-44.
https://doi.org/10.1016/j.tox.2007.06.089.
Hsieh HI, Chen PC, Wong RH, et al. 2011. Mortality from liver cancer and leukaemia among polyvinyl
chloride workers in Taiwan: an updated study. Occup Environ Med 68(2):120-125.
https://doi.org/10.1136/oem.2010.056978.
Hsu YH, Chuang HC, Lee YH, et al. 2019. Induction of fibrosis and autophagy in kidney cells by vinyl
chloride. Cells 8(6):601. https://doi.org/10.3390/cells8060601.
Huang PC, Liu LH, Shie RH, et al. 2016. Assessment of urinary thiodiglycolic acid exposure in school-
aged children in the vicinity of a petrochemical complex in central Taiwan. Environ Res 150:566-
572.
ht
tps://doi.org/10.1016/j.envres.2015.11.027.
Huberman E, Bartsch H, Sachs L. 1975. Mutation induction in Chinese hamster V79 cells by two vinyl
chloride metabolites, chloroethylene oxide and 2-chloroacetaldehyde. Int J Cancer 16(4):639-644.
https://doi.org/10.1002/ijc.2910160414.
Hultmark D, Sundh K, Johansson L, et al. 1979. Ethanol inhibition of vinyl chloride metabolism in
isolated rat hepatocytes. Chem Biol Interact 25(1):1-6. https://doi.org/10.1016/0009-
2797(79)90064-4.
Hussain S, Osterman-Golkar S. 1976. Comment on the mutagenic effectiveness of vinyl chloride
metabolites. Chem Biol Interact 12(3-4):265-267. https://doi.org/10.1016/0009-2797(76)90042-9.
Hüttner E, Holzapfel B. 1996. HPRT mutant frequencies and detection of large deletions by multiplex-
PCR in human lymphocytes of vinyl chloride exposed and non-exposed populations. Toxicol Lett
88(1-3):175-183. https://doi.org/10.1016/0378-4274(96)03735-6.
Hüttner E, Nikolova T. 1998. Cytogenetic analysis of peripheral lymphocytes in a population exposed to
vinyl chloride through an accidental release into the environment. Toxicol Lett 96-97:143-148.
https://doi.org/10.1016/S0378-4274(98)00061-7.
VINYL CHLORIDE 191
8. REFERENCES
Hüttner E, Nikolova T, Foth H. 1998. Genotoxic effects in humans after an accidental vinyl chloride
exposure. Naunyn Schmiedebergs Arch Pharmakol 357(4):R141.
Hüttner E, Gotze A, Nikolova T. 1999. Chromosomal aberrations in humans as genetic endpoints to
assess the impact of pollution. Mutat Res 45:251-257. https://doi.org/10.1016/S1383-
5718(99)00130-8.
IARC. 2012. Vinyl chloride. In: IARC Monographs on the evaluation of carcinogenic risks to humans.
Volume 100F. Chemical agents and related occupations. Lyon, France: International Agency for
Research on Cancer, 451-478. https://publications.iarc.fr/123. February 4, 2021.
ICIS. 2006. Chemical profile: Vinyl chloride. ICIS Chemical Business.
https://www.icis.com/explore/resources/news/2006/10/06/2016491/chemical-profile-vinyl-chloride/.
October 26, 2021.
Infante PF. 1976. Oncogenic and mutagenic risks in communities with polyvinyl chloride production
facilities. Ann N Y Acad Sci 271:49-57. https://doi.org/10.1111/j.1749-6632.1976.tb23092.x.
Infante PF, Wagoner JK, McMichael AJ, et al. 1976a. Genetic risks of vinyl chloride. Lancet
307(7962):734-735. https://doi.org/10.1016/s0140-6736(76)93103-2.
Infante PF, Wagoner JK, Waxweiler RJ. 1976b. Carcinogenic, mutagenic and teratogenic risks
associated with vinyl chloride. Mutat Res 41:131-141. https://doi.org/10.1016/0027-
5107(76)90083-x.
Ivanetich KM, Aronson I, Katz ID. 1977. The interaction of vinyl chloride with rat hepatic microsomal
cytochrome P-450 in vitro. Biochem Biophys Res Commun 74(4):1411-1418.
https://doi.org/10.1016/0006-291x(77)90599-x.
Jacobsen JS, Perkins CP, Callahan JT, et al. 1989. Mechanisms of mutagenesis by chloroacetaldehyde.
Genetics 121(2):213-222. https://doi.org/10.1093/genetics/121.2.213.
Jaeger RJ, Reynolds ES, Conolly RB, et al. 1974. Acute hepatic injury by vinyl chloride in rats
pretreated with phenobarbital. Nature 252(5485):724-726. https://doi.org/10.1038/252724a0.
Jaeger RJ, Murphy SD, Reynolds ES, et al. 1977. Chemical modification of acute hepatotoxicity of vinyl
chloride monomer in rats. Toxicol Appl Pharmacol 41(3):597-607. https://doi.org/10.1016/s0041-
008x(77)80013-6.
Jayson MIV, Bailey AJ, Black C, et al. 1976. Clinical aspects of vinyl chloride disease: Collagen studies
in acroosteolysis. Proc R Soc Med 69:295-297. https://doi.org/10.1177/003591577606900424.
Jedrychowski RA, Sokal JA, Chmielnicka J. 1984. Influence of exposure mode on vinyl chloride action.
Arch Toxicol 55(3):195-198. https://doi.org/10.1007/BF00316128.
Jedrychowski RA, Sokal JA, Chmielnicka J. 1985. Comparison of the impact of continuous and
intermittent exposure to vinyl chloride, including phenobarbital effect. J Hyg Epidemiol Microbiol
Immunol 29(2):111-120.
Ji F, Wang W, Xia ZL, et al. 2010. Prevalence and persistence of chromosomal damage and susceptible
genotypes of metabolic and DNA repair genes in Chinese vinyl chloride-exposed workers.
Carcinogenesis 31(4):648-653. https://doi.org/10.1093/carcin/bgq015.
Jia C, Foran J. 2013. Air toxics concentrations, source identification, and health risks: An air pollution
hot spot in southwest Memphis, TN. Atmos Environ 81:112-116.
https://doi.org/10.1016/j.atmosenv.2013.09.006.
Jia J, Chen SQ, Pan WZ, et al. 2022. Mechanism of subchronic vinyl chloride exposure combined with a
high-fat diet on hepatic steatosis. J Appl Toxicol 42(3):490-505. https://doi.org/10.1002/jat.4234.
Jiao J, Feng NN, Li Y, et al. 2012. Estimation of a safe level for occupational exposure to vinyl chloride
using a benchmark dose method in central China. J Occup Health 54(4):263-270.
https://doi.org/10.1539/joh.11-0157-oa.
John JA, Smith FA, Schwetz BA. 1981. Vinyl chloride: inhalation teratology study in mice, rats and
rabbits. Environ Health Perspect 41:171-177. https://doi.org/10.1289/ehp.8141171.
John JA, Smith FA, Leong BK, et al. 1977. The effects of maternally inhaled vinyl chloride on
embryonal and fetal development in mice, rats, and rabbits. Toxicol Appl Pharmacol 39(3):497-513.
https://doi.org/10.1016/0041-008x(77)90141-7.
VINYL CHLORIDE 192
8. REFERENCES
Jones DB, Smith PM. 1982. Progression of vinyl chloride induced hepatic fibrosis to angiosarcoma of
the liver. Br J Ind Med 39(3):306-307. https://doi.org/10.1136/oem.39.3.306.
Jones RD, Smith DM, Thomas PG. 1988. A mortality study of vinyl chloride monomer workers
employed in the United Kingdom in 1940-1974. Scand J Work Environ Health 14(3):153-160.
https://doi.org/10.5271/sjweh.1937.
Jury WA, Spencer WF, Farmer WJ. 1984. Behavior assessment model for trace organics in soil: III.
Application of screening model. J Environ Qual 13:573-579.
https://doi.org/10.2134/jeq1984.00472425001300040013x.
Kaelin BR, McKenzie CM, Hempel KW, et al. 2020. Adipose tissue-liver crosstalk during pathologic
changes caused by vinyl chloride metabolites in mice. Toxicol Appl Pharmacol 399:115068.
https://doi.org/10.1016/j.taap.2020.115068.
Kandala JC, Mrema JE, DeAngelo A, et al. 1990. 2-Chloroacetaldehyde and 2-chloroacetal are potent
inhibitors of DNA synthesis in animal cells. Biochem Biophys Res Commun 167(2):457-463.
https://doi.org/10.1016/0006-291x(90)92045-2.
Kappus H, Bolt HM, Buchter A, et al. 1976. Liver microsomal uptake of [14C]vinyl chloride and
transformation to protein alkylating metabolites in vitro. Toxicol Appl Pharmacol 37(3):461-471.
https://doi.org/10.1016/0041-008x(76)90208-8.
Kielhorn J, Melber C, Wahnschaffe U, et al. 2000. Vinyl chloride: still a cause for concern. Environ
Health Perspect 108(7):579-588. https://doi.org/10.1289/ehp.00108579.
Knight KR, Gibbons R. 1987. Increased collagen synthesis and cross-link formation in the skin of rats
exposed to vinyl chloride monomer. Clin Sci (Lond) 72(6):673-678.
https://doi.org/10.1042/cs0720673.
Konkle SL, Zierold KM, Taylor KC, et al. 2020. National secular trends in ambient air volatile organic
compound levels and biomarkers of exposure in the United States. Environ Res 182:108991.
https://doi.org/10.1016/j.envres.2019.108991.
Kotseva K. 1996. Five year follow-up study of the incidence of arterial hypertension and coronary heart
disease in vinyl chloride monomer and polyvinyl chloride production workers. Int Arch Occup
Environ Health 68(6):377-379. https://doi.org/10.1007/bf00377854.
Kowalczyk P, Ciesla JM, Saparbaev M, et al. 2006. Sequence-specific p53 gene damage by
chloroacetaldehyde and its repair kinetics in Escherichia coli. Acta Biochim Pol 53(2):337-347.
https://doi.org/10.18388/abp.2006_3347.
Krajewski J, Dobecki M, Gromiec J. 1980. Retention of vinyl chloride in the human lung. Br J Ind Med
37(4):373-374. https://doi.org/10.1136/oem.37.4.373.
Krishnan K, Andersen ME, Clewell HJ, et al. 1994. Physiologically based pharmacokinetic modeling of
chemical mixtures. In: Yang RSA, ed. Toxicology of chemical mixtures. New York, NY:
Academic Press, 399-437.
Krock R. 2018. Vinyl Institute comments on Cicalese et al.: "An ecological study of the association
between air pollution and hepatocellular carcinoma incidence in Texas". Liver Cancer 7(3):295-296.
https://doi.org/10.1159/000486432.
Kucerova M, Polivkova Z, Batora J. 1979. Comparative evaluation of the frequency of chromosomal
aberrations and the SCE numbers in peripheral lymphocytes of workers occupationally exposed to
vinyl chloride monomer. Mutat Res 67(1):97-100. https://doi.org/10.1016/0165-1218(79)90105-8.
Kuchenmeister F, Wang M, Klein RG, et al. 1996. Transport of reactive metabolites of procarcinogens
between different liver cell types, as demonstrated by the single cell microgel electrophoresis assay.
Toxicol Lett 88(1 to 3):29-34. https://doi.org/10.1016/0378-4274(96)03714-9.
Kudo YY, Yamada S, Nakamura I, et al. 1990. On the changes in peripheral red cells in mice exposed to
vinyl chloride monomer. Pol J Occup Med 3(3):301-310.
Kumar AK, Balachandar V, Arun M, et al. 2013. A comprehensive analysis of plausible genotoxic
covariates among workers of a polyvinyl chloride plant exposed to vinyl chloride monomer. Arch
Environ Contam Toxicol 64(4):652-658. https://doi.org/10.1007/s00244-012-9857-1.
VINYL CHLORIDE 193
8. REFERENCES
Kwok ESC, Atkinson R. 1995. Estimation of hydroxyl radical reaction rate constants for gas-phase
organic compounds using a structure-reactivity relationship: An update. Atmos Environ
29(14):1685-1695. https://doi.org/10.1016/1352-2310(95)00069-B
Laib RJ. 1982. Specific covalent binding and toxicity of aliphatic halogenated xenobiotics. Q Rev Drug
Metab Drug Interact 4(1):1-48. https://doi.org/10.1515/dmdi.1982.4.1.1.
Laib RJ. 1986. The role of cyclic base adducts in vinyl-chloride-induced carcinogenesis: studies on
nucleic acid alkylation in vivo. IARC Sci Publ 70(70):101-108.
Laib RJ, Bolt HM. 1977. Alkylation of RNA by vinyl chloride metabolites in vitro and in vivo:
Formation of 1-N-6-ethenoadenosine. Toxicology 8:185-195. https://doi.org/10.1016/0300-
483X(77)90007-5.
Laib RJ, Stockle G, Bolt HM, et al. 1979. Vinyl chloride and trichloroethylene: comparison of alkylating
effects of metabolites and induction of preneoplastic enzyme deficiencies in rat liver. J Cancer Res
Clin Oncol 94(2):139-147. https://doi.org/10.1007/BF00422494.
Laib RJ, Gwinner LM, Bolt HM. 1981. DNA alkylation by vinyl chloride metabolites: Etheno
derivatives or 7-alkylation of guanine? Chem Biol Interact 37(1-2):219-231.
https://doi.org/10.1016/0009-2797(81)90179-4.
Laib RJ, Klein KP, Bolt HM. 1985. The rat liver foci bioassay: I. Age-dependence of induction by vinyl
chloride of ATPase-deficient foci. Carcinogenesis 6(1):65-68. https://doi.org/10.1093/carcin/6.1.65.
Laib RJ, Bolt HM, Cartier R, et al. 1989. Increased alkylation of liver DNA and cell turnover in young
versus old rats exposed to vinyl chloride correlates with cancer susceptibility. Toxicol Lett 45(2-
3):231-239. https://doi.org/10.1016/0378-4274(89)90014-3.
Lang AL, Chen L, Poff GD, et al. 2018. Vinyl chloride dysregulates metabolic homeostasis and
enhances diet-induced liver injury in mice. Hepatol Commun 2(3):270-284.
https://doi.org/10.1002/hep4.1151.
Lang AL, Krueger AM, Schnegelberger RD, et al. 2019. Rapamycin attenuates liver injury caused by
vinyl chloride metabolite chloroethanol and lipopolysaccharide in mice. Toxicol Appl Pharmacol
382:114745. https://doi.org/10.1016/j.taap.2019.114745.
Lang AL, Goldsmith WT, Schnegelberger RD, et al. 2020. Vinyl chloride and high-fat diet as a model of
environment and obesity interaction. J Vis Exp 155:e60351. https://doi.org/10.3791/60351.
Langard S, Rosenberg J, Andersen A, et al. 2000. Incidence of cancer among workers exposed to vinyl
chloride in polyvinyl chloride manufacture. Occup Environ Med 57:65-68.
https://doi.org/10.1136/oem.57.1.65.
Langauer-Lewowicka H, Dudziak Z, Byczkowska Z, et al. 1976. Cryoglobulinemia in Raynaud's
phenomenon due to vinyl chloride. Int Arch Occup Environ Health 35:197-207.
https://doi.org/10.1007/BF00378274.
Langauer-Lewowicka H, Kurzbauer H, Byczkowska Z, et al. 1983. Vinyl chloride disease-neurological
disturbances. Int Arch Occup Environ Health 52(2):151-157. https://doi.org/10.1007/BF00405418.
Laplanche A, Clavel F, Contassot JC, et al. 1987. Exposure to vinyl chloride monomer: report on a
cohort study. Br J Ind Med 44(10):711-715. https://doi.org/10.1136/oem.44.10.711.
Laplanche A, Clavel-Chapelon F, Contassot JC, et al. 1992. Exposure to vinyl chloride monomer: results
of a cohort study after a seven year follow up. The French VCM Group. Br J Ind Med 49(2):134-
137. https://doi.org/10.1136/oem.49.2.134.
Laumbach AD, Lee S, Wong J, et al. 1977. Studies on the mutagenicity of vinyl chloride metabolites
and related chemicals. Prev Detect Cancer Proc Int Symp 1976 1:155-170.
Laval J, Saparbaev M. 2001. Enzymology of the repair in human cells and in Escherichia coli K12 of
mutagenic ethenoadducts. Mutat Res 483:S35.
Lee CC, Bhandari JC, Winston JM, et al. 1977a. Inhalation toxicity of vinyl chloride and vinylidene
chloride. Environ Health Perspect 21:25-32. https://doi.org/10.1289/ehp.772125.
Lee FI, Harry DS, Adams WG, et al. 1977b. Screening for liver disease in vinyl chloride workers. Br J
Ind Med 34(2):142-147. https://doi.org/10.1136/oem.34.2.142.
VINYL CHLORIDE 194
8. REFERENCES
Lee CC, Bhandari JC, Winston JM, et al. 1978. Carcinogenicity of vinyl chloride and vinylidene
chloride. J Toxicol Environ Health 4(1):15-30. https://doi.org/10.1080/15287397809529640.
Lee FI, Smith PM, Bennett B, et al. 1996. Occupationally related angiosarcoma of the liver in the United
Kingdom 1972-1994. Gut 39(2):312-318. https://doi.org/10.1136/gut.39.2.312.
Lee CC, Shen Y, Hsu CW, et al. 2020. Reduced adiponectin:leptin ratio associated with inhalation
exposure to vinyl chloride monomer. Sci Total Environ 703:135488.
https://doi.org/10.1016/j.scitotenv.2019.135488.
Lei YC, Yang HT, Ma YC, et al. 2004. DNA single strand breaks in peripheral lymphocytes associated
with urinary thiodiglycolic acid levels in polyvinyl chloride workers. Mutat Res 561(1-2):119-126.
https://doi.org/10.1016/j.mrgentox.2004.04.002.
Lelbach WK. 1996. A 25-year follow-up study of heavily exposed vinyl chloride workers in Germany.
Am J Ind Med 29(5):446-458. https://doi.org/10.1002/(SICI)1097-0274(199605)29:5<446::AID-
AJIM3>3.0.CO;2-K.
Lester D, Greenberg LA, Adams WR. 1963. Effects of single and repeated exposures of humans and rats
to vinyl chloride. Am Ind Hyg Assoc J 24:265-275. https://doi.org/10.1080/00028896309342963.
Lewis RJ. 1996. Vinyl chloride. In: Sax's dangerous properties of industrial materials. 9
th
ed. New
York, NY: Van Nostrand Reinhold, 3377-3378.
Lewis R. 2001. Use of rank-order analysis of ordinal exposure data: Application to vinyl chloride
exposure. Appl Occup Environ Hyg 16:188-191. https://doi.org/10.1080/104732201460325.
Lewis R, Rempala G. 2003. A case-cohort study of angiosarcoma of the liver and brain cancer at a
polymer production plant. J Occup Environ Med 45(5):538-545.
Lewis RJ, Rempala G, Dell LD, et al. 2003. Vinyl chloride and liver and brain cancer at a polymer
production plant in Louisville, Kentucky. J Occup Environ Med 45(5):533-537.
https://doi.org/10.1097/01.jom.0000058348.05741.1d.
Li Y, Marion MJ, Asherova M, et al. 1998. Mutant p21ras in vinyl chloride exposed workers.
Biomarkers 3(6):433-439. https://doi.org/10.1080/135475098231075.
Li Y, Marion MJ, Rundle A, et al. 2003a. A common polymorphism in XRCC1 as a biomarker of
susceptibility for chemically induced genetic damage. Biomarkers 8(5):408-414.
https://doi.org/10.1080/13547500310001619301.
Li Y, Marion MJ, Ho R, et al. 2003b. Polymorphisms for vinyl chloride metabolism in French vinyl
chloride workers. Int J Occup Med Environ Health 16(1):55-59.
Li Y, Lee S, Marion MJ, et al. 2005a. Polymorphisms of microsomal epoxide hydrolase in French vinyl
chloride workers. Int J Occup Med Environ Health 18(2):133-138.
Li Y, Zhou M, Marion MJ, et al. 2005b. Polymorphisms in glutathione S-transferases in French vinyl
chloride workers. Biomarkers 10(1):72-79. https://doi.org/10.1080/13547500500070364.
Li Y, Marion MJ, Zipprich J, et al. 2006. The role of XRCC1 polymorphisms in base excision repair of
etheno-DNA adducts in French vinyl chloride workers. Int J Occup Med Environ Health 19(1):45-
52. https://doi.org/10.2478/v10001-006-0006-9.
Li Y, Long C, Lin G, et al. 2009a. Effect of the XRCC1 codon 399 polymorphism on the repair of vinyl
chloride metabolite-induced DNA damage. J Carcinog 8:14. https://doi.org/10.4103/1477-
3163.56290.
Li Y, Marion MJ, Zipprich J, et al. 2009b. Gene-environment interactions between DNA repair
polymorphisms and exposure to the carcinogen vinyl chloride. Biomarkers 14(3):148-155.
https://doi.org/10.1080/13547500902811266.
Li Y, Feng NN, Zhang GH, et al. 2013. Polymorphisms in the p53 pathway genes and micronucleus
occurrence in Chinese vinyl chloride-exposed workers. Int J Occup Med Environ Health 26(6):825-
836. https://doi.org/10.2478/s13382-013-0155-6.
Liang Y, Lang AL, Zhang J, et al. 2018. Exposure to vinyl chloride and its influence on western diet-
induced cardiac remodeling. Chem Res Toxicol 31(6):482-493.
https://doi.org/10.1021/acs.chemrestox.8b00043.
VINYL CHLORIDE 195
8. REFERENCES
Liber HL, Kaufmann W, Marion M, et al. 1999. Exposure to vinyl chloride is associated with a
persistent increase in HRPT-mutants in the peripheral blood lymphocytes. Proc Am Assoc Cancer
Res 40:508.
Lilis R, Anderson H, Nicholson WJ, et al. 1975. Prevalence of disease among vinyl chloride and
polyvinyl chloride workers. Ann NY Acad Sci 246:22-41. https://doi.org/10.1111/j.1749-
6632.1975.tb51078.x.
Lilis R, Anderson H, Miller A, et al. 1976. Pulmonary changes among vinyl chloride polymerization
workers. Chest 69:299-303. https://doi.org/10.1378/chest.69.2_Supplement.299.
Lim GQ, John K. 2020. Impact of energy production in the Barnett Shale gas region on the measured
ambient hydrocarbon concentrations in Denton, Texas. Atmos Pollut Res 11(2):409-418.
https://doi.org/10.1016/j.apr.2019.11.013.
Liss GM, Greenberg RA, Tamburro CH. 1985. Use of serum bile acids in the identification of vinyl
chloride hepatotoxicity. Am J Med 78:68-76. https://doi.org/10.1016/0002-9343(85)90464-4.
Liu S, He L, Bannister OB, et al. 2023. Western diet unmasks transient low-level vinyl chloride-induced
tumorigenesis; potential role of the (epi-)transcriptome. Toxicol Appl Pharmacol 468:116514.
https://doi.org/10.1016/j.taap.2023.116514.
Lloyd MH, Gauld S, Copland L, et al. 1984. Epidemiological study of the lung function of workers at a
factory manufacturing polyvinyl chloride. Br J Ind Med 41:328-333.
https://doi.org/10.1136/oem.41.3.328.
Lopez V, Chamoux A, Tempier M, et al. 2013. The long-term effects of occupational exposure to vinyl
chloride monomer on microcirculation: a cross-sectional study 15 years after retirement. BMJ Open
3(6) https://doi.org/10.1136/bmjopen-2013-002785.
Loprieno N, Barale R, Baroncelli S, et al. 1976. Evaluation of the genetic effects induced by vinyl
chloride monomer (VCM) under mammalian metabolic activation: Studies in vitro and in vivo.
Mutat Res 40(2):85-95. https://doi.org/10.1016/0165-1218(76)90002-1.
Loprieno N, Barale R, Baroncelli S, et al. 1977. Induction of gene mutations and gene conversions by
vinyl chloride metabolites in yeast. Cancer Res 37(1):253-257.
Lu PY, Metcalf RL, Plummer N, et al. 1977. The environmental fate of three carcinogens: benzo-(α)-
pyrene, benzidine, and vinyl chloride evaluated in laboratory model ecosystems. Arch Environ
Contam Toxicol 6(2-3):129-142. https://doi.org/10.1007/BF02097756.
Lu C, Bjerg PL, Zhang F, et al. 2011. Sorption of chlorinated solvents and degradation products on
natural clayey tills. Chemosphere 83(11):1467-1474.
https://doi.org/10.1016/j.chemosphere.2011.03.007.
Luo JC, Liu HT, Cheng TJ, et al. 1998. Plasma Asp13-Ki-ras oncoprotein expression in vinyl chloride
monomer workers in Taiwan. J Occup Environ Med 40(12):1053-1058.
Luo JC, Liu HT, Cheng TJ, et al. 1999. Plasma p53 protein and anti-p53 antibody expression in vinyl
chloride monomer workers in Taiwan. J Occup Environ Med 41(6):521-526.
https://doi.org/10.1097/00043764-199906000-00020.
Luo JC, Cheng TJ, Du CL, et al. 2003. Molecular epidemiology of plasma oncoproteins in vinyl chloride
monomer workers in Taiwan. Cancer Detect Prev 27:94-101. https://doi.org/10.1016/S0361-
090X(03)00021-7.
Luong C, Zhang K. 2017. An assessment of emission event trends within the Greater Houston area
during 2003-2013. Air Qual Atmos Health 10(5):543-554. https://doi.org/10.1007/s11869-016-
0449-5.
Lyman WJ, Reehl WF, Rosenblatt DH. 1982. Regression equations for the estimation of K
oc
. In:
Handbook of chemical property estimation methods. Environmental behavior of organic
compounds. New York, NY: McGraw Hill Book Co., 4-9.
Maciejewska AM, Ruszel KP, Nieminuszczy J, et al. 2010. Chloroacetaldehyde-induced mutagenesis in
Escherichia coli: The role of AlkB protein in repair of 3,N
4
-ethenocytosine and 3,N
4
-α-
hydroxyethanocytosine. Mutat Res 684(1-2):24-34. https://doi.org/10.1016/j.mrfmmm.2009.11.005.
VINYL CHLORIDE 196
8. REFERENCES
Magnavita N, Bergamaschi A, Garcovich A, et al. 1986. Vasculitic purpura in vinyl chloride disease: a
case report. Angiology 37(5):382-388. https://doi.org/10.1177/000331978603700508.
Magnusson J, Ramel C. 1978. Mutagenic effects of vinyl chloride on Drosophila melanogaster with and
without pretreatment with sodium phenobarbiturate. Mutat Res 57(3):307-312.
https://doi.org/10.1016/0027-5107(78)90215-4.
Malaveille C, Bartsch H, Barbin A, et al. 1975. Mutagenicity of vinyl chloride, chloroethyleneoxide,
chloroacetaldehyde and chloroethanol. Biochem Biophys Res Commun 63(2):363-370.
https://doi.org/10.1016/0006-291x(75)90697-x.
Maltoni C, Lefemine G. 1975. Carcinogenicity bioassays of vinyl chloride: current results. Ann N Y
Acad Sci 246:195-218. https://doi.org/10.1111/j.1749-6632.1975.tb51094.x.
Maltoni C, Cotti G. 1988. Carcinogenicity of vinyl chloride in Sprague-Dawley rats after prenatal and
postnatal exposure. Ann N Y Acad Sci 534:145-159. https://doi.org/10.1111/j.1749-
6632.1988.tb30108.x.
Maltoni C, Lefemine G, Ciliberti A, et al. 1981. Carcinogenicity bioassays of vinyl chloride monomer: a
model of risk assessment on an experimental basis. Environ Health Perspect 41:3-29.
https://doi.org/10.1289/ehp.81413.
Maricq HR, Johnson MN, Whetstone CL, et al. 1976. Capillary abnormalities in polyvinyl chloride
production workers. Examination by in vivo microscopy. JAMA 236(12):1368-1371.
https://doi.org/10.1001/jama.1976.03270130030022.
Marion MJ. 1998. Critical genes as early warning signs: Example of vinyl chloride. Toxicol Lett 102-
103:603-607. https://doi.org/10.1016/S0378-4274(98)00255-0.
Marion MJ, Boivin-Angele S. 1999. Vinyl chloride-specific mutations in humans and animals. IARC
Sci Publ 150(150):315-324.
Marion MJ, Froment O, Trepo C. 1991. Activation of Ki-ras gene by point mutation in human liver
angiosarcoma associated with vinyl chloride exposure. Mol Carcinog 4(6):450-454.
https://doi.org/10.1002/mc.2940040607.
Markowitz SS, McDonald CJ, Fethiere W, et al. 1972. Occupational acroosteolysis. Arch Dermatol
106(2):219-223. https://doi.org/10.1001/archderm.1972.01620110051012.
Maroni M, Fanetti AC. 2006. Liver function assessment in workers exposed to vinyl chloride. Int Arch
Occup Environ Health 79(1):57-65. https://doi.org/10.1007/s00420-005-0018-y.
Maroni M, Mocci F, Visentin S, et al. 2003. Periportal fibrosis and other liver ultrasonography findings
in vinyl chloride workers. Occup Environ Med 60(1):60-65. https://doi.org/10.1136/oem.60.1.60.
Marsh GM, Towle KM. 2018. Methodological limitations to the analysis by Cicalese et al. Liver Cancer
7(3):299-300. https://doi.org/10.1159/000489022.
Marsh GM, Youk AO, Buchanich JM, et al. 2007a. Mortality patterns among industrial workers exposed
to chloroprene and other substances. I. General mortality patterns. Chem Biol Interact 166(1-
3):285-300. https://doi.org/10.1016/j.cbi.2006.08.011.
Marsh GM, Youk AO, Buchanich JM, et al. 2007b. Mortality patterns among industrial workers exposed
to chloroprene and other substances. II. Mortality in relation to exposure. Chem Biol Interact
166(1-3):301-316. https://doi.org/10.1016/j.cbi.2006.08.012.
Marsh GM, Kruchten A, Buchanich JM. 2021. Mortality patterns among industrial workers exposed to
chloroprene and other substances: Extended follow-up. J Occup Environ Med 63(2):126-138.
https://doi.org/10.1097/jom.0000000000002093.
Marsteller HJ, Lelbach WK, Muller R, et al. 1975. Unusual splenomegalic liver disease as evidenced by
peritoneoscopy and guided liver biopsy among polyvinyl chloride production workers. Ann NY
Acad Sci 246:95-134. https://doi.org/10.1111/j.1749-6632.1975.tb51085.x.
Mastrangelo G, Manno M, Marcer G, et al. 1979. Polyvinyl chloride pneumoconiosis: epidemiological
study of exposed workers. J Occup Med 21(8):540-542.
Mastrangelo G, Fedeli U, Fadda E, et al. 2003. Lung cancer risk in workers exposed to poly(vinyl
chloride) dust: a nested case-referent study. Occup Environ Med 60(6):423-428.
https://doi.org/10.1136/oem.60.6.423.
VINYL CHLORIDE 197
8. REFERENCES
Mastrangelo G, Fedeli U, Fadda E, et al. 2004. Increased risk of hepatocellular carcinoma and liver
cirrhosis in vinyl chloride workers: synergistic effect of occupational exposure with alcohol intake.
Environ Health Perspect 112(11):1188-1192. https://doi.org/10.1289/ehp.6972.
Mastrangelo G, Cegolon L, Fadda E, et al. 2013. Comment to "Vinyl chloride exposure and cirrhosis: a
systematic review and meta-analysis". Dig Liver Dis 45(8):701-702.
https://doi.org/10.1016/j.dld.2013.02.006.
Mastromatteo E, Fisher AM, Christie H, et al. 1960. Acute inhalation toxicity of vinyl chloride to
laboratory animals. Am Ind Hyg Assoc J 21:394-398. https://doi.org/10.1080/00028896009344092.
Matsuda T, Yagi T, Kawanishi M, et al. 1995. Molecular analysis of mutations induced by 2-
chloroacetaldehyde, the ultimate carcinogenic form of vinyl chloride, in human cells using shuttle
vectors. Carcinogenesis 16(10):2389-2394. https://doi.org/10.1093/carcin/16.10.2389.
McCann J, Simmon V, Streitwieser D, et al. 1975. Mutagenicity of chloroacetaldehyde, a possible
metabolic product of 1,2-dichloroethane (ethylene dichloride), chloroethanol (ethylene
chlorohydrin), vinyl chloride, and cyclophosphamide. Proc Natl Acad Sci U S A 72(8):3190-3193.
https://doi.org/10.1073/pnas.72.8.3190.
McCarthy MC, Hafner HR, Montzka SA. 2006. Background concentrations of 18 air toxics for North
America. J Air Waste Manag Assoc 56(1):3-11. https://doi.org/10.1080/10473289.2006.10464436.
McClain C, Lewis R, Reynolds L, et al. 2002. The serum hyaluronic concentration is a screening test for
angiosarcoma in plant workers exposed to vinyl chloride. Hepatology 36(4):706A.
McMahon PB, Lindsey BD, Conlon MD, et al. 2019. Hydrocarbons in upland groundwater, Marcellus
Shale Region, Northeastern Pennsylvania and Southern New York, U.S.A. Environ Sci Technol
53(14):8027-8035. https://doi.org/10.1021/acs.est.9b01440.
McNeal TP, Olivo C, Begley TH. 2003. Determination of residual vinyl chloride and vinylidene
chloride in food packages containing polyvinyl chloride and Saran resins. In: FDA science:
Protecting America's health: 2003 FDA Science Forum: April 24-25, 2003. Washington, DC: U.S.
Food and Drug Administration., 40-41.
Meylan WM, Howard PH, Boethling RS, et al. 1999. Improved method for estimating
bioconcentration/bioaccumulation factor from octanol/water partition coefficient. Environ Toxicol
Chem 18(4):664-672. https://doi.org/10.1002/etc.5620180412.
Micu D, Mihailescu E, Vilau C, et al. 1985. The value of some cytoenzymochemical investigations of
the leukocytes and platelets in estimating the effects of occupational exposure to benzene, vinyl
chloride and carbon disulphide. Med Interne 23(2):115-120.
Miller JJ, Beizer MB. 1985. Air quality in residences adjacent to an active hazardous waste disposal site.
In: Proceedings: 78
th
APCA Annual Meeting, June 16-21, 1985 Detroit, Michigan. Air Pollution
Control Association, 2-17.
Mirkova E, Mihaylova A, Nosko M. 1978. [Embryotoxic and teratogenic effects in vinyl chloride]. Hig
Zdraveopaz 21:440-443. (Bulgarian)
Monson RR, Peters JM, Johnson MN. 1975. Proportional mortality among vinyl chloride workers.
Environ Health Perspect 11:75-77. https://doi.org/10.1016/S0140-6736(74)91771-1.
Morinello EJ, Ham AJL, Ranasinghe A, et al. 2002a. Molecular dosimetry and repair of N2,3
ethenoguanine in rats exposed to vinyl chloride. Cancer Res 62:5189-5195.
Morinello EJ, Koe H, Ranasinghe A, et al. 2002b. Differential induction of N2,3-ethenoguanine in rat
brain and liver after exposure to vinyl chloride. Cancer Res 62:5183-5188.
Müller JPH, Korte F. 1977. Beiträge zur okologischen chemie CXXXVI kurze mitteilung zur nicht
sensibilisierten photooxidation von chloräthylenen. Chemosphere 6(6):341-346.
https://doi.org/10.1016/0045-6535(77)90098-4.
Müller G, Norpoth K, Wickramasinghe RH. 1979. An analytical method using GC-MS for the
quantitative determination of urinary thiodiglycolic acid. Int Arch Occup Environ Health 44:185-
191. https://doi.org/10.1007/BF00381133.
VINYL CHLORIDE 198
8. REFERENCES
Mumtaz MM, Ray M, Crowell SR, et al. 2012. Translational research to develop a human PBPK models
tool kit-volatile organic compounds (VOCs). J Toxicol Environ Health A 75(1):6-24.
https://doi.org/10.1080/15287394.2012.625546.
Mundt KA, Dell LD, Austin RP, et al. 2000. Historical cohort study of 10,109 men in the North
American vinyl chloride industry, 1942-1972: Update of cancer mortality to 31 December 1995.
Occup Environ Med 57(11):774-781. https://doi.org/10.1136/oem.57.11.774.
Mundt KA, Dell LD, Crawford L, et al. 2017. Quantitative estimated exposure to vinyl chloride and risk
of angiosarcoma of the liver and hepatocellular cancer in the US industry-wide vinyl chloride cohort:
mortality update through 2013. Occup Environ Med 74(10):709-716.
https://doi.org/10.1136/oemed-2016-104051.
Mutlu E, Collins LB, Stout MD, et al. 2010. Development and application of an LC-MS/MS method for
the detection of the vinyl chloride-induced DNA adduct N(2),3-ethenoguanine in tissues of adult and
weanling rats following exposure to [(13)C(2)]-VC. Chem Res Toxicol 23(9):1485-1491.
https://doi.org/10.1021/tx1001767.
Mutlu E, Jeong YC, Collins LB, et al. 2012. A new LC-MS/MS method for the quantification of
endogenous and vinyl chloride-induced 7-(2-Oxoethyl)guanine in Sprague-Dawley rats. Chem Res
Toxicol 25(2):391-399. https://doi.org/10.1021/tx200447w.
NAS/NRC. 2006. Human biomonitoring for environmental chemicals. Washington, DC: The National
Academies Press, National Research Council. https://doi.org/10.17226/11700.
Nelson YM, Jewell WJ. 1993. Vinyl chloride biodegradation with methanotrophic attached films. J
Environ Eng ASCE 119(5):890-907. https://doi.org/10.1061/(ASCE)0733-9372(1993)119:5(890).
NIOSH. 1977. A cross-sectional epidemiologic survey of vinyl chloride workers. Cincinnati, OH:
National Institute for Occupational Safety and Health. PB274193.
https://ntrl.ntis.gov/NTRL/dashboard/searchResults/titleDetail/PB274193.xhtml. August 29, 2021.
NIOSH. 1986. Chemical review: Vinyl chloride. Dangerous properties of industrial materials report.
Vol. 6. Washington, DC: National Institute for Occupational Safety and Health. 13-43.
NIOSH00188891.
NIOSH. 1990. Vinyl chloride. In: NIOSH pocket guide to chemical hazards. Washington, DC:
National Institute for Occupational Safety and Health, 4-5, 224-225.
NIOSH. 2019. Vinyl chloride. NIOSH pocket guide to chemical hazards. National Institute for
Occupational Safety and Health. https://www.cdc.gov/niosh/npg/npgd0658.html. February 4, 2021.
Nivard MJ, Vogel EW. 1999. Genetic effects of exocyclic DNA adducts in vivo: heritable genetic
damage in comparison with loss of heterozygosity in somatic cells. IARC Sci Publ 150(150):335-
349.
NLM. 2023. PubChem: Vinyl chloride. National Library of Medicine.
https://pubchem.ncbi.nlm.nih.gov/compound/Vinyl-chloride. October 4, 2023.
Norpoth K, Heger M, Mueller G, et al. 1986. Investigations on metabolism genotoxic effects and
carcinogenicity of 2,2-dichlorodiethyl ether. J Cancer Res Clin Oncol 112:125-130.
https://doi.org/10.1007/BF00404394.
NTP. 2013. Draft OHAT approach for systematic review and evidence integration for literature-based
health assessments - February 2013. National Toxicology Program.
https://ntp.niehs.nih.gov/ntp/ohat/evaluationprocess/draftohatapproach_february2013.pdf. March 24,
2021.
NTP. 2015. Handbook for conducting a literature-based health assessment using OHAT approach for
systematic review and evidence integration. National Toxicology Program. PB2016102134.
https://ntrl.ntis.gov/NTRL/dashboard/searchResults/titleDetail/PB2016102134.xhtml. October 3,
2022.
NTP. 2021. Vinyl halides (selected). In: Report on carcinogens. 15
th
ed. National Toxicology
Program, https://ntp.niehs.nih.gov/sites/default/files/ntp/roc/content/profiles/vinylhalides.pdf. July
19, 2023.
VINYL CHLORIDE 199
8. REFERENCES
NTSB. 2023. Norfolk Southern Railway train derailment with subsequent hazardous material release and
fires. National Transportation Safety Board. Preliminary Report: RRD23MR005.
https://www.ntsb.gov/investigations/Pages/RRD23MR005.aspx. September 5, 2023.
Oesch F, Doerjer G. 1982. Detection of N2,3-ethanoguanine in DNA after treatment with
chloroacetaldehyde in vitro. Carcinogenesis 3(6):663-665. https://doi.org/10.1093/carcin/3.6.663.
Ojajarvi A, Partanen T, Ahlbom A, et al. 2001. Risk of pancreatic cancer in workers exposed to
chlorinated hydrocarbon solvents and related compounds: a meta-analysis. Am J Epidemiol
153(9):841-850. https://doi.org/10.1093/aje/153.9.841.
OSHA. 2021a. Occupational safety and health standards. Subpart Z: Vinyl chloride. Occupational
Safety and Health Administration. Code of Federal Regulations. 29 CFR 1910.1017.
https://www.govinfo.gov/content/pkg/CFR-2021-title29-vol6/pdf/CFR-2021-title29-vol6-sec1910-
1017.pdf. July 19, 2023.
OSHA. 2021b. Occupational safety and health standards for shipyard employment. Toxic and hazardous
substances. Vinyl chloride. Occupational Safety and Health Administration. Code of Federal
Regulations. 29 CFR 1915.1017. https://www.govinfo.gov/content/pkg/CFR-2021-title29-
vol7/pdf/CFR-2021-title29-vol7-sec1915-1017.pdf. July 19, 2023.
OSHA. 2021c. Safety and health regulations for construction. Toxic and Hazardous Substances. Vinyl
chloride. Occupational Safety and Health Administration. Code of Federal Regulations. 29 CFR
1926.1117. https://www.govinfo.gov/content/pkg/CFR-2021-title29-vol8/pdf/CFR-2021-title29-
vol8-sec1926-1117.pdf. July 19, 2023.
Oster RH, Carr CJ, et al. 1947. Anesthesia; narcosis with vinyl chloride. Anesthesiology 8(4):359-361.
https://doi.org/10.1097/00000542-194707000-00003.
Osterman-Golkar S, Jultmark D, Segerback D, et al. 1977. Alkylation of DNA and proteins in mice
exposed to vinyl chloride. Biochem Biophys Res Commun 76:259-266.
https://doi.org/10.1016/0006-291X(77)90720-3.
Ostlere LS, Harris D, Buckley C, et al. 1992. Atypical systemic sclerosis following exposure to vinyl
chloride monomer. A case report and review of the cutaneous aspects of vinyl chloride disease. Clin
Exp Dermatol 17(3):208-210. https://doi.org/10.1111/j.1365-2230.1992.tb00210.x.
Ott MG, Langer RR, Holder BB. 1975. Vinyl chloride exposure in a controlled industrial environment.
A long-term mortality experience in 594 employees. Arch Environ Health 30(7):333-339.
https://doi.org/10.1080/00039896.1975.10666716.
Pan SY, Ugnat AM, Mao Y, et al. 2005. Occupational risk factors for brain cancer in Canada. J Occup
Environ Med 47(7):704-717. https://doi.org/10.1097/01.jom.0000165747.95801.c5.
Pan W, Yu S, Jia J, et al. 2021. Deregulation of the cell cycle and related microRNA expression induced
by vinyl chloride monomer in the hepatocytes of rats. Toxicol Ind Health 37(6):365-376.
https://doi.org/10.1177/07482337211015591.
Pandya GA, Moriya M. 1996. 1,N6-ethenodeoxyadenosine, a DNA adduct highly mutagenic in
mammalian cells. Biochemistry 35(35):11487-11492. https://doi.org/10.1021/bi960170h.
Park KK, Liem A, Stewart BC, et al. 1993. Vinyl carbamate epoxide, a major strong electrophilic,
mutagenic and carcinogenic metabolite of vinyl carbamate and ethyl carbamate (urethane).
Carcinogenesis 14(3):441-450. https://doi.org/10.1093/carcin/14.3.441.
Patty FA, Yapt WP, Waite CP. 1930. Acute response of guinea pigs to vapors of some new commercial
organic compounds. V. Vinyl chloride. Public Health Rep 45:1963-1971.
https://doi.org/10.2307/4581959.
Perry RA, Atkinson R, Pitts JN. 1977. Rate constants for the reaction of hydroxyl radicals with vinyl
fluoride, vinyl chloride, and vinyl bromide over the temperature range 229-426 K. J Chem Phys
67:458 462.
Perticoni GF, Abbritti G, Cantisani TA, et al. 1986. Polyneuropathy in workers with long exposure to
vinyl chloride. Electrophysiological study. Electromyogr Clin Neurophysiol 26(1):41-47.
Peter S, Ungvary G. 1980. Lack of mutagenic effect of vinyl chloride monomer in the mammalian spot
test. Mutat Res 77(2):193-196. https://doi.org/10.1016/0165-1218(80)90139-1.
VINYL CHLORIDE 200
8. REFERENCES
Pettit BR. 1986. The analysis of thiodiglycollic acid by selected ion monitoring. Clin Chim Acta
156(1):85-90. https://doi.org/10.1016/0009-8981(86)90182-8.
Phelps TJ, Malachowsky K, Schram RM, et al. 1991. Aerobic mineralization of vinyl chloride by a
bacterium of the order Actinomycetales. Appl Environ Microbiol 57(4):1252-1254.
https://doi.org/10.1128/AEM.57.4.1252-1254.1991.
Picciano DJ, Flake RE, Gay PC, et al. 1977. Vinyl chloride cytogenetics. J Occup Med 19(8):527-530.
Pirastu R, Comba P, Reggiani A, et al. 1990. Mortality from liver disease among Italian vinyl chloride
monomer/polyvinyl chloride manufacturers. Am J Ind Med 17:155-161.
https://doi.org/10.1002/ajim.4700170202.
Plugge H, Safe S. 1977. Vinyl chloride metabolism - a review. Chemosphere 6:309-325.
Poncelet F, de Meester C, Duverger-van Bogaert M, et al. 1980. Influence of experimental factors on the
mutagenicity of vinylic monomers. Arch Toxicol Suppl 4:63-66. https://doi.org/10.1007/978-3-642-
67729-8_14.
Popper H, Thomas LB. 1975. Alterations of liver and spleen among workers exposed to vinyl chloride.
Ann NY Acad Sci 246:172-194. https://doi.org/10.1111/j.1749-6632.1975.tb51093.x
Popper H, Maltoni C, Selikoff IJ. 1981. Vinyl chloride-induced hepatic lesions in man and rodents. A
comparison. Liver 1(1):7-20. https://doi.org/10.1111/j.1600-0676.1981.tb00018.x.
Pottenger LH, Andrews LS, Bachman AN, et al. 2014. An organizational approach for the assessment of
DNA adduct data in risk assessment: case studies for aflatoxin B1, tamoxifen and vinyl chloride.
Crit Rev Toxicol 44(4):348-391. https://doi.org/10.3109/10408444.2013.873768.
Pourquier P, Bjornsti MA, Pommier Y. 1998. Induction of topoisomerase I cleavage complexes by the
vinyl chloride adduct 1,N6-ethenoadenine. J Biol Chem 273(42):27245-27249.
https://doi.org/10.1074/jbc.273.42.27245.
Poynter JN, Richardson M, Roesler M, et al. 2017. Chemical exposures and risk of acute myeloid
leukemia and myelodysplastic syndromes in a population-based study. Int J Cancer 140(1):23-33.
https://doi.org/10.1002/ijc.30420.
Pratt GC, Palmer K, Wu CY, et al. 2000. An assessment of air toxics in Minnesota. Environ Health
Perspect 108(9):815-825. https://doi.org/10.1289/ehp.00108815.
Preston BJ, Jones KL, Grainger RG. 1976. Clinical aspects of vinyl chloride disease: Acro-osteolysis.
Proc R Soc Med 69:284-306. https://doi.org/10.1177/003591577606900419.
Prodan L, Suciu I, Pislaru V, et al. 1975. Experimental acute toxicity of vinyl chloride
(monochloroethene). Ann N Y Acad Sci 246:154-158. https://doi.org/10.1111/j.1749-
6632.1975.tb51090.x.
Puentes Jacome LA, Wang PH, Molenda O, et al. 2019. Sustained dechlorination of vinyl chloride to
ethene in dehalococcoides-enriched cultures grown without addition of exogenous vitamins and at
low pH. Environ Sci Technol 53(19):11364-11374. https://doi.org/10.1021/acs.est.9b02339.
Purchase IFH, Richardson CR, Anderson D. 1975. Chromosomal and dominant lethal effects of vinyl
chloride. Lancet 306(7931):410-411. https://doi.org/10.1016/S0140-6736(75)92927-X.
Purchase IFH, Richardson CR, Anderson D, et al. 1978. Chromosomal analysis in vinyl chloride
exposed workers. Mutat Res 57:325-334. https://doi.org/10.1016/0027-5107(78)90283-X.
Qiu YL, Wang W, Wang T, et al. 2008. Genetic polymorphisms, messenger RNA expression of p53,
p21, and CCND1, and possible links with chromosomal aberrations in Chinese vinyl chloride-
exposed workers. Cancer Epidemiol Biomarkers Prev 17(10):2578-2584.
https://doi.org/10.1158/1055-9965.EPI-07-2925.
Qiu YL, Wang W, Wang T, et al. 2011a. DNA repair gene polymorphisms and micronucleus
frequencies in Chinese workers exposed to vinyl chloride monomer. Int J Hyg Environ Health
214(3):225-230. https://doi.org/10.1016/j.ijheh.2010.12.001.
Qiu YL, Sun P, Wang W, et al. 2011b. Messenger RNA expression and genetic polymorphisms of cell
cycle control genes and chromosomal aberrations in Chinese vinyl chloride monomer-exposed
workers. J Occup Environ Med 53(12):1442-1446.
https://doi.org/10.1097/JOM.0b013e3182363bfb.
VINYL CHLORIDE 201
8. REFERENCES
Qiu YL, Xu ZB, Wang Q, et al. 2019. Association between methylation of DNA damage response-
related genes and DNA damage in hepatocytes of rats following subchronic exposure to vinyl
chloride. Chemosphere 227:323-328. https://doi.org/10.1016/j.chemosphere.2019.04.058.
Quan H, Ma T, Zhao X, et al. 2014. Vinyl chloride monomer (VCM) induces high occurrence of neural
tube defects in embryonic mouse brain during neurulation. Cell Molec Neurobiol 34(4):619-630.
https://doi.org/10.1007/s10571-014-0049-6.
Radike MJ, Stemmer KL, Bingham E. 1981. Effect of ethanol on vinyl chloride carcinogenesis. Environ
Health Perspect 41:59-62. https://doi.org/10.1289/ehp.814159.
Rannug U, Johansson A, Ramel C, et al. 1974. The mutagenicity of vinyl chloride after metabolic
activation. Ambio 3:194-197.
Rannug U, Gothe R, Wachtmeister CA. 1976. The mutagenicity of chloroethylene oxide,
chloroacetaldehyde, 2-chloroethanol and chloroacetic acid, conceivable metabolites of vinyl
chloride. Chem Biol Interact 12(3-4):251-263. https://doi.org/10.1016/0009-2797(76)90041-7.
Reitz RH, Gargas ML, Andersen ME, et al. 1996. Predicting cancer risk from vinyl chloride exposure
with a physiologically based pharmacokinetic model. Toxicol Appl Pharmacol 137(2):253-267.
https://doi.org/10.1006/taap.1996.0079.
RePORTER. 2023. Vinyl chloride. National Institutes of Health, Research Portfolio Online Reporting
Tools. http://projectreporter.nih.gov/reporter.cfm. March 24, 2021.
Reynolds ES, Jaeger RJ, Murphy SD. 1975a. Acute liver injury by vinyl chloride: involvement of
endoplasmic reticulum in phenobarbital-pretreated rats. Environ Health Perspect 11:227-233.
https://doi.org/10.1289/ehp.7511227.
Reynolds ES, Moslen MT, Szabo S, et al. 1975b. Hepatotoxicity of vinyl chloride and 1,1-
dichloroethylene. Role of mixed function oxidase system. Am J Pathol 81(1):219-236.
Richards PM, Ewald JM, Zhao W, et al. 2022. Natural biodegradation of vinyl chloride and cis-
dichloroethene in aerobic and suboxic conditions. Environ Sci Pollut Res Int 29(37):56154-56167.
https://doi.org/10.1007/s11356-022-19755-1.
Richardson CR, Styles JA, Bennett IP. 1983. Activity of vinyl chloride monomer in the mouse
micronucleus assay. Mutat Res 122(2):139-142. https://doi.org/10.1016/0165-7992(83)90051-9.
Rinsky RA, Ott G, Ward E, et al. 1988. Study of mortality among chemical workers in the Kanawha
Valley of West Virginia. Am J Ind Med 13:429-438. https://doi.org/10.1002/ajim.4700130403.
Rioux KL, Delaney S. 2020. 1,N(6)-Ethenoadenine: From molecular to biological consequences. Chem
Res Toxicol 33(11):2688-2698. https://doi.org/10.1021/acs.chemrestox.0c00326.
Rodrigues EG, Herrick RF, Stewart J, et al. 2020. Case-control study of brain and other central nervous
system cancer among workers at semiconductor and storage device manufacturing facilities. Occup
Environ Med 77(4):238-248. https://doi.org/10.1136/oemed-2019-106120.
Rooney AA, Boyles AL, Wolfe MS, et al. 2014. Systematic review and evidence integration for
literature-based environmental health science assessments. Environ Health Perspect 122(7):711-718.
https://doi.org/10.1289/ehp.1307972.
Rosenman KD, Rizzo JE, Conomos MG, et al. 1989. Central nervous system malformations in relation
to two polyvinyl chloride production facilities. Arch Environ Health 44(5):279-282.
https://doi.org/10.1080/00039896.1989.9935894.
Rossabi S, Choudoir M, Helmig D, et al. 2018. Volatile organic compound emissions from soil
following wetting events. J Geophys Res Biogeosci 123(6):1988-2001.
https://doi.org/10.1029/2018jg004514.
Ruckart PZ, Bove FJ, Maslia M. 2013. Evaluation of exposure to contaminated drinking water and
specific birth defects and childhood cancers at Marine Corps Base Camp Lejeune, North Carolina: a
case-control study. Environ Health 12:104. https://doi.org/10.1186/1476-069X-12-104.
Ruiz P, Ray M, Fisher J, et al. 2011. Development of a human Physiologically Based Pharmacokinetic
(PBPK) Toolkit for environmental pollutants. Int J Mol Sci 12(11):7469-7480.
https://doi.org/10.3390/ijms12117469.
VINYL CHLORIDE 202
8. REFERENCES
Rusyn I, Arzuaga X, Cattley RC, et al. 2021. Key characteristics of human hepatotoxicants as a basis for
identification and characterization of the causes of liver toxicity. Hepatology 74(6):3486-3496.
https://doi.org/10.1002/hep.31999.
Saad AAA, Mohsen MA, Kandil SM, et al. 2017. Predictive values of some atherogenic risk factors in
young workers occupationally exposed to vinyl chloride and heavy metals. Arabian J Chem
10(1):100-108. https://doi.org/10.1016/j.arabjc.2014.01.003.
Sabadie N, Malaveille C, Camus AM, et al. 1980. Comparison of the hydroxylation of benzo(a)pyrene
with the metabolism of vinyl chloride, N-nitrosomorpholine, and N-nitroso-N'methylpiperazine to
mutagens by human and rat liver microsomal fractions. Cancer Res 409:119-126.
Sabel GV, Clark TP. 1984. Volatile organic compounds as indicators of municipal solid waste leachate
contamination. Waste Manage Res 2:119-130. https://doi.org/10.1016/0734-242X(84)90135-6.
Saito T, Barbin A, Omori Y, et al. 1997. Connexin 37 mutations in rat hepatic angiosarcomas induced
by vinyl chloride. Cancer Res 57(3):375-377.
Sakabe H. 1975. Bone lesions among polyvinyl chloride production workers in Japan. Ann NY Acad
Sci 246:78-79. https://doi.org/10.1111/j.1749-6632.1975.tb51082.x
Sakuratani Y, Kasai K, Noguchi Y, et al. 2007. Comparison of predictivities of Log P calculation
models based on experimental data for 134 simple organic compounds. QSAR Comb Sci 26(1):109-
116. https://doi.org/10.1002/qsar.200630019.
Salmon AG. 1976. Cytochrome P-450 and the metabolism of vinyl chloride. Cancer Lett 2(2):109-113.
https://doi.org/10.1016/s0304-3835(76)80019-5.
Sal'nikova LS, Kotsovskaya IA. 1980. [Effect of vinyl chloride on embryogenesis of rats]. Gig Tr Prof
Zabol 3:46-47. (Russian)
Saurin JC, Taniere P, Mion F, et al. 1997. Primary hepatocellular carcinoma in workers exposed to vinyl
chloride: a report of two cases. Cancer 79(9):1671-1677. https://doi.org/10.1002/(sici)1097-
0142(19970501)79:9<1671::aid-cncr6>3.0.co;2-f.
Scarselli A, Corfiati M, Di Marzio D, et al. 2022. The impact of vinyl chloride exposure on the health of
Italian workers: an evaluation from SIREP compliance data. Arch Environ Occup Health 77(5):372-
381. https://doi.org/10.1080/19338244.2021.1900045.
Scelo G, Constantinescu V, Csiki I, et al. 2004. Occupational exposure to vinyl chloride, acrylonitrile
and styrene and lung cancer risk (Europe). Cancer Causes Control 15(5):445-452.
https://doi.org/10.1023/B:CACO.0000036444.11655.be.
Schaffner F. 1978. Effect of long-term vinyl chloride exposure in mouse liver structure. In: Remmer H,
Bolt HM, Bannasch P, et al., eds. Primary liver tumors: Proceedings of the 25
th
Falk Symposium,
on the occasion of the 5
th
centennial celebrations of the Eberhard-Karls-Universität Tübingen held at
the Schwartzwaldhotel Titisee, West Germany, October 3-5, 1977. Baltimore, MD: University Park
Press, 189-199.
Schindler J, Li Y, Marion MJ, et al. 2007. The effect of genetic polymorphisms in the vinyl chloride
metabolic pathway on mutagenic risk. J Hum Genet 52(5):448-455. https://doi.org/10.1007/s10038-
007-0134-5.
Schoental R, Magee PN. 1957. Chronic liver changes in rats after a single dose of lasiocarpine, a
pyrrolizidine (Senecio) alkaloid. J Pathol Bacteriol 74(2):305-319.
https://doi.org/10.1002/path.1700740208.
Schoental R, Magee PN. 1959. Further observations on the subacute and chronic liver changes in rats
after a single dose of various pyrrolizidine (Senecio) alkaloids. J Pathol Bacteriol 78(2):471-482.
https://doi.org/10.1002/path.1700780213.
SERDP/ESTCP. 2012. Elucidation of the mechanisms and environmental relevance of cis-
dichloroethene and vinyl chloride biodegradation. Alexandria, VA: Department of Defense
Strategic Environmental Research and Development Program/Environmental Security Technology
Certification Program. https://www.serdp-estcp.org/content/download/17268/193091/file/ER-1557-
FR.pdf. March 24, 2021.
VINYL CHLORIDE 203
8. REFERENCES
Serratrice J, Granel B, Pache X, et al. 2001. A case of polymyositis with anti-histidyl-t-RNA synthetase
(Jo-1) antibody syndrome following extensive vinyl chloride exposure. Clin Rheumatol 20(5):379-
382. https://doi.org/10.1007/s100670170032.
Shahin MM. 1976. The non-mutagenicity and recombinogenicity of vinyl chloride in the absence of
metabolic activation. Mutat Res 40:269-272. https://doi.org/10.1016/0165-1218(76)90053-7.
Sharma RP, Gehring PJ. 1979. Immunologic effects of vinyl chloride in mice. Ann N Y Acad Sci
320:551-563. https://doi.org/10.1111/j.1749-6632.1979.tb56633.x.
Sharma RP, Yakel HO, Gehring PJ. 1980. Immunotoxicologic studies with vinyl chloride in rabbits and
mice. Int J Immunopharmacol 2(4):295-299. https://doi.org/10.1016/0192-0561(80)90029-6.
Short RD, Minor JL, Winston JM, et al. 1977. A dominant lethal study in male rats after repeated
exposures to vinyl chloride or vinylidene chloride. J Toxicol Environ Health 3(5-6):965-968.
https://doi.org/10.1080/15287397709529630.
Shumate AM, Taylor J, McFarland E, et al. 2017. Medical response to a vinyl chloride release from a
train derailment: New Jersey, 2012. Disaster Med Public Health Prep 11(5):538-544.
https://doi.org/10.1017/dmp.2016.191.
Simmon VF, Kauhanen K, Tardiff RG. 1977. Mutagenic activity of chemicals identified in drinking
water. In: Scott D, Bridges BA, Sobels FH, eds. Progress in genetic toxicology: Proceedings of the
second international conference on environmental mutagens; Edinburgh, July 11-15, 1977.
Amsterdam, Netherlands: Elsevier/North Holland Biomedical Press, 249-258.
Simonato L, L'Abbe KA, Andersen A, et al. 1991. A collaborative study of cancer incidence and
mortality among vinyl chloride workers. Scand J Work Environ Health 17(3):159-169.
Singer B. 1996. DNA damage: chemistry, repair, and mutagenic potential. Regul Toxicol Pharmacol
23:2-13. https://doi.org/10.1006/rtph.1996.0002.
Singer B, Hang B. 1999. Mammalian enzymatic repair of etheno- and para-benzoquinone exocyclic
adducts derived from the carcinogens vinyl chloride and benzene. IARC Sci Publ 150(150):233-247.
Singer B, Spengler SJ, Chavez F, et al. 1987. The vinyl chloride-derived nucleoside, N2,3-
ethenoguanosine, is a highly efficient mutagen in transcription. Carcinogenesis 8:745-747.
https://doi.org/10.1093/carcin/8.5.745.
Sinués B, Sanz A, Bernal ML, et al. 1991. Sister chromatid exchanges, proliferating rate index, and
micronuclei in biomonitoring of internal exposure to vinyl chloride monomer in plastic industry
workers. Toxicol Appl Pharmacol 108(1):37-45. https://doi.org/10.1016/0041-008x(91)90266-h.
Smith LR, Dragun J. 1984. Degradation of volatile chlorinated aliphatic priority pollutants in
groundwater. Environ Int 10:291-298. https://doi.org/10.1016/0160-4120(84)90134-X.
Smith SJ, Li Y, Whitley R, et al. 1998. Molecular epidemiology of p53 protein mutations in workers
exposed to vinyl chloride. Am J Epidemiol 147(3):302-308.
https://doi.org/10.1093/oxfordjournals.aje.a009450.
Smits TH, Assal A, Hunkeler D, et al. 2011. Anaerobic degradation of vinyl chloride in aquifer
microcosms. J Environ Qual 40(3):915-922. https://doi.org/10.2134/jeq2010.0403.
Smulevich VB, Fedotova IV, Filatova VS. 1988. Increasing evidence of the rise of cancer in workers
exposed to vinyl chloride. Br J Ind Med 45:93-97. https://doi.org/10.1136/oem.45.2.93.
Soini Y, Welsh JA, Ishak KG, et al. 1995. p53 Mutations in primary hepatic angiosarcomas not
associated with vinyl chloride exposure. Carcinogenesis 16(11):2879-2881.
https://doi.org/10.1093/carcin/16.11.2879.
Sokal JA, Baranski B, Majka J, et al. 1980. Experimental studies on the chronic toxic effects of vinyl
chloride in rats. J Hyg Epidemiol Microbiol Immunol 24(3):285-294.
Spirtas R, McMichael AJ, Gamble J, et al. 1975. The association of vinyl chloride exposures with
morbidity symptoms. Am Ind Hyg Assoc J 36(10):779-789.
https://doi.org/10.1080/0002889758507339.
Squillace PJ, Moran MJ, Price CV. 2004. VOCs in shallow groundwater in new residential/commercial
areas of the United States. Environ Sci Technol 38(20):5327-5338.
https://doi.org/10.1021/es0349756.
VINYL CHLORIDE 204
8. REFERENCES
SRI. 1990a. Vinyl chloride. In: 1990 Directory of chemical producers. Menlo Park, CA: SRI
International, 1064.
SRI. 1990b. Vinyl chloride. In: 1990 Directory of chemical producers. Supplement. Menlo Park, CA:
SRI International, 66.
SRI. 1993. Vinyl chloride. In: 1993 Directory of chemical producers. Menlo Park, CA: SRI
International, 1006.
SRI. 1994. Vinyl chloride. In: 1994 Directory of chemical producers. Menlo Park, CA: SRI
International, 994.
Staib F, Hussain SP, Hofseth LJ, et al. 2003. TP53 and liver carcinogenesis. Hum Mutat 21(3):201-216.
https://doi.org/10.1002/humu.10176.
Stallones RA. 1987. The use and abuse of subgroup analysis in epidemiological research. Prev Med
16:183-194. https://doi.org/10.1016/0091-7435(87)90082-X.
Stephens RD, Ball NB, Mar DM. 1986. A multimedia study of hazardous waste landfill gas migration.
In: Cohen Y, ed. Pollutants in a multimedia environment. New York, NY: Plenum Press, 265-287.
Suciu I, Drejman I, Valeskai M. 1963. [Investigation of the diseases caused by vinyl chloride]. Med
Interna 15:967-977. (Spanish)
Suciu I, Prodan L, Ilea E, et al. 1975. Clinical manifestations in vinyl chloride poisoning. Ann N Y
Acad Sci 246:53-69. https://doi.org/10.1111/j.1749-6632.1975.tb51080.x.
Sugaya N, Inoue K, Tahara M, et al. 2023. Analysis and risk assessment of vinyl chloride emitted from
aerosol products. J Environ Sci Health A Tox Hazard Subst Environ Eng 58(4):284-294.
https://doi.org/10.1080/10934529.2023.2173925.
Suzuki Y. 1978. Pulmonary tumors induced in mice by vinyl chloride monomer. Environ Res 16(1-
3):285-301. https://doi.org/10.1016/0013-9351(78)90163-9.
Suzuki Y. 1980. Nonneoplastic effects of vinyl chloride in mouse lung. Environ Res 21(1):235-253.
https://doi.org/10.1016/0013-9351(80)90026-2.
Suzuki Y. 1981. Neoplastic and nonneoplastic effects of vinyl chloride in mouse lung. Environ Health
Perspect 41:31-52. https://doi.org/10.1289/ehp.814131.
Suzuki Y. 1983. Neoplastic effect of vinyl chloride in mouse lung-lower doses and short-term exposure.
Environ Res 32(1):91-103. https://doi.org/10.1016/0013-9351(83)90195-0.
Swartz MD, Cai Y, Chan W, et al. 2015. Air toxics and birth defects: a Bayesian hierarchical approach
to evaluate multiple pollutants and spina bifida. Environ Health 14:16.
https://doi.org/10.1186/1476-069X-14-16.
Sweeney LM, Gearhart JM. 2020. Examples of physiologically based pharmacokinetic modeling applied
to risk assessment. In: Fisher JW, Gearhart JM, Lin Z, eds. Physiologically based pharmacokinetic
(PBPK) modeling. Academic Press: 281-299. https://doi.org/10.1016/B978-0-12-818596-4.00011-
4.
Swenberg JA, Fedtke N, Ciroussel F, et al. 1992. Etheno adducts formed in DNA of vinyl chloride-
exposed rats are highly persistent in liver. Carcinogenesis 13(4):727-729.
https://doi.org/10.1093/carcin/13.4.727.
Swenberg JA, Bogdanffy MS, Ham A, et al. 1999. Formation and repair of DNA adducts in vinyl
chloride- and vinyl fluoride-induced carcinogenesis. IARC Sci Publ 150(150):29-43.
Swenberg JA, Ham A, Koc H, et al. 2000. DNA adducts: effects of low exposure to ethylene oxide,
vinyl chloride and butadiene. Mutat Res 464(1):77-86. https://doi.org/10.1016/s1383-
5718(99)00168-0.
Swenberg JA, Lu K, Moeller BC, et al. 2011. Endogenous versus exogenous DNA adducts: their role in
carcinogenesis, epidemiology, and risk assessment. Toxicol Sci 120(Suppl 1):S130-145.
https://doi.org/10.1093/toxsci/kfq371.
Tabershaw IR, Gaffey WR. 1974. Mortality study of workers in the manufacture of vinyl chloride and
its polymers. J Occup Med 16(8):509-518.
VINYL CHLORIDE 205
8. REFERENCES
Talbott EO, Marshall LP, Rager JR, et al. 2015. Air toxics and the risk of autism spectrum disorder: the
results of a population based case-control study in southwestern Pennsylvania. Environ Health
14:80. https://doi.org/10.1186/s12940-015-0064-1.
Tamburro CH, Makk L, Popper H. 1984. Early hepatic histologic alterations among chemical (vinyl
monomer) workers. Hepatology 4:413-418. https://doi.org/10.1002/hep.1840040310.
Tan YM, Chan M, Chukwudebe A, et al. 2020. PBPK model reporting template for chemical risk
assessment applications. Regul Toxicol Pharmacol 115:104691.
https://doi.org/10.1016/j.yrtph.2020.104691.
ten Berge WF. 1987. Incidence of cancer among vinylchloride and polyvinylchloride workers: Further
evidence for an association with malignant melanoma. Br J Ind Med 44:718-719.
https://doi.org/10.1136/oem.44.10.718.
Teta MJ, Schnatter AR, Ott MG, et al. 1990. Mortality surveillance in a large chemical company: the
Union Carbide Corporation experience, 1974-1983. Am J Ind Med 17(4):435-447.
https://doi.org/10.1002/ajim.4700170403.
Theriault G, Allard P. 1981. Cancer mortality of a group of Canadian workers exposed to vinyl chloride
monomer. J Occup Med 23(10):671-676. https://doi.org/10.1097/00043764-198110000-00009.
Theriault G, Iturra H, Gingras S. 1983. Evaluation of the association between birth defects and exposure
to ambient vinyl chloride. Teratology 27:359-370. https://doi.org/10.1002/tera.1420270310.
Thomas TL, Stewart PA, Stemhagen A, et al. 1987. Risk of astrocytic brain tumors associated with
occupational chemical exposures. A case-referent study. Scand J Work Environ Health 13(5):417-
423. https://doi.org/10.5271/sjweh.2024.
Thornton SR, Schroeder RE, Robison RL, et al. 2002. Embryo-fetal developmental and reproductive
toxicology of vinyl chloride in rats. Toxicol Sci 68(1):207-219.
https://doi.org/10.1093/toxsci/68.1.207.
Til HP, Immel HR, Feron VJ. 1983. Lifespan oral carcinogenicity study of vinyl chloride in rats. Final
report. Civo Institutes; TNO. Report No. V 93.285/291099.
Til HP, Feron VJ, Immel HR. 1991. Lifetime (149-week) oral carcinogenicity study of vinyl chloride in
rats. Food Chem Toxicol 29(10):713-718. https://doi.org/10.1016/0278-6915(91)90130-y.
Torkelson TR, Oyen F, Rowe VK. 1961. The toxicity of vinyl chloride as determined by repeated
exposure of laboratory animals. Am Ind Hyg Assoc J 22:354-361.
https://doi.org/10.1080/00028896109343421.
Towle KM, Benson SM, Egnot NS, et al. 2021. An ecological evaluation of vinyl chloride exposure and
liver cancer incidence and mortality in Texas. J Clin Transl Hepatol 9(1):99-105.
https://doi.org/10.14218/jcth.2020.00073.
TRI21. 2023a. Vinyl chloride. TRI explorer: release reports. U.S. Environmental Protection Agency.
https://enviro.epa.gov/triexplorer/tri_release.chemical. July 10, 2023.
TRI21. 2023b. Vinyl chloride. TRI explorer: release trends report. U.S. Environmental Protection
Agency. https://enviro.epa.gov/triexplorer/tri_release.trends. July 10, 2023.
Trivers GE, Cawley HL, DeBenedetti VM, et al. 1995. Anti-p53 antibodies in sera of workers
occupationally exposed to vinyl chloride. J Natl Cancer Inst 87(18):1400-1407.
https://doi.org/10.1093/jnci/87.18.1400.
U.S. Air Force. 1990. Development and validation of methods for applying pharmacokinetic data in risk
assessment. Volume V of VII. Vinyl chloride. Wright-Patterson AFB, OH: U.S. Air Force.
ADA237369. AAMRL-TR-90-072.
https://ntrl.ntis.gov/NTRL/dashboard/searchResults/titleDetail/ADA237369.xhtml. March 26, 2021.
Uche UI, Evans S, Rundquist S, et al. 2021. Community-level analysis of drinking water data highlights
the importance of drinking water metrics for the state, federal environmental health justice priorities
in the United States. Int J Environ Res Public Health 18(19):10401.
https://doi.org/10.3390/ijerph181910401.
VINYL CHLORIDE 206
8. REFERENCES
Ungvary G, Hudak A, Tatrai E, et al. 1978. Effects of vinyl chloride exposure alone and in combination
with trypan blue-applied systematically during all thirds of pregnancy on the fetuses of CFY rats.
Toxicology 11:45-54. https://doi.org/10.1016/S0300-483X(78)90389-X.
USGS. 2006. Volatile organic compounds in the nation's ground water and drinking-water supply wells.
Reston, VA: U.S. Geological Survey. Circular 1292.
https://pubs.usgs.gov/circ/circ1292/pdf/circular1292.pdf. March 24, 2021.
USGS. 2014. Anthropogenic organic compounds in source water of select community water systems in
the United States, 2002-10. U.S. Geological Survey. PB2015101729. Scientific Investigations
Report 2014-5139.
https://ntrl.ntis.gov/NTRL/dashboard/searchResults/titleDetail/PB2015101729.xhtml. August 26,
2021.
USITC. 1994. Synthetic organic chemicals: United States production and sales; 1992. Washington, DC:
United States International Trade Commission. USITC Publication 2720.
Vaglenov A, Lalchev S, Nosko M, et al. 1999. Chromosome aberrations and micronuclei in plastic
industry workers exposed to vinyl chloride monomer. Cytogenet Cell Genet 85(1-2):103.
Veltman G, Lange CE, Juhe S, et al. 1975. Clinical manifestations and course of vinyl chloride disease.
Ann N Y Acad Sci 246:6-17. https://doi.org/10.1111/j.1749-6632.1975.tb51076.x.
Verschueren K. 1983. Vinyl chloride. In: Handbook of environmental data on organic chemicals. 2
nd
ed. New York, NY: Van Nostrand Reinhold Company, 1185-1186.
Victorin K, Stahlberg M. 1988. A method for studying the mutagenicity of some gaseous compounds in
Salmonella typhimurium. Environ Mol Mutagen 11:65-77. https://doi.org/10.1002/em.2850110108.
Vihko R, Vihko P, Maentausta O, et al. 1984. Assessment of early hepatotoxicity. In: Aitio A,
Riihimaki V, Vainio J, eds. Biological monitoring and surveillance of workers exposed to
chemicals. Washington, DC: Hemisphere Publishing Co., 309-313.
Viola PL. 1970. Pathology of vinyl chloride. Med Lav 61(3):147-180.
Viola PL, Bigotti A, Caputo A. 1971. Oncogenic response of rat skin, lungs, and bones to vinyl chloride.
Cancer Res 31(5):516-522.
Voigt MD. 2005. Alcohol in hepatocellular cancer. Clin Liver Dis 9(1):151-169.
https://doi.org/10.1016/j.cld.2004.10.003.
Wackett LP, Brusseau GA, Householder SR, et al. 1989. Survey of microbial oxygenases:
trichloroethylene degradation by propane-oxidizing bacteria. Appl Environ Microbiol 55(11):2960-
2964. https://doi.org/10.1128/AEM.55.11.2960-2964.1989.
Wagnerova M, Wagner V, Znojemska S, et al. 1988. Factors of humoral resistance in workers exposed
to vinyl chloride with a view to smoking habits. J Hyg Epidemiol Microbiol Immunol 32(3):265-
272.
Wahlang B, Hardesty JE, Head KZ, et al. 2020. Hepatic injury caused by the environmental toxicant
vinyl chloride is sex-dependent in mice. Toxicol Sci 174(1):79-91.
https://doi.org/10.1093/toxsci/kfz236.
Walker AE. 1976. Clinical aspects of vinyl chloride disease: skin. Proc R Soc Med 69(4):286-289.
https://doi.org/10.1177/003591577606900420.
Wallace LA, Pellizzari E, Hartwell T, et al. 1984. Personal exposure to volatile organic compounds. I.
Direct measurements in breathing-zone air, drinking water, food, and exhaled breath. Environ Res
35(1):293-319. https://doi.org/10.1016/0013-9351(84)90137-3.
Walles SA, Holmberg B, Svensson K, et al. 1988. Induction of single-strand breaks in liver DNA of
mice after inhalation of vinyl chloride. IARC Sci Publ 89(89):227-231.
Walter RK, Lin PH, Edwards M, et al. 2011. Investigation of factors affecting the accumulation of vinyl
chloride in polyvinyl chloride piping used in drinking water distribution systems. Water Res
45(8):2607-2615. https://doi.org/10.1016/j.watres.2011.02.016.
Wang AH, Zhu SM, Qiu YL, et al. 2008. CYP2E1 mRNA expression, genetic polymorphisms in
peripheral blood lymphocytes and liver abnormalities in Chinese VCM-exposed workers. Int J
Occup Med Environ Health 21(2):141-146. https://doi.org/10.2478/v10001-008-0016-x.
VINYL CHLORIDE 207
8. REFERENCES
Wang Q, Ji F, Sun Y, et al. 2010a. Genetic polymorphisms of XRCC1, HOGG1 and MGMT and
micronucleus occurrence in Chinese vinyl chloride-exposed workers. Carcinogenesis 31(6):1068-
1073. https://doi.org/10.1093/carcin/bgq075.
Wang W, Qiu YL, Ji F, et al. 2010b. Genetic polymorphisms in metabolizing enzymes and susceptibility
of chromosomal damage induced by vinyl chloride monomer in a Chinese worker population. J
Occup Environ Med 52(2):163-168. https://doi.org/10.1097/JOM.0b013e3181cac00b.
Wang W, Qiu YL, Jiao J, et al. 2011. Genotoxicity in vinyl chloride-exposed workers and its implication
for occupational exposure limit. Am J Ind Med 54(10):800-810. https://doi.org/10.1002/ajim.20990.
Wang Q, Tan HS, Ma XM, et al. 2013a. Estimation of benchmark dose for micronucleus occurrence in
Chinese vinyl chloride-exposed workers. Int J Hyg Environ Health 216(1):76-81.
https://doi.org/10.1016/j.ijheh.2012.02.008.
Wang Q, Tan HS, Zhang F, et al. 2013b. Polymorphisms in BER and NER pathway genes: effects on
micronucleus frequencies among vinyl chloride-exposed workers in Northern China. Mutat Res
754(1-2):7-14. https://doi.org/10.1016/j.mrgentox.2013.03.007.
Wang Q, Zhang L, Chen SQ, et al. 2019a. Role of endoplasmic reticulum stress and oxidative stress in
vinyl chloride-induced hepatic steatosis in mice. Toxicol Appl Pharmacol 381:114730.
https://doi.org/10.1016/j.taap.2019.114730.
Wang CW, Liao KW, Chan CC, et al. 2019b. Association between urinary thiodiglycolic acid level and
hepatic function or fibrosis index in school-aged children living near a petrochemical complex.
Environ Pollut 244:648-656. https://doi.org/10.1016/j.envpol.2018.10.012.
Ward AM. 1976. Clinical aspects of vinyl chloride disease: Evidence of an immune complex disorder in
vinyl chloride workers. Proc R Soc Med 69:289-290.
https://doi.org/10.1177/003591577606900421.
Ward E, Boffetta P, Andersen A, et al. 2001. Update of the follow-up of mortality and cancer incidence
among European workers employed in the vinyl chloride industry. Epidemiology 12:710-718.
Watanabe PG, Gehring PJ. 1976. Dose-dependent fate of vinyl chloride and its possible relationship to
oncogenicity in rats. Environ Health Perspect 17:145-152. https://doi.org/10.1289/ehp.7617145.
Watanabe PG, McGowan GR, Gehring PJ. 1976a. Fate of [14C]vinyl chloride after single oral
administration in rats. Toxicol Appl Pharmacol 36(2):339-352. https://doi.org/10.1016/0041-
008x(76)90013-2.
Watanabe PG, McGowan GR, Madrid EO, et al. 1976b. Fate of [14C]vinyl chloride following inhalation
exposure in rats. Toxicol Appl Pharmacol 37(1):49-59. https://doi.org/10.1016/s0041-
008x(76)80007-5.
Watanabe PG, Zempel JA, Gehring PJ. 1978a. Comparison of the fate of vinyl chloride following single
and repeated exposure in rats. Toxicol Appl Pharmacol 44(2):391-399.
https://doi.org/10.1016/0041-008x(78)90199-0.
Watanabe PG, Zempel JA, Pegg DG, et al. 1978b. Hepatic macromolecular binding following exposure
to vinyl chloride. Toxicol Appl Pharmacol 44(3):571-579. https://doi.org/10.1016/0041-
008x(78)90265-x.
Watson WP, Aston JP, Barlow T, et al. 1999. Detection of 1,N6-etheno-2’-deoxyadenosine and 3,N4
etheno-2’-deoxycytidine occurring endogenously in DNA. IARC Sci Publ 150(150):63-73.
Waxweiler RJ, Stringer W, Wagner JK, et al. 1976. Neoplastic risk among workers exposed to vinyl
chloride. Ann NY Acad Sci 271:40-48. https://doi.org/10.1111/j.1749-6632.1976.tb23091.x.
Weber H, Reinl W, Greiser E. 1981. German investigations on morbidity and mortality of workers
exposed to vinyl chloride. Environ Health Perspect 41:95-99. https://doi.org/10.1289/ehp.814195.
Weihrauch M, Lehnert G, Kockerling F, et al. 2000. p53 Mutation pattern in hepatocellular carcinoma in
workers exposed to vinyl chloride. Cancer 88(5):1030-1036. https://doi.org/10.1002/(SICI)1097-
0142(20000301)88:5<1030::AID-CNCR12>3.0.CO;2-4.
Weihrauch M, Benicke M, Lehnert G, et al. 2001a. Frequent K-ras-2 mutations and p16INK4A
methylation in hepatocellular carcinomas in workers exposed to vinyl chloride. Br J Cancer
84(7):982 989. https://doi.org/10.1054/bjoc.2000.1675.
VINYL CHLORIDE 208
8. REFERENCES
Weihrauch M, Benick M, Lehner G, et al. 2001b. High prevalence of K-ras-2 mutations in
hepatocellular carcinomas in workers exposed to vinyl chloride. Int Arch Occup Environ Health
74(6):405-410. https://doi.org/10.1007/s004200100244.
Weihrauch M, Bader M, Lehnert G, et al. 2002. Mutation analysis of K-ras-2 in liver angiosarcoma and
adjacent nonneoplastic liver tissue from patients occupationally exposed to vinyl chloride. Environ
Mol Mutagen 40(1):36-40. https://doi.org/10.1002/em.10084.
Wen-Bin M, Wei W, Yu-Lan Q, et al. 2009. Micronucleus occurrence related to base excision repair
gene polymorphisms in Chinese workers occupationally exposed to vinyl chloride monomer. J
Occup Environ Med 51(5):578-585. https://doi.org/10.1097/JOM.0b013e3181990d19.
Westrick JJ, Mello JW, Thomas RF. 1984. The groundwater supply survey. J Am Water Works Assoc
76(5):52-59. https://doi.org/10.1002/j.1551-8833.1984.tb05334.x.
WHO. 1999. Environmental health criteria on vinyl chloride. Geneva, Switzerland: World Health
Organization. http://www.inchem.org/pages/ehc.html. March 25, 2021.
WHO. 2000. Air quality guidelines for Europe, 2
nd
edition. World Health Organization.
https://apps.who.int/iris/handle/10665/107335. November 29, 2022.
WHO. 2010. Guidelines for indoor air quality: Selected pollutants. World Health Organization.
https://www.who.int/publications/i/item/9789289002134. July 16, 2023.
WHO. 2022. Guidelines for drinking-water quality. Fourth edition incorporating the first and second
addenda. World Health Organization. https://www.who.int/publications/i/item/9789240045064.
July 16, 2023.
Whysner J, Conaway CC, Verna L, et al. 1996. Vinyl chloride mechanistic data and risk assessment:
DNA reactivity and cross-species quantitative risk extrapolation. Pharmacol Ther 71(1-2):7-28.
https://doi.org/10.1016/0163-7258(96)00060-5.
Wilken JA, Graziano L, Vaouli E, et al. 2015. Exposures and symptoms among workers after an offsite
train derailment and vinyl chloride release. Am J Disaster Med 10(2):153-165.
https://doi.org/10.5055/ajdm.2015.0198.
Williamson J, Kavanagh B. 1987. Vinyl chloride monomer and other contaminants in PVC welding
fumes. Am Ind Hyg Assoc J 48(5):432-436. https://doi.org/10.1080/15298668791384995.
Wilson RH, McCormick WE, Tatum CF, et al. 1967. Occupational acroosteolysis. Report of 31 cases.
JAMA 201(8):577-581. https://doi.org/10.1001/jama.201.8.577.
Wisniewska-Knypl JM, Klimczak J, Kolakowski J. 1980. Monooxygenase activity and ultrastructural
changes of liver in the course of chronic exposure of rats to vinyl chloride. Int Arch Occup Environ
Health 46(3):241-249. https://doi.org/10.1007/BF00380014.
Withey JR. 1976. Pharmacodynamics and uptake of vinyl chloride monomer administered by various
routes to rats. J Toxicol Environ Health 1:381-394. https://doi.org/10.1080/15287397609529338.
Woldbaek T, Klaboe P. 1978. The photochemical reaction of vinyl chloride with oxygen studied by
infrared spectroscopy. Spectrochim Acta 34(5):481-487. https://doi.org/10.1016/0584-
8539(78)80043-9.
Wong O, Whorton MD. 1993. Letter to the editor. Diagnostic bias in occupational epidemiologic
studies: An example based on the vinyl chloride literature. Am J Ind Med 24:251-256.
https://doi.org/10.1002/ajim.4700240215.
Wong O, Whorton MD, Foliart DE, et al. 1991. An industry-wide epidemiologic study of vinyl chloride
workers, 1942-1982. Am J Ind Med 20(3):317-334. https://doi.org/10.1002/ajim.4700200305.
Wong RH, Wang JD, Hsieh LL, et al. 1998. Effects on sister chromatid exchange frequency of aldehyde
dehydrogenase 2 genotype and smoking in vinyl chloride workers. Mutat Res 420(1-3):99-107.
https://doi.org/10.1016/S1383-5718(98)00150-8.
Wong RH, Chen PC, Du Cl, et al. 2002a. An increased standardized mortality ratio for liver cancer
among polyvinyl chloride workers in Taiwan. Occup Environ Med 59:405-409.
https://doi.org/10.1136/oem.59.6.405.
VINYL CHLORIDE 209
8. REFERENCES
Wong RH, Du CL, Wang JD, et al. 2002b. XRCC1 and CYP2E1 polymorphisms as susceptibility
factors of plasma mutant p53 protein and anti-p53 antibody expression in vinyl chloride monomer-
exposed polyvinyl chloride workers. Cancer Epidemiol Biomarkers Prev 11:475-482.
Wong RH, Chen PC, Wang JD, et al. 2003a. Interaction of vinyl chloride monomer exposure and
hepatitis B viral infection on liver cancer. J Occup Environ Med 45(4):379-383.
https://doi.org/10.1097/01.jom.0000063622.37065.fd.
Wong RH, Wang JD, Hsieh LL, et al. 2003b. XRCC1, CYP2E1 and ALDH2 genetic polymorphisms
and sister chromatid exchange frequency alterations amongst vinyl chloride monomer-exposed
polyvinyl chloride workers. Arch Toxicol 77(8):433-440. https://doi.org/10.1007/s00204-003-0467-
6.
WQP. 2021. Vinyl chloride. Water quality portal: 2011 to 2021 data. Advisory Committee on Water
Information (ACWI); Agricultural Research Service (ARS); Environmental Protection Agency
(EPA); National Water Quality Monitoring Council (NWQMC); United States Geological Survey
(USGS). https://www.waterqualitydata.us/portal/. March 24, 2021.
WQP. 2023. Vinyl chloride. Water quality portal: 2022 to 2023 data. Advisory Committee on Water
Information (ACWI); Agricultural Research Service (ARS); Environmental Protection Agency
(EPA); National Water Quality Monitoring Council (NWQMC); United States Geological Survey
(USGS). https://www.waterqualitydata.us/portal/. June 29, 2023.
Wu W, Steenland K, Brown D, et al. 1989. Cohort and case-control analyses of workers exposed to
vinyl chloride: an update. J Occup Med 31(6):518-523. https://doi.org/10.1097/00043764-
198906000-00007.
Wu F, Liu J, Qiu YL, et al. 2013. Correlation of chromosome damage and promoter methylation status
of the DNA repair genes MGMT and hMLH1 in Chinese vinyl chloride monomer (VCM)-exposed
workers. Int J Occup Med Environ Health 26(1):173-182. https://doi.org/10.2478/s13382-013-
0079-1.
Xiao Z, Jiang W, Chen D, et al. 2020. Bioremediation of typical chlorinated hydrocarbons by microbial
reductive dechlorination and its key players: A review. Ecotoxicol Environ Saf 202:110925.
https://doi.org/10.1016/j.ecoenv.2020.110925.
Yang X, Wang K, Xiao D, et al. 2000. Development of a fluorescent optode membrane for sodium ion
based on the calix[4]arene and tetraphenylporphine. Talanta 52(6):1033-1039.
https://doi.org/10.1016/s0039-9140(00)00469-0.
Yoon M, Madden MC, Barton HA. 2007. Extrahepatic metabolism by CYP2E1 in PBPK modeling of
lipophilic volatile organic chemicals: impacts on metabolic parameter estimation and prediction of
dose metrics. J Toxicol Environ Health A 70(18):1527-1541.
https://doi.org/10.1080/15287390701384684.
Yuan TH, Chen JL, Shie RH, et al. 2020. Liver fibrosis associated with potential vinyl chloride and
ethylene dichloride exposure from the petrochemical industry. Sci Total Environ 739:139920.
https://doi.org/10.1016/j.scitotenv.2020.139920.
Yun B, Guo J, Bellamri M, et al. 2020. DNA adducts: Formation, biological effects, and new
biospecimens for mass spectrometric measurements in humans. Mass Spectrom Rev 39(1-2):55-82.
https://doi.org/10.1002/mas.21570.
Zalesak M, Ruzicka J, Vicha R, et al. 2021. Examining aerobic degradation of chloroethenes mixture in
consortium composed of Comamonas testosteroni RF2 and Mycobacterium aurum L1.
Chemosphere 269:128770. https://doi.org/10.1016/j.chemosphere.2020.128770.
Zelko IN, Taylor BS, Das TP, et al. 2022. Effect of vinyl chloride exposure on cardiometabolic toxicity.
Environ Toxicol 37(2):245-255. https://doi.org/10.1002/tox.23394.
Zenzen V, Diekmann J, Gerstenberg B, et al. 2012. Reduced exposure evaluation of an electrically
heated cigarette smoking system. Part 2: Smoke chemistry and in vitro toxicological evaluation
using smoking regimens reflecting human puffing behavior. Regul Toxicol Pharmacol 64(2
Suppl):S11-S34. https://doi.org/10.1016/j.yrtph.2012.08.004.
VINYL CHLORIDE 210
8. REFERENCES
Zhang J, Hatakeyama S, Akimoto H. 1983. Rate constants of the reaction of ozone with trans-1,2-
dichloroethene and vinyl chloride in air. Int J Chem Kinet 15(7):655-668.
https://doi.org/10.1002/kin.550150707.
Zhang W, Rieger R, Iden C, et al. 1995. Synthesis of 3,N
4
-etheno, 3,N
4
-etheno and 3-(2-hydroxyetheyl)
derivatives of 2’-deoxycytidine and their incorporation into oligomeric DNA. Chem Res Toxicol
8(1):148-156. https://doi.org/10.1021/tx00043a020.
Zhao MY, Ying CJ, Shao N, et al. 1994. The study of health effects of vinyl chloride air pollution on
population. Biomed Environ Sci 7(2):136-143.
Zhao SF, Zhang XC, Zhang LF, et al. 1996. Application of the whole embryo culture system in
screening industrial teratogens. Teratology 53(2):97.
Zheng GQ, Zhang GH, Xu XW, et al. 2017. Association of telomere length with chromosomal damage
among Chinese workers exposed to vinyl chloride monomer. J Occup Environ Med 59(12):e252-
e256. https://doi.org/10.1097/jom.0000000000001177.
Zheng GQ, Zhang GH, Wu HT, et al. 2019. Relative telomere length and gene expression of shelterin
complex proteins among vinyl chloride monomer-exposed workers in China. Environ Mol Mutagen
60(4):361-367. https://doi.org/10.1002/em.22270.
Zhu S, Wang A, Xia Z. 2005b. Polymorphisms of DNA repair gene XPD and DNA damage of workers
exposed to vinylchloride monomer. Int J Hyg Environ Health 208(5):383-390.
https://doi.org/10.1016/j.ijheh.2005.05.002.
Zhu SM, Ren XF, Wan JX, et al. 2005a. Evaluation in vinyl chloride monomer-exposed workers and the
relationship between liver lesions and gene polymorphisms of metabolic enzymes. World J
Gastroenterol 11(37):5821-5827. https://doi.org/10.3748/wjg.v11.i37.5821.
Zhu SM, Xia ZL, Wang AH, et al. 2008. Polymorphisms and haplotypes of DNA repair and xenobiotic
metabolism genes and risk of DNA damage in Chinese vinyl chloride monomer (VCM)-exposed
workers. Toxicol Lett 178(2):88-94. https://doi.org/10.1016/j.toxlet.2008.02.009.
Zielinski B, Hergenhahn M. 2001. 2-Chloroacetaldehyde induces εdA DNA adducts in DNA of Raji
cells as demonstrated by an improved HPLC-fluorimetry method. Fresenius J Anal Chem
370(1):97-100. https://doi.org/10.1007/s002160100776.
VINYL CHLORIDE A-1
APPENDIX A. ATSDR MINIMAL RISK LEVEL WORKSHEETS
MRLs are derived when reliable and sufficient data exist to identify the target organ(s) of effect or the
most sensitive health effect(s) for a specific duration for a given route of exposure. An MRL is an
estimate of the daily human exposure to a hazardous substance that is likely to be without appreciable risk
of adverse noncancer health effects over a specified route and duration of exposure. MRLs are based on
noncancer health effects only; cancer effects are not considered. These substance-specific estimates,
which are intended to serve as screening levels, are used by ATSDR health assessors to identify
contaminants and potential health effects that may be of concern at hazardous waste sites. It is important
to note that MRLs are not intended to define clean-up or action levels.
MRLs are derived for hazardous substances using the NOAEL/uncertainty factor approach. They are
below levels that might cause adverse health effects in the people most sensitive to such chemical-
induced effects. MRLs are derived for acute (1–14 days), intermediate (15–364 days), and chronic
(≥365 days) durations and for the oral and inhalation routes of exposure. Currently, MRLs for the dermal
route of exposure are not derived because ATSDR has not yet identified a method suitable for this route
of exposure. MRLs are generally based on the most sensitive substance-induced endpoint considered to
be of relevance to humans. LOAELs for serious health effects (such as irreparable damage to the liver or
kidneys, or serious birth defects) are not used as a basis for establishing MRLs. Exposure to a level above
the MRL does not mean that adverse health effects will occur.
MRLs are intended only to serve as a screening tool to help public health professionals decide where to
look more closely. They may also be viewed as a mechanism to identify those hazardous waste sites that
are not expected to cause adverse health effects. Most MRLs contain a degree of uncertainty because of
the lack of precise toxicological information on the people who might be most sensitive (e.g., infants,
elderly, nutritionally or immunologically compromised) to the effects of hazardous substances. ATSDR
uses a conservative (i.e., protective) approach to address this uncertainty consistent with the public health
principle of prevention. Although human data are preferred, MRLs often must be based on animal studies
because relevant human studies are lacking. In the absence of evidence to the contrary, ATSDR assumes
that humans are more sensitive to the effects of hazardous substance than animals and that certain persons
may be particularly sensitive. Thus, the resulting MRL may be as much as 100-fold below levels that
have been shown to be nontoxic in laboratory animals.
VINYL CHLORIDE A-2
APPENDIX A
Proposed MRLs undergo a rigorous review process: Health Effects/MRL Workgroup reviews within the
Office of Innovation and Analytics, Toxicology Section, expert panel peer reviews, and agency-wide
MRL Workgroup reviews, with participation from other federal agencies and comments from the public.
They are subject to change as new information becomes available concomitant with updating the
toxicological profiles. Thus, MRLs in the most recent toxicological profiles supersede previously
published MRLs. For additional information regarding MRLs, please contact the Office of Innovation
and Analytics, Toxicology Section, Agency for Toxic Substances and Disease Registry, 1600 Clifton
Road NE, Mailstop S106-5, Atlanta, Georgia 30329-4027.

VINYL CHLORIDE A-3
APPENDIX A
MINIMAL RISK LEVEL (MRL) WORKSHEET
Chemical Name: Vinyl chloride
CAS Numbers: 75-01-4
Date: January 2024
Profile Status: Final
Route: Inhalation
Duration: Acute
MRL: 0.5 ppm; 1.3 mg/m
3
Critical Effect: Delayed ossification
References: John et al. 1977, 1981
Point of Departure: NOAEL of 50 ppm; NOAEL
HEC
= 15 ppm
Uncertainty Factor: 30
LSE Graph Key: 14
Species: Mouse
MRL Summary: An acute-duration inhalation MRL of 0.5 ppm (1.3 mg/m
3
) was derived for vinyl
chloride based on a developmental endpoint of delayed ossification NOAEL of 50 ppm and a LOAEL of
500 ppm for mice administered vinyl chloride for 7 hours/day on GDs 6–15 (John et al. 1977, 1981). The
inhalation concentration of 50 ppm was duration adjusted (NOAEL
ADJ
) to a continuous exposure of
15 ppm. The partition coefficient in mice is greater than that in humans; therefore, a default value of 1 is
used for the ratio resulting in a NOAEL
HEC
of 15 ppm. The NOAEL
HEC
of 15 ppm was divided by a total
uncertainty factor of 30 (3 for extrapolation from animals to humans with dosimetric adjustment and
10 for human variability).
Selection of the Critical Effect: Available data indicate that developmental effects are the most sensitive
target for toxic effects following acute-duration inhalation exposure to vinyl chloride (Table A-1).
Delayed ossification was observed in both mice and rabbits at 500 ppm, which is the lowest LOAEL
identified for developmental effects (John et al. 1977, 1981). The mouse study included a lower
concentration (50 ppm), which was a NOAEL. Exposure of pregnant rats to 2,500 ppm 7 hours/day over
GDs 6–15 resulted in ureter dilatation in the offspring (John et al. 1977, 1981).
Relative kidney weight was increased by 20% in pregnant rats exposed to ≥100 ppm vinyl chloride
6 hours/day on GDs 6–19 (Thornton et al. 2002). This endpoint was not chosen as the basis of the acute-
duration inhalation MRL because absolute kidney weights were similar to controls and no other
parameters were available to evaluate the potential for renal toxicity (i.e., no clinical chemistry,
urinalysis, or histopathology data). A number of studies in animals identified acute-duration LOAELs for
frank narcosis and severe lung, liver, and kidney damage following exposures of 10,000–400,000 ppm of
vinyl chloride (Table 2-1).
Table A-1. Summary of Candidate Critical Effects for Acute-Duration Inhalation
MRL for Vinyl Chloride
Species
Duration
NOAEL (ppm)
LOAEL (ppm)
Effect
Reference
Developmental effects
a
Rat (Sprague-
Dawley)
GDs 6–15
10 days
7 hours/day
500
2,500
Ureter dilatation
(developmental)
John et al.
1977, 1981
VINYL CHLORIDE A-4
APPENDIX A
Table A-1. Summary of Candidate Critical Effects for Acute-Duration Inhalation
MRL for Vinyl Chloride
Species
Duration
NOAEL (ppm)
LOAEL (ppm)
Effect
Reference
Mouse (CF-1)
GDs 6–15
10 days
7 hours/day
50
500
Delayed
ossification
John et al.
1977, 1981
Rabbit (New
Zealand)
GDs 6–18
13 days
7 hours/day
ND
500
Delayed
ossification
John et al.
1977, 1981
Hepatic effects
Rat (Sprague-
Dawley)
GDs 6–15
10 days
7 hours/day
500
2,500
9 and 10%
increase in
absolute and
relative liver
weight,
respectively
John et al.
1977, 1981
Renal effects
Rat (Sprague-
Dawley)
GDs 6–19
4–6 hours/day
10
100
20% increase in
relative kidney
weight
Thornton et al.
2002
a
Selected critical effect.
GD = gestational day; LOAEL = lowest-observed-adverse-effect level; MRL = Minimal Risk Level; ND = not
determined; NOAEL = no -observed-adverse-effect level
Selection of the Principal Study: The study by John et al. (1977, 1981) was selected as the principal
study for the derivation of an acute-duration inhalation MRL based on the NOAEL of 50 ppm for delayed
ossification. This study identified the lowest LOAEL for developmental endpoints (500 ppm).
Summary of the Principal Study:
John JA, Smith FA, Leong BKJ, et al. 1977. The effects of maternally inhaled vinyl chloride on
embryonal and fetal development in mice, rats, and rabbits. Toxicol Appl Pharmacol 39:497-513.
John JA, Smith FA, Schwetz BA. 1981. Vinyl chloride: Inhalation teratology study in mice, rats, and
rabbits. Environ Health Perspect 41:171-177.
CF-1 mice (19–26 per group) were exposed to vinyl chloride at concentrations of 0, 50, or 500 ppm for
7 hours/day on GDs 6–15 (John et al. 1977, 1981). Concurrent control groups (47 animals total) were
used, one for each dose level. Control groups were sham-exposed to filtered room air. Whole body
exposure was conducted in chambers of 3.7 m
3
volume under dynamic conditions. Animals were
observed daily for clinical signs, and maternal body weights were measured several times during
gestation. Animals were euthanized on GD 18 by carbon dioxide inhalation. Maternal liver weight was
measured and uterine horns were examined. Fetuses were weighed, measured (crown-rump length),
sexed, and subjected to gross and histopathological examinations.
No adverse maternal or fetal effects were noted at 50 ppm, with the exception of a slight increase in
crown-rump length that was not observed at 500 ppm. Maternal body weight gain decreased along with
food consumption at 500 ppm. At 500 ppm, delayed ossification of the skull and sternebrae was
VINYL CHLORIDE A-5
APPENDIX A
observed. The increase in resorptions at 500 ppm was considered to have been within historical control
limits. Significant changes in the percentage of implantations resorbed, litter size, and fetal body weight
would not have been observed at 500 ppm if comparison had been made to the other control group (the
sham-exposed group for the 50-ppm concentration). There was frank maternal toxicity at 500 ppm (17%
death). The data for delayed ossification are not amenable to benchmark dose (BMD) modeling, because
only one of two dose groups showed a response that was different from controls. A LOAEL of 500 ppm
and a NOAEL of 50 ppm were identified based on delayed ossification in fetuses.
Selection of the Point of Departure for the MRL: The NOAEL of 50 ppm was selected as the POD.
Adjustment for Intermittent Exposure: The intermittent exposure duration of 7 hours/day was duration-
adjusted (NOAEL
ADJ
) to continuous exposure according to the following equation:
NOAEL
ADJ
= NOAEL (50 ppm) x 7 hours/24 hours per day = 14.58 ppm.
Human Equivalent Concentration: Following EPA (1994) methodology, the human equivalent
concentration (NOAEL
HEC
) for an extrarespiratory effect produced by a category 3 gas, such as vinyl
chloride, is calculated by multiplying the duration-adjusted animal NOAEL by the ratio of the blood:gas
partition coefficients in animals and humans ([H
b/g
]
A
/ [H
b/g
]
H
). Since the partition coefficient in mice is
greater than that in humans a default value of 1 is used for the ratio resulting in a NOAEL
HEC
of
14.58 ppm.
Uncertainty Factor: The NOAEL
HEC
was divided by a total uncertainty factor (UF) of 30:
• 3 for extrapolation from animals to humans with dosimetric adjustment
• 10 for human variability
MRL = NOAEL
HEC
÷ (UF)
14.58 ppm ÷ (3 x 10) = 0.486 ppm ≈ 0.5 ppm
Other Additional Studies or Pertinent Information that Lend Support to this MRL: Delayed
ossification (500 ppm, the lowest concentration tested) was the only developmental effect observed in a
rabbit developmental study (John et al. 1977, 1981).
Agency Contacts (Chemical Managers): Rae Benedict
VINYL CHLORIDE A-6
APPENDIX A
MINIMAL RISK LEVEL (MRL) WORKSHEET
Chemical Name: Vinyl chloride
CAS Numbers: 75-01-4
Date: January 2024
Profile Status: Final
Route: Inhalation
Duration: Intermediate
MRL: 0.02 ppm; 0.05 mg/m
3
Critical Effect: Increased incidence of centrilobular hypertrophy
Reference: Thornton et al. 2002
Point of Departure: BMCL
10
: 2.05 ppm (BMCL
HEC
: 0.5 ppm)
Uncertainty Factor: 30
LSE Graph Key: 28
Species: Rat
MRL Summary: An intermediate-duration inhalation MRL of 0.02 ppm (0.05 mg/m
3
) was derived for
vinyl chloride based on the benchmark concentration lower confidence limit 10%
(BMCL
10
) of 2.05 ppm
for the increased incidence of centrilobular hypertrophy of the liver in F1 female rats exposed for 16–
19 weeks, including exposure during gestation and lactation (Thornton et al. 2002). The BMCL
10
was
adjusted to continuous duration exposure and converted to a human equivalent concentration
(BMCL
10HEC
) of 0.5125 ppm. A total uncertainty factor of 30 (3 for species extrapolation using a
dosimetric conversion and 10 for human variability) was applied to the BMCL
10HEC
to derive the MRL of
0.02 ppm.
Selection of the Critical Effect: No dose-response data are available for humans. Available data indicate
that the liver is the most sensitive endpoint for toxic effects following intermediate-duration inhalation
exposure to vinyl chloride (Table A-2). Liver effects observed at the lowest LOAEL concentration of
approximately 10 ppm include increased liver weight (Bi et al. 1985; Thornton et al. 2002) and
centrilobular hypertrophy (Thornton et al. 2002). Fatty liver changes were also observed in two studies of
rats exposed to 50 ppm for 10 months (Sokal et al. 1980; Wisniewska-Knypl et al. 1980) and one study in
mice exposed to 286.7 ppm for 16 weeks (Wang et al. 2019a). Centrilobular degeneration and necrosis
was observed in rabbits exposed to 200 ppm for 6 months (Torkelson et al. 1961). Adverse
histopathological changes in the liver of rats and mice exposed to 2,000–3,000 ppm were observed in
several other intermediate-duration inhalation studies (Lester et al. 1963; Schaffner 1978; Torkelson et al.
1961; Wisniewska-Knypl et al. 1980).
Table A-2. Summary of Candidate Critical Effects for Intermediate-Duration
Inhalation MRL for Vinyl Chloride
Species
Duration
NOAEL (ppm)
LOAEL (ppm)
Effect
Reference
Hepatic effects
a
Rat (Wistar)
3, 6 months
6 days/week
6 hours/day
ND
11.1
Increased relative
liver weight at
6 months
Bi et al. 1985
Rat (Wistar)
10 months
5 days/week
5 hours/day
ND
50
Fatty changes
Sokal et al.
1980
Rat (Sprague-
Dawley)
2 generations
16 weeks (M)
ND
10
a
Centrilobular
hypertrophy in F1
Thornton et al.
2002

VINYL CHLORIDE A-7
APPENDIX A
Table A-2. Summary of Candidate Critical Effects for Intermediate-Duration
Inhalation MRL for Vinyl Chloride
Species
Duration
NOAEL (ppm)
LOAEL (ppm)
Effect
Reference
19 weeks (F)
4-6 hours/day
female rats
Rat (NS)
6 months
5 days/week
0.5–
7 hours/day
ND
100
Increased relative
liver weight
Torkelson et al.
1961
Rabbit (NS)
6 months
5 days/week
7 hours/day
100
200
Centrilobular
degeneration and
necrosis
Torkelson et al.
1961
Rat (Wistar)
10 months
5 days/week
5 hours/day
ND
50
Fatty changes
Wisniewska-
Knypl et al.
1980
Mouse
(C57BL/6N)
16 weeks
5 days/week
2 hours/day
57.3
286.7
Fat droplets,
eosinophilic
changes, nuclear
condensation; at
1,433.6 ppm:
Steatosis, large
lipid droplets,
hepatic edema,
cytoplasmic
loosening, and
hepatocyte nuclear
fragmentation
Wang et al.
2019a
Reproductive effects
Rat (Wistar)
3, 6 months
6 days/week
6 hours/day
100
Decreased testes
weight with
testicular necrosis
at 6 months
Bi et al. 1985
Renal effects
Rat (Wistar)
3, 6 months
6 days/week
6 hours/day
2,918
Increased relative
kidney weight at
3 months
Bi et al. 1985
Rat (Wistar)
10 months
5 days/week
5 hours/day
50
500
Increased relative
kidney weight
Sokal et al.
1980
Immunological effects
Rat (Wistar)
10 months
5 days/week
5 hours/day
ND
50
Increased relative
spleen weight
Sokal et al.
1980

VINYL CHLORIDE A-8
APPENDIX A
Table A-2. Summary of Candidate Critical Effects for Intermediate-Duration
Inhalation MRL for Vinyl Chloride
Species
Duration
NOAEL (ppm)
LOAEL (ppm)
Effect
Reference
Mouse (CD-1)
2–8 weeks
5 days/week
6 hours/day
ND
10
Increased
spontaneous
lymphocyte
proliferation
Sharma and
Gehring 1979
Rabbit (New
Zealand)
8 weeks
5 days/week
6 hours/day
ND
10
Increased
spontaneous
lymphocyte
proliferation
Sharma et al.
1980
a
Selected critical effect.
F = female(s); LOAEL = lowest-observed-adverse-effect level; M = male(s); MRL = Minimal Risk Level; ND = not
determined; NOAEL = no-observed-adverse-effect level
Testicular lesions characterized as degenerative seminiferous tubule changes or spermatogenic epithelial
necrosis were observed in male rats exposed for 6–10 months to 100–500 ppm vinyl chloride (Bi et al.
1985; Sokal et al. 1980). Decreased white blood cell counts resulted from exposure of rats to 20,000 ppm
for 3 months (Lester et al. 1963), while increased lymphocyte proliferation resulted in mice and
immunized rabbits exposed to 10 ppm for up to 8 weeks (Sharma and Gehring 1979; Sharma et al. 1980).
Exposures of 10–20,000 ppm resulted in increases and decreases in various relative organ weights (Bi et
al. 1985; Sokal et al. 1980; Sharma et al. 1980), including the liver (Bi et al. 1985; Sharma and Gehring
1979; Thornton et al. 2002; Torkelson et al. 1961).
Selection of the Principal Study: Thornton et al. (2002) was chosen as the principal study for derivation
of the intermediate-duration inhalation MRL. The study identified the lowest LOAEL for critical liver
effects including centrilobular hypertrophy and increased liver weight in rats. The study provided data for
centrilobular hypertrophy in F1 offspring, a minimally adverse effect in a sensitive subpopulation
(offspring) of the target organ (liver) that is sensitive to both inhalation and oral exposures. A
hematological effect was also observed at 10 ppm in mice (Sharma and Gehring 1979) and immunized
rabbits (Sharma et al. 1980). However, these studies were not selected as a principal study due to the
short exposure duration (2–8 weeks) and lack of other study support.
Summary of the Principal Study:
Thornton SR, Schroeder RE, Robison RL, et al. 2002. Embryo-fetal developmental and reproductive
toxicology of vinyl chloride in rats. Toxicol Sci 68:207-219.
Groups of male and female Sprague-Dawley rats (30/sex/group) were exposed to vinyl chloride vapor
concentrations of 0, 10, 100, or 1,100 ppm, 6 hours/day for 10 weeks prior to mating and during a 3-week
mating period. F0 males were exposed during the gestational period and sacrificed following the
completion of parturition. F0 females were exposed during gestation and lactation (with the exception of
a break in exposure from GD 21 through postnatal day 4 to allow for delivery of litters). All F0 rats were
observed twice daily for clinical signs. Body weights and food consumption were monitored. F1 litters
were examined for live and dead pups and on lactation day 4, litters were culled to eight pups (equal
numbers of male and female pups where possible). All F0 female rats (including those that did not
produce offspring) were sacrificed after the F1 rats had been weaned. Reproductive tissues, adrenal
glands, brain, kidneys, liver, lungs, spleen, thymus, mammary glands, nasal tissues, pituitary, and trachea

VINYL CHLORIDE A-9
APPENDIX A
from each of the F0 rats were individually weighed and subjected to histopathologic examinations. At
weaning, 15 male and female F1 rats/group were selected for gross and microscopic examinations. Other
F1 rats were randomly selected to form groups of 30/sex/group, and these F1 rats were subjected to the
same treatment as the F0 rats during the production of an F2 generation. At weaning, 15 male and female
F2 rats/group were subjected to gross and microscopic examinations. Sperm parameters were assessed in
15 F0 and 15 F1 male rats of each exposure group.
Absolute and relative mean liver weights were significantly increased at all exposure levels in F0 males
and in 100- and 1,100-ppm F1 males. Slight centrilobular hypertrophy, considered to be a minimal
adverse effect, was noted in the livers of all 1,100-ppm male and female F0 and F1 rats, most 100-ppm
male and female F0 and F1 rats, and in 2/30 and 6/30 of the 10-ppm F0 and F1 female rats, respectively.
No incidences of centrilobular hypertrophy were found in any of the control rats. Compared to an
incidence of 0/30 for this lesion in controls, the incidence of 6/30 in the 10-ppm F1 female rats exceeded
the level of statistical significance (p<0.05 according to Fisher’s Exact Test performed by ATSDR).
Selection of the Point of Departure for the MRL: The BMCL
10
value of 2.05 ppm for increased
incidence of centrilobular hypertrophy in the liver of F1 female rats was selected as the basis of the MRL.
BMD modeling was performed for the candidate liver endpoints in Table A-3 when data were amenable
to modeling. Data modeled are shown in Tables A-4 and A-5. The data were fit to all available
dichotomous or continuous models in EPA’s Benchmark Dose Software (BMDS) (version 3.2) using a
benchmark response (BMR) of 1 standard deviation (liver weight data) or 10% extra risk (centrilobular
hypertrophy). Adequate model fit was judged by four criteria: goodness-of-fit statistics (p-value >0.1),
visual inspection of the dose-response curve, BMCL that is not 10 times lower than the lowest non-zero
dose, and scaled residual within ±2 units at the data point (except the control) closest to the predefined
BMR. Among all of the models providing adequate fit to the data, the lowest BMCL (95% lower
confidence limit on the BMD) was selected as the POD when the difference between the BMCLs
estimated from these models was ≥3 fold; otherwise, the BMCL from the model with the lowest
Akaike Information Criterion (AIC) was chosen. ATSDR follows EPA BMD Guidance (EPA 2012) that
compares the fold difference in BMCL values of acceptable models to select the most appropriate model.
Table A-3. Summary of Candidate Critical Liver Effects for Intermediate-Duration
Inhalation MRL for Vinyl Chloride
a
Effect
Sex/generation
NOAEC (ppm)
LOAEC (ppm)
Absolute liver weight
F0 males
ND
10
F1 males
10
100
Relative liver weight
F0 males
10
100
F1 males
10
100
Centrilobular hypertrophy
F0 females
10
100
F1 females
ND
10
a
Thornton et al. (2002); exposure occurred 10 weeks prior to mating and during a 3-week mating period; F0 males
were further exposed during the gestational period and F0 females were further exposed during gestation and
lactation.
LOAEC = lowest-observed-adverse-effect level; MRL = Minimal Risk Level; NOAEC = no-observed-adverse-effect
level; ND = not determined

VINYL CHLORIDE A-10
APPENDIX A
Table A-4. Absolute and Relative Liver Weight in F0 And F1 Male Rats Following
Inhalation Exposure to Vinyl Chloride
a
Endpoint
Exposure concentration (ppm)
0
10
100
1,100
Number of animals
15
15
15
15
Absolute liver weight (g)
F0 males
14.32±2.13
b
16.20±2.19
c
16.22±1.59
d
16.72±0.86
d
F1 males
14.13±2.36
15.07±2.74
16.62±2.27
c
17.01±1.49
d
Relative liver weight
F0 males
2.83±0.26
3.05±0.29
c
3.09±0.20
c
3.26±0.19
d
F1 males
2.98±0.33
3.01±0.19
3.32±0.36
d
3.38±0.19
d
a
Exposure for 6 hours/day for 10 weeks prior to mating and during mating and gestation (males and females) and
lactation (females).
b
Mean±standard deviation.
c
Statistically significantly (p<0.05) different from controls.
d
Statistically significantly (p<0.01) different from controls.
Source: Thornton et al. 2002
Table A-5. Incidences of Centrilobular Hypertrophy in the Liver for F0 And F1
Female Rats Following Inhalation Exposure to Vinyl Chloride
a
Exposure concentration (ppm)
0
10
100
1,100
F0 females
F1 females
0/30
0/30
2/30
6/30
b
26/30
b
30/30
b
30/30
b
30/30
b
a
Exposure for 6 hours/day for 10 weeks prior to mating and during mating and gestation (males and females) and
lactation (females).
b
Statistically significantly (p<0.05) different from controls according to Fisher’s Exact Test performed by ATSDR.
Source: Thornton et al. 2002
None of the BMD models (with constant variance or nonconstant variance) provided adequate fit to the
data for increased absolute liver weight in F0 males or to relative liver weight in F1 males. Therefore, a
NOAEL/LOAEL approach was used for these endpoints.
For absolute liver weight in F1 males, the BMD software (BMDS) could not adequately fit the full
dataset, but it was able to provide an adequate fit after dropping the highest dose (1,100 ppm). Dropping
the highest dose (or doses) is a valid technique in this case. First, the dataset had enough non-zero dose
groups with significant responses to remove the highest dosage without loss of BMD trend. Second, the
POD for this dataset would visually be in the lower dose groups, but the high dose group is very far away
from these lower groups. This situation can lead to models straining to fit the high group (because of
leverage) at the cost of losing adequate fit of lower groups. With the highest dose dropped, five
frequentist, constant variance models provided adequate fit to the data. BMCLs for models providing
adequate fit were sufficiently close (differed by <3-fold), so the simplest model with the lowest AIC was
selected (Linear). The restricted linear model estimated a BMC
1SD
and BMCL
1SD
of 110 and 68 ppm,

VINYL CHLORIDE A-11
APPENDIX A
respectively. BMDS states a warning when fitting the reduced dataset, as the estimated BMD was higher
than the new highest dose (100 ppm), which normally raises extrapolation error concerns. However, the
estimated BMD (109.8) was still less than the removed high dose, so the estimate would not be much of
an extrapolation. Since BMD falls well below the dropped dose of 1,100 ppm, the extrapolation warning
(BMD>higher dose) may not be a concern. The results of the BMD modeling are summarized in
Table A-6.
Table A-6. Model Predictions (Constant Variance) for Absolute Liver Weight in F1
Male Rats Following Inhalation Exposure to Vinyl Chloride
a
Model
BMC
1SD
b
(ppm)
BMCL
1SD
b
(ppm)
p-Value
c
Scaled residuals
d
AIC
Dose near
BMC
Dose near
control
Highest dose dropped from dataset
Exponential (model 2)
e
109.69
70.36
0.40
212.52
-0.06
-0.57
Exponential (model 3)
e
109.72
70.36
0.40
212.52
-0.05
-0.57
Exponential (model 4)
e
NA
213.80
-3.3x10
-6
-4.1x10
-6
Exponential (model 5)
e
NA
213.80
-5.8x10
-8
-2.7x10
-7
Hill
e
<0.0001
215.80
-0.00023
-9.7x10
-5
Polynomial (2-degree)
e
109.77
67.61
0.41
212.49
-0.06
-0.55
Power
f
109.77
67.61
0.41
212.49
-0.06
-0.55
Linear
e,g
109.77
67.61
0.41
212.49
-0.06
-0.55
a
Exposure for 6 hours/day for 10 weeks prior to mating and during mating and gestation (males and females) and
lactation (females).
b
BMC and BMCL values for models that do not provide adequate fit are not included in the table.
c
Values <0.1 fail to meet conventional goodness-of-fit criteria.
d
Scaled residuals at concentrations immediately below and above the BMC.
e
Power restricted to ≥1.
f
Coefficients restricted to be positive.
g
Selected model. For the full dataset, none of the models provided adequate fit to the variance data (constant or
nonconstant). With the highest dose dropped, c
onstant variance models provided adequate fit to the variance data.
With constant variance model applied, all models provided adequate fit to the means except for the Hill and
Exponential 4 and 5 models. BMCLs for models providing adequate fit were sufficiently close (differed by <3-fold),
so the simplest model with the lowest AIC is selected (Linear).
AIC = Akaike Information Criterion; BMC = benchmark concentration (maximum likelihood estimate of the exposure
concentration associated with the selected benchmark response); BMCL = 95% lower confidence limit on the BMC
(subscripts denote benchmark response); NA = not applicable (Goodness of fit test cannot be calculated);
SD = standard deviation
Source: Thornton et al. 2002
For relative liver weight in F0 males, no constant variance models provided an adequate fit to the dataset
with the nonconstant variance model applied, only the Hill and Exponential 4 and 5 models provided
adequate fit to the data. The BMD computation failed for the Hill model; the lower limit included zero
and the BMDL was not estimated. BMCLs for models providing adequate fit were sufficiently close
(differed by <3-fold), so the model with the lowest AIC was selected (Exponential 4). The Exponential 4
model estimated a BMC
1SD
and BMCL
1SD
of 216 and 72 ppm, respectively. The results of the BMD
modeling are summarized in Table A-7.

VINYL CHLORIDE A-12
APPENDIX A
Table A-7. Model Predictions (Nonconstant Variance) for Relative Liver Weight in
F0 Male Rats Following Inhalation Exposure to Vinyl Chloride
a
Model
BMC
1SD
b
(ppm)
BMCL
1SD
b
(ppm)
p-Value
c
Scaled residuals
d
AIC
Dose near
BMC
Dose near
control
Exponential (model 2)
e
0.02
8.08
-0.07
-2.16
Exponential (model 3)
e
0.02
8.08
-0.08
-2.16
Exponential (model 4)
e,f
216.31
71.99
0.11
5.08
-0.40
-1.20
Exponential (model 5)
e
225.86
70.96
0.11
5.09
-0.38
-1.22
Hill
d
246.14
0
0.14
4.72
-0.57
-1.04
Polynomial (3-degree)
e
0.02
8.03
-0.09
-2.15
Polynomial (2-degree)
e
0.02
8.03
-0.09
-2.15
Power
e
0.02
8.03
-0.09
-2.15
Linear
g
0.02
8.03
-0.09
-2.15
a
Exposure for 6 hours/day for 10 weeks prior to mating and during mating and gestation (males and females) and
lactation (females).
b
BMC and BMCL values for models that do not provide adequate fit are not included in the table.
c
Values <0.1 fail to meet conventional goodness-of-fit criteria.
d
Scaled residuals at concentrations immediately below and above the BMC.
e
Power restricted to ≥1.
f
Selected model. None of the constant variance models provided adequate fit to the data. With the nonconstant
variance model applied, only the Hill and Exponential 4 and 5 models provided adequate fit to the data. The BMC
computation failed for the Hill model; the lower limit included zero and the BMCL was not estimated; therefore, the
Hill model was unusable. BMCLs for models providing adequate fit were sufficiently close (differed by <3-fold), so
the model with the lowest AIC was selected (Exponential 4).
g
Coefficients restricted to be positive.
AIC = Akaike Information Criterion; BMC = benchmark concentration (maximum likelihood estimate of the exposure
concentration associated with the selected benchmark response); BMCL = 95% lower confidence limit on the BMC
(subscripts denote benchmark response); SD = standard deviation
Source: Thornton et al. 2002
For the incidence of centrilobular hypertrophy in the liver in F0 females, all models provided an adequate
fit to the data except for the Probit model. BMCLs for models providing an adequate fit were not
sufficiently close (differed by >3-fold), so the model with the lowest BMCL was selected (1-degree
multistage). The 1-degree multistage model estimated a BMC
10
and BMCL
10
of 6.16 and 4.4 ppm,
respectively. The results of the BMD modeling are summarized in
Table A-8.

VINYL CHLORIDE A-13
APPENDIX A
Table A-8. Results from BMD Analysis of Incidences of Centrilobular
Hypertrophy in the Liver in F0 Female Rats Following Inhalation
Exposure to Vinyl Chloride
a
Model
BMC
10
b
(ppm)
BMCL
10
b
(ppm)
p-Value
c
Scaled residuals
d
AIC
Dose near
BMC
Dose near
control
Gamma
e
13.01
5.89
1.00
44.26
0.0006
-0.0032
Logistic
31.04
20.79
0.54
44.13
0.7257
-0.8500
Log-Logistic
f
12.64
6.89
0.98
42.34
0.0301
-0.0007
Log-Probit
12.14
7.58
0.97
44.26
0.0028
-0.0007
Multistage (1-degree)
g,h
6.16
4.40
0.31
45.03
-1.3638
-0.0007
Multistage (2-degree)
h
14.06
5.78
1.00
44.26
1.71x10
-5
-0.0007
Multistage (3-degree)
h
14.92
5.76
1.00
42.26
2.16x10
-6
-0.0007
Probit
0.01
55.73
-1.1734
-1.9041
Weibull
e
12.79
5.85
0.90
44.27
-0.1025
-0.0017
Dichotomous Hill
12.64
6.89
0.98
42.34
0.0301
-0.0007
a
Exposure for 6 hours/day for 10 weeks prior to mating and during mating and gestation (males and females) and
lactation (females).
b
BMC and BMCL values for models that do not provide adequate fit are not included in the table.
c
Values <0.1 fail to meet conventional χ
2
goodness-of-fit criteria.
d
Scaled residuals at doses immediately below and above the BMC.
e
Power restricted to ≥1.
f
Slope restricted to ≥1.
g
Selected model. All models provided adequate fit to the data except for the Probit model. BMCLs for models
providing adequate fit differed by >3-fold; therefore, the model with the lowest BMCL was selected (1-degree
Multistage).
h
Betas restricted to ≥0.
AIC = Akaike Information Criterion; BMC = benchmark concentration (maximum likelihood estimate of the dose
associated with the selected benchmark response); BMCL
10
= 95% lower confidence limit on the BMC (subscripts
denote benchmark response: i.e., 10 = dose associated with 10% extra risk)
Source: Thornton et al. 2002
For the incidence of centrilobular hypertrophy in the liver in F1 females, all models provided an adequate
fit to the data except for the Probit model. The BMD computation failed for the Weibull model and a
BMCL was not estimated; this model was deemed unusable. BMCLs for models providing an adequate
fit were not sufficiently close (differed by >3-fold), so the model with the lowest BMCL was selected
(1-degree multistage). The 1-degree multistage model estimated a BMC
10
and BMCL
10
of 3.03 and
2.05 ppm, respectively. The results of the BMD modeling are summarized in
Table A-9.

VINYL CHLORIDE A-14
APPENDIX A
Table A-9. Results from BMD Analysis of Incidences of Centrilobular
Hypertrophy in the Liver in F1 Female Rats Following Inhalation
Exposure to Vinyl Chloride
a
Model
BMC
10
b
(ppm)
BMCL
10
b
(ppm)
p-Value
c
Scaled residuals
d
AIC
Dose near
BMC
Dose near
control
Gamma
e
6.53
3.10
0.98
34.11
-0.0241
-0.0007
Logistic
11.34
7.58
0.41
36.75
0.9450
-1.4034
Log-Logistic
f
8.21
5.21
1.00
32.04
-0.0021
-0.0007
Log-Probit
8.59
5.09
1.00
34.02
7.296x10
-11
-0.0007
Multistage (1-degree)
g,h
3.03
2.05
0.33
37.28
-0.0007
-0.0007
Multistage (2-degree)
h
6.75
2.72
1.00
34.02
-2.32x10
-8
-0.0007
Multistage (3-degree)
h
6.76
2.61
1.00
36.02
3.527x10
-8
-0.0007
Probit
0.001
60.13
-0.4459
-2.6297
Weibull
e
5.11
0.00
0.84
34.65
-0.2606
-0.0007
Dichotomous Hill
8.21
5.21
1.00
34.04
-0.0021
-0.0007
a
Exposure for 6 hours/day for 10 weeks prior to mating and during mating and gestation (males and females) and
lactation (females).
b
BMC and BMCL values for models that do not provide adequate fit are not included in the table.
c
Values <0.1 fail to meet conventional χ
2
goodness-of-fit criteria.
d
Scaled residuals at doses immediately below and above the BMC.
e
Power restricted to ≥1.
f
Slope restricted to ≥1.
g
Selected model. All models provided adequate fit to the data except for the Probit model and the Weibull model did
not estimate a BMCL. BMCLs for models providing adequate fit differed by >3-fold; therefore, the model with the
lowest BMCL was selected (1-degree Multistage).
h
Betas restricted to ≥0.
AIC = Akaike Information Criterion; BMC = benchmark concentration (maximum likelihood estimate of the dose
associated with the selected benchmark response); BMCL
10
= 95% lower confidence limit on the BMC (subscripts
denote benchmark response: i.e., 10 = dose associated with 10% extra risk)
Source: Thornton et al. 2002
Table A-10 summarizes the potential candidate PODs for the intermediate-duration inhalation MRL for
vinyl chloride. Based on the lowest available critical values (BMC, NOAEL), centrilobular hypertrophy
(in F1 females) was identified as the critical effect following intermediate-duration inhalation exposure to
vinyl chloride. The 1-degree multistage model fit to the centrilobular hypertrophy data in F1 female rats
is presented in Figure A-1. The corresponding BMCL
10
of 2.05 is used as the POD in further
calculations.

VINYL CHLORIDE A-15
APPENDIX A
Table A-10. Candidate Points of Departure for the Intermediate-Duration
Inhalation MRL
Endpoint
NOAEC
(ppm)
LOAEC
(ppm)
BMC
10
(ppm)
BMCL
10
(ppm)
Increased absolute liver weight
F0 males
ND
10
Increased absolute liver weight
F1 males
110
68
Increased relative liver weight
F0 males
216
72
Increased relative liver weight
F1 males
10
100
Centrilobular hypertrophy
F0 females
6.16
4.4
Centrilobular hypertrophy
F1 females
3.03
2.05
BMC = benchmark concentration; BMCL = 95% lower confidence limit on the BMC; LOAEL = lowest-observed-
adverse-effect level; MRL = Minimal Risk Level; NOAEL = no-observed-adverse-effect level
Figure A-1. Fit of 1-Degree Multistage Model to Data for Incidences of
Centrilobular Hypertrophy in the Liver in F1 Female Rats Following
Inhalation Exposure to Vinyl Chloride (Thornton et al. 2002)
0
0.1
0.2
0.3
0.4
0.5
0.6
0.7
0.8
0.9
1
0 200 400 600 800 1000
Response
Dose
Frequentist Multistage Degree 1 Model with BMR of 10% Extra
Risk for the BMD and 0.95 Lower Confidence Limit for the BMDL
Estimated Probability
Response at BMD
Linear Extrapolation
Data
BMD
BMDL
VINYL CHLORIDE A-16
APPENDIX A
Calculations
Adjustment for Intermittent Exposure: The intermittent exposure duration of 6 hours/day was duration-
adjusted (BMCL
10ADJ
) to continuous exposure according to the following equation:
BMCL
10ADJ
= BMCL
10
(2.05 ppm) x 6 hours/24 hours per day = 0.5125 ppm
Human Equivalent Concentration: Following EPA (1994) methodology, the human equivalent
concentration (BMCL
10HEC
) for an extrarespiratory effect produced by a category 3 gas, such as vinyl
chloride, is calculated by multiplying the animal BMCL
10ADJ
by the ratio of the blood:gas partition
coefficients in animals and humans [(H
b/g
)
A
/ H
b/g
)
H
]. Since the partition coefficient in rats is greater than
that in humans, a default value of 1 is used for the ratio and the animal BMCL
10ADJ
is equivalent to the
BMCL
10HEC
. Several PBPK models are available for vinyl chloride; however, none of these models
included an evaluation of exposure during mating, gestation, or lactation. Therefore, PBPK models could
not be used to calculate a BMCL
10HEC
from the Thornton et al. (2002) study. The intermediate-duration
inhalation MRL of 0.02 ppm was derived by dividing the BMCL
10HEC
of 0.5125 ppm for centrilobular
hypertrophy in female Sprague-Dawley rats by a factor of 30 (3 for species extrapolation using a
dosimetric conversion and 10 for human variability).
Uncertainty Factor: The BMCL
10
was divided by a total uncertainty factor (UF) of 30:
• 3 for extrapolation from animals to humans with dosimetric adjustment
• 10 for human variability
MRL = BMCL
10HEC
÷ (UF)
0.5125 ppm ÷ (3 x 10) = 0.017 ≈ 0.02 ppm
Other Additional Studies or Pertinent Information that Lend Support to this MRL: Liver enlargement
and/or histopathological changes have been noted in a number of intermediate-duration inhalation studies
in animals (Bi et al. 1985; Lester et al. 1963; Schaffner 1978; Sokal et al. 1980; Torkelson et al. 1961;
Wisniewska-Knypl et al. 1980). The studies by Thornton et al. (2002) and Bi et al. (1985) show these
effects at a somewhat lower dosage. In support of using an effect level of 10 ppm, there was also a
finding of immunostimulation in mice and immunized rabbits at 10 ppm (Sharma and Gehring 1979;
Sharma et al. 1980).
Agency Contacts (Chemical Managers): Rae Benedict
VINYL CHLORIDE A-17
APPENDIX A
MINIMAL RISK LEVEL (MRL) WORKSHEET
Chemical Name: Vinyl chloride
CAS Numbers: 75-01-4
Date: January 2024
Profile Status: Final
Route: Inhalation
Duration: Chronic
MRL Summary: There are insufficient data for derivation of a chronic-duration inhalation MRL for
vinyl chloride.
Rationale for Not Deriving an MRL: In the absence of exposure level data, the human database did not
provide a suitable LOAEL or NOAEL for derivation of a chronic-duration inhalation MRL. The animal
database mostly reported cancer and death. One study (Bi et al. 1985) reported body weight, organ
weight, reproductive (histological), and cancer effects. A NOAEL (11.1 ppm) and a LOAEL (105.6 ppm)
were identified for testicular effects (increases in the number of degenerative seminiferous tubule
changes) in a chronic-duration inhalation study (Bi et al. 1985). However, the results of the Thornton et
al. (2002) study for intermediate-duration exposure suggest that liver effects (increased liver weight,
centrilobular hypertrophy) would occur at lower concentrations (10 ppm) than the reported testicular
effects. Bi et al. (1985) did not report noncancer liver histopathology; therefore, this study cannot be used
to derive a chronic-duration inhalation MRL. Though several other chronic-duration studies did report
carcinogenicity in rats chronically exposed to 5–250 ppm vinyl chloride (Drew et al. 1983; Lee et al.
1977a, 1978; Maltoni et al. 1981), they did not report the incidence of noncancerous or precancerous
histopathological lesions in any tissue. Therefore, no chronic-duration inhalation MRL was derived for
vinyl chloride.
Agency Contacts (Chemical Managers): Rae Benedict
VINYL CHLORIDE A-18
APPENDIX A
MINIMAL RISK LEVEL (MRL) WORKSHEET
Chemical Name: Vinyl chloride
CAS Numbers: 75-01-4
Date: January 2024
Profile Status: Final
Route: Oral
Duration: Acute
MRL Summary: There are insufficient data for derivation of an acute-duration oral MRL for vinyl
chloride.
Rationale for Not Deriving an MRL: No acute-duration oral MRLs was derived for vinyl chloride
because of an absence of data on the effects of oral exposure to vinyl chloride for this duration category.
Agency Contacts (Chemical Managers): Rae Benedict
VINYL CHLORIDE A-19
APPENDIX A
MINIMAL RISK LEVEL (MRL) WORKSHEET
Chemical Name: Vinyl chloride
CAS Numbers: 75-01-4
Date: January 2024
Profile Status: Final
Route: Oral
Duration: Intermediate
MRL Summary: There are insufficient data for derivation of an intermediate-duration oral MRL for
vinyl chloride.
Rationale for Not Deriving an MRL: No intermediate-duration oral MRLs was derived for vinyl
chloride because of an absence of data on the effects of oral exposure to vinyl chloride for this duration
category.
Agency Contacts (Chemical Managers): Rae Benedict

VINYL CHLORIDE A-20
APPENDIX A
MINIMAL RISK LEVEL (MRL) WORKSHEET
Chemical Name: Vinyl chloride
CAS Numbers: 75-01-4
Date: January 2024
Profile Status: Final
Route: Oral
Duration: Chronic
MRL: 0.003 mg/kg/day (3 µg/kg/day)
Critical Effect: Liver cell polymorphisms
References: Til et al. 1983, 1991
Point of Departure: NOAEL of 0.17 mg/kg/day (NOAEL
HED
of 0.09 mg/kg/day)
Uncertainty Factor: 30
LSE Graph Key: 5
Species: Rat
MRL Summary: A chronic-duration oral MRL of 0.003 mg/kg/day (3 µg/kg/day) is proposed for vinyl
chloride based on a NOAEL of 0.17 mg/kg/day and a LOAEL of 1.7 mg/kg/day for liver cell
polymorphisms in rats administered vinyl chloride for 149 weeks (Til et al. 1983,1991). The PBPK-
modeled equivalent human NOAEL associated with the rat NOAEL (NOAEL
HED
) of 0.17 mg/kg/day was
0.09 mg/kg/day. The NOAEL
HED
was divided by a total uncertainty factor of 30 (3 for species
extrapolation using a dosimetric conversion and 10 for human variability) to arrive at an MRL of
0.003 mg/kg/day.
Selection of the Critical Effect: No dose-response data are available for humans. Available data indicate
that the liver is the most sensitive endpoint for toxic effects following chronic-duration oral exposure to
vinyl chloride (Table A-11). A number of effects were observed in rats given 1.7 mg/kg/day, including
hepatocellular alterations (Feron et al. 1981), liver cell polymorphisms, and increased mortality (Til et al.
1983, 1991). Liver cell polymorphism is related to cytotoxicity and is considered a nonneoplastic lesion
(Schoental and Magee 1957, 1959). The LOAEL of 1.7 mg/kg/day for liver cell polymorphism (in both
sexes) and hepatic cysts in female rats was the lowest identified LOAEL and was associated with the
lowest identified NOAEL (0.17 mg/kg/day) for any chronic effect. Chronic gavage doses of 3 mg/kg/day
vinyl chloride in rats resulted in increased mottled appearance and hemorrhagic liver patches (Knight and
Gibbons 1987). Doses of 14.1 mg/kg/day in female rats resulted in extensive hepatic necrosis, 100%
early mortality, humpback position, lethargy, and emaciation (Feron et al. 1981). Decreased blood
clotting time was also observed in rats given 14.1 mg/kg/day (Feron et al. 1981). Increased collagen
deposition and skin thickness were seen in rats chronically gavaged with 30 mg/kg/day (Knight and
Gibbons 1987).
Table A-11. Summary of Candidate Critical Effects for Chronic-Duration Oral
MRL for Vinyl Chloride
Species
Duration/route
NOAEL
(mg/kg/day)
LOAEL
(mg/kg/day)
Effect
Reference
Hepatic effects
Rat (Wistar)
84 weeks–
2.7 years
5 days/week
4 hours/day
(F),
(GO)
ND
1.7
Cellular alteration
Feron et al.
1981

VINYL CHLORIDE A-21
APPENDIX A
Table A-11. Summary of Candidate Critical Effects for Chronic-Duration Oral
MRL for Vinyl Chloride
Species
Duration/route
NOAEL
(mg/kg/day)
LOAEL
(mg/kg/day)
Effect
Reference
Rat (Wistar)
149 weeks
4 hours/day (F)
0.17
a
1.7
Liver cell
polymorphism
Til et al. 1983,
1991
Rat (Wistar)
2 years
1 time/day (GO)
3
Mottled appearance
and hemorrhagic
patches
Knight and
Gibbons 1987
Hematological
Rat (Wistar)
84 weeks–
2.7 years
5 days/week
4 hours/day
(F),
(GO)
5
14.1
Decreased clotting
time
Feron et al.
1981
Neurological
Rat (Wistar)
84 weeks–
2.7 years
5 days/week
4 hours/day
(F),
(GO)
5
14.1
Humpback position,
lethargy, emaciation
Feron et al.
1981
Dermal effects
Rat (Wistar)
2 years
1 time/day (GO)
30
Increased skin
thickness, collagen
Knight and
Gibbons 1987
F = female(s); G = gavage (no vehicle); GO = gavage (oil vehicle); LOAEL = lowest-observed-adverse-effect level;
M = male(s); MRL = Minimal Risk Level; NOAEL = no-observed-adverse-effect level; ND = not determined
Selection of the Principal Study: The study by Til et al. (1983,1991) was selected as the principal study
for the derivation of a chronic-duration oral MRL based on the NOAEL of 0.17 mg/kg/day for liver cell
polymorphisms. This study identified the lowest LOAEL (1.7 mg/kg/day) for the critical effect.
Summary of the Principal Study:
Til HP, Immel HR, Feron VJ. 1983. Lifespan oral carcinogenicity study of vinyl chloride in rats. Final
report. Civo Institutes, TNO. Report No. V 93.285/291099.
Til HP, Feron VJ, Immel HR. 1991. Lifetime (149-week) oral carcinogenicity study of vinyl chloride in
rats. Food Chem Toxicol 29:713-718.
Groups of Wistar rats (100/sex/group in controls and the two lowest exposure groups; 50/sex at the
highest exposure level) were administered vinyl chloride in the daily diet at intended initial dietary
concentrations of 0, 0.46, 4.6, or 46 ppm for 149 weeks. Due to rapid evaporative loss of vinyl chloride
from the food, liquid vinyl chloride was mixed with PVC granules to produce a mixture in which vinyl
chloride was effectively encapsulated in PVC granules (Feron et al. 1975). The study authors trained the
rats to a feeding schedule of 4 hours/day prior to the initiation of exposure to vinyl chloride in the diet.
The authors noted that food consumption per hour was fairly constant during the 4-hour feeding period.
Loss of vinyl chloride from food during the first hour, the second hour, and the final 2 hours was
calculated. Periodic food intake measurements were made for the first hour, the second hour, and the

VINYL CHLORIDE A-22
APPENDIX A
final 2 hours. Based on these measurements, the study authors calculated the average oral intake of the
combined sexes during the daily 4-hour feeding periods to be 0, 0.018, 0.17, and 1.7 mg/kg/day for the 0-,
0.49-, 4.49-, and 44.1-ppm groups, respectively. Measurements of vinyl chloride in the feces were made
periodically at 1 hour prior to the feeding period, the end of the 4-hour feeding period, and 4 and 9 hours
later. The study authors considered the vinyl chloride content in the feces to have remained encapsulated
in the PVC granules and thus not to have been available for absorption from the gastrointestinal tract.
The amount of vinyl chloride in the feces was subtracted from the calculated daily oral intake of vinyl
chloride to arrive at what the study authors termed “actual oral exposure levels” of 0, 0.014, 0.13, and
1.3 mg/kg/day for the 0-, 0.49-, 4.49-, and 44.1-ppm groups, respectively. The incidence of cell
polymorphism was recorded by sex and estimated absorbed dose group (Table A-12). Results of
toxicokinetic assessments for vinyl chloride indicate that, following absorption, vinyl chloride and its
metabolites are not excreted in appreciable amounts in the feces. Types and incidences of neoplastic and
nonneoplastic liver lesions were determined at the end of the study.
Effects noted in study and corresponding doses: The critical nonneoplastic effect was determined to be
liver cell polymorphism, which was classified by severity (slight, moderate, severe). The incidences of
this lesion are listed in Table A-12.
Selection of the Point of Departure for the MRL: A LOAEL of 1.7 mg/kg/day was identified for
statistically significantly increased incidences of liver cell polymorphism in male and female rats. The
NOAEL for nonneoplastic liver effects is 0.17 mg/kg/day. An increase in the incidence of female rats
with many hepatic cysts was also observed at the highest dose (1.7 mg/kg/day). Other histopathologic
lesions, described as hepatic foci of cellular alteration, were observed at all dose levels in female rats and
in high-dose male rats, but were not used to derive an MRL because they are considered to be
preneoplastic lesions. MRLs are protective only for non-neoplastic effects and do not reflect cancer risk.
EPA (2000) applied the Clewell et al. (1995) PBPK model for vinyl chloride to the low-, mid-, and high-
dose groups (estimated absorbed doses of 0.014, 0.13, and 1.3 mg/kg/day, respectively) to generate dose
metrics of 0.3, 3, and 30 mg vinyl chloride metabolites/L liver, respectively. The EPA approach was
reviewed and was considered appropriate for deriving the chronic-duration oral MRL.
Table A-12. Incidences of Male and Female Wistar Rats Exhibiting Slight,
Moderate, or Severe Liver Cell Polymorphism Following Daily
Oral Exposure to Vinyl Chloride in the Diet for 149 Weeks
Estimated oral intake, absorbed (mg/kg/day)
Males
Females
0
0.014
0.13
1.3
0
0.014
0.13
1.3
Number of rats
examined
99
99
99
49
98
100
96
49
Slight
Moderate
Severe
27
4
1
23
4
1
26
7
1
19
10
a
3
46
14
2
41
13
3
49
8
4
23
15
b
9
c
a
Significantly different from controls according to Fisher’s exact test (p<0.001).
b
Significantly different from controls according to Fisher’s exact test (p<0.05).
c
Significantly different from controls according to Fisher’s exact test (p<0.0001).
Source: Til et al. 1983, 1991
VINYL CHLORIDE A-23
APPENDIX A
The dose metric, “number of rats examined,” and the “moderate” and “severe” polymorphism categories
(Table A-12) were used in modeling. The “number of rats examined” were summed, regardless of sex,
for each dose group, resulting in a low-dose, mid-dose, and high-dose groups. For example, the low-dose
group males numbered 99 and the low-dose females numbered 100 to result in 199 rats that were
examined in that group (Tables A-12 and A-13). Likewise, the “moderate” and “severe” cell
polymorphism incidence data were combined (i.e., summed) for each group, regardless of sex, resulting
in one data category of moderate+severe (Table A-13). The moderate+severe polymorphism data had one
control group and three exposure groups (low-dose, mid-dose, and high-dose). These combinations
resulted in the following cell polymorphism data that were used for modeling: 21/197 controls,
21/199 low-dose, 20/196 mid-dose, and 37/98 high-dose rats) (Til et al. 1983, 1991).
Table A-13. Incidences of Male and Female Wistar Rats Exhibiting Moderate or
Severe Liver Cell Polymorphism Following Daily Oral Exposure
to Vinyl Chloride in the Diet for 149 Weeks
Estimated oral intake, absorbed (mg/kg/day)
0
0.014
0.13
1.3
Dose metric (mg metabolite/L liver)
0
0.3
3
30
Number of rats
examined
197 (99, 98)
a
199 (99, 100)
195 (99, 96)
98 (49, 49)
Moderate+severe
cell polymorphism
21 (4, 1, 14, 2)
b
21 (4, 1, 13, 3)
20 (7, 1, 8, 4)
37 (10, 3, 15, 9)
a
Data in parentheses are the incidence numbers for males and females taken from Table A-12.
b
Data in parentheses are moderate and severe cell polymorphism incidence numbers for males and females.
Source: Til et al. 1983, 1991
The resulting incidence data for each dose metric (0.3, 3, and 30 mg metabolite/L liver) were subjected to
BMD modeling in order to statistically identify a threshold response for vinyl chloride-induced effects.
The resulting dose metric values are shown in Table A-14.

VINYL CHLORIDE A-24
APPENDIX A
Table A-14. LED
10
Values Generated from Various Models to Liver Cell
Polymorphism Incidence Data from Oral Exposure of Male and
Female Rats to Vinyl Chloride in the Diet for 149 Weeks in the
Study of Til et al. (1991)
Model
LED
10
(mg/L liver)
a
p-Value
Weibull (power ≥1)
24.0
0.88
Gammahit
21.4
0.88
Quantal quadratic
13.8
0.96
Logistic
12.9
0.47
Multistage
11.8
0.79
Probit
11.6
0.44
Quantal linear
6.5
0.46
NOAEL
LOAEL
3.00 (0.13 mg/kg/day)
29.9 (1.3 mg/kg/day)
a
LED
10
is the lower 95% confidence limit of a 10% change in numbers exhibiting polymorphism evaluated as either
moderate or severe. The NOAEL and LOAEL are shown for comparison.
Source: EPA 2000
Although all models provided adequate fit to the data, the LED
10
values ranged from 6.5 to 24.01 mg/L
liver (nearly a 4-fold range) and all modeled LED
10
values were higher than the NOAEL of the study.
Because there was no biological reason to choose the results of one model over another and the dose-
response characteristics present additional uncertainty due to the large gaps between dose levels, the
BMD modeling results were not used to derive the POD. Assuming that all dietary vinyl chloride was
absorbed, the human equivalent dose of 0.09 mg/kg/day, calculated from the rat NOAEL of
0.17 mg/kg/day (Til et al. 1983, 1991), served as the basis for the chronic-duration oral MRL for vinyl
chloride. The chronic-duration oral MRL of 0.003 mg/kg/day was derived by dividing the PBPK-
modeled equivalent human NOAEL of 0.09 mg/kg/day for liver cell polymorphisms by a factor of
30 (3 for species extrapolation using a dosimetric conversion and 10 for human variability).
Human Equivalent Concentration: In deriving the MRL, the rat NOAEL of 0.17 mg/kg/day was
converted to a human equivalent dose using the PBPK models described in Clewell et al. (2001) and EPA
(2000) to extrapolate from rats to humans. Source code and parameter values for running the rat and
human models in ACSL were transcribed from Appendix C of EPA (2000). Parameter values used in the
interspecies extrapolation are presented in Table A-15. Accuracy of the implementation of the model in
ACSL (v. 11.8.4) was checked against observations reported in Gehring et al. (1978), also reported in
Clewell et al. (2001) (results shown in Figure A-2). The visual fit of the observed and predicted values
appears adequately good at low doses. The total amount of vinyl chloride metabolized in 24 hours per L
of liver volume was the rat internal dose metric that was used in determining the human dose that would
result in an equivalent human dose metric. One kilogram of liver was assumed to have an approximate
volume of 1 L. Exposures in the Til et al. (1983, 1991) rat dietary study were simulated as 4-hour oral
exposures, for which the average daily dose (ADD) was equivalent to the NOAEL dose for liver effects
(ADD=0.17 mg/kg/day). This dose was uniformly distributed over a 4-hour period (i.e.,
0.0425 mg/kg/hour for 4 hours, followed by 16 hours at 0 mg/kg/hour). Dose metrics reflect the
cumulative amount of vinyl chloride metabolized over the 24-hour period.

VINYL CHLORIDE A-25
APPENDIX A
Table A-15. Parameter Values for Rat and Human Models
Parameter
Definition
Model
Rat
Human
BW
Body weight (kg)
0.377 (M)
0.204 (F)
70
VLC
Liver volume (fraction of body)
0.05
0.026
VFC
Fat volume (fraction of body)
0.12
0.19
VSC
Slowly-perfused tissue volume (fraction of body)
0.75
0.63
VRC
Rapidly-perfused tissue volume (fraction of body)
0.05
0.064
QCC
Cardiac output (L/hour-kg body weight)
18.0
16.5
QPC
Alveolar ventilation rate (L/hour-kg body weight)
21.0
24.0
QLC
Liver blood flow (fraction of cardiac output)
0.25
0.26
QFC
Fat blood flow (fraction of cardiac output)
0.09
0.05
QSC
Slowly-perfused blood flow (fraction of cardiac output)
0.15
0.19
QRC
Rapidly-perfused blood flow (fraction of cardiac output)
0.51
0.5
PB
Blood:air partition coefficient
2.4
1.16
PL
Liver:blood partition coefficient
0.7
1.45
PF
Fat:blood partition coefficient
10.0
20.7
PS
Slowly-perfused partition coefficient
4.0
0.83
PR
Rapidly-perfused partition coefficient
0.7
1.45
VMAX1C
Maximum rate of oxidative metabolism
(mg/hour-kg body weight)
4.0
4.0
VMAX2C
Maximum rate of oxidative metabolism
(mg/hour-kg body weight)
2.0
0.1
KM1
Michaelis-Menten coefficient for oxidative metabolism (mg/L)
0.1
0.1
KM2
Michaelis-Menten coefficient for oxidative metabolism (mg/L)
10.0
10.0
KCO2C
Rate constant for formation of CO
2
from oxidative metabolite
(hour
-1
)
1.6
1.6
KGSMC
Rate constant for conjugation with GSH (hour
-1
)
0.13
0.13
KFEEC
Rate constant for conjugation, not with GSH (hour
-1
)
35.0
35.0
CGSZ
Initial GSH concentration in liver (µmol/L)
5,800
5,800
KBC
Rate constant for GSH catabolism (hour
-1
)
0.12
0.12
KS
Coefficient controlling resynthesis of GSH (µmol/L)
2,000
2,000
KZC
Zero-order rate constant for resynthesis of GSH (µmol/hour)
28.5
28.5
Ka
Gastrointestinal absorption rate constant (hour
-1
)
3.0
F= female; GSH = glutathione; M = male
Source: EPA 2000

VINYL CHLORIDE A-26
APPENDIX A
Figure A-2. Predicted and Observed Relationship Between Air Exposure
Concentration and Rate Metabolism of Vinyl Chloride in Rats*
0
2000
4000
6000
8000
10000
12000
1 10 100 1000 10000
Exposure Concentration (ppm)
Total Metabolized (ug/6hr)
Gehring et al. 1978
Model (BW=0.200)
*Measurements of metabolites (non-volatile
14
C in carcass) were made immediately following a
6-hour exposure to [
14
C]vinyl chloride in air. Circles represent observations (±standard deviation);
the line shows the corresponding simulations.
The human model was run iteratively, varying the ADD, until the model converged with the internal dose
estimate shown in row 1 in Table A-7 (rat, male). The value for the Km1 for oxidative metabolism in
humans was assumed to be equal to the Km1 value for rats (0.1 mg/L) (EPA 2000). The human ADD
was assumed to be uniformly distributed over a 24-hour period. The resulting HED was 0.09 mg/kg/day
(Table A-16). Additional simulations were performed assuming that the ADD was distributed over a
12-hour period (to simulate exposure from drinking water or food during the day only). The resulting
dose metrics were very similar to the 24-hour estimates (data not shown).
Table A-16. Summary of Internal Dose Predictions and Corresponding Human
and Rat Equivalent Doses
Species
BW
Km1
ED
EF1
EF2
ADD
DM
(kg)
mg/L
(week)
(day/week)
(hour/day)
(mg/kg/day)
(mg/L)
Wistar rat
Male
0.377
0.1
149
7
4
0.17
3.16
Female
0.204
0.1
149
7
4
0.17
3.16
Human
70
0.1
3,640
7
24
0.09
3.16
ADD = average daily administered dose; BW = body weight; DM = dose metric equals the total amount of metabolite
formed in 24 hours per L of liver; ED = exposure duration; EF = exposure frequency; Km1 = Michaelis-Menten
constant for oxidative metabolism
VINYL CHLORIDE A-27
APPENDIX A
The NOAEL
HED
of 0.09 mg/kg/day, associated with the rat NOAEL of 0.17 mg/kg/day (Til et al. 1983,
1991), served as the basis for the chronic-duration oral MRL for vinyl chloride; the LOAEL
HED
is
1.07 mg/kg/day.
Uncertainty Factor: The PBPK-modeled equivalent human NOAEL of 0.09 mg/kg/day was divided by a
total uncertainty factor (UF) of 30:
• 3 for extrapolation from animals to humans with dosimetric adjustment
• 10 for human variability
MRL = NOAEL
HED
÷ (UF)
0.09 mg/kg/day ÷ (3 x 10) = 0.003 mg/kg/day
Other Additional Studies or Pertinent Information that Lend Support to this MRL: This MRL is
reinforced by a study by Feron et al. (1981) in which rats were fed diets containing PVC powder.
Increased areas of cellular alteration (consisting of clear foci, basophilic foci, and eosinophilic foci) were
observed in the liver of rats at an oral intake of vinyl chloride monomer of 1.8 mg/kg/day.
Agency Contacts (Chemical Managers): Rae Benedict

VINYL CHLORIDE B-1
APPENDIX B. LITERATURE SEARCH FRAMEWORK FOR VINYL CHLORIDE
The objective of the toxicological profile is to evaluate the potential for human exposure and the potential
health hazards associated with inhalation, oral, or dermal/ocular exposure to vinyl chloride.
B.1 LITERATURE SEARCH AND SCREEN
A literature search and screen were conducted to identify studies examining health effects, toxicokinetics,
mechanisms of action, susceptible populations, biomarkers, chemical interactions, physical and chemical
properties, production, use, environmental fate, environmental releases, and environmental and biological
monitoring data for vinyl chloride. ATSDR primarily focused on peer-reviewed articles without
publication date or language restrictions. Foreign language studies are reviewed based on available
English-language abstracts and/or tables (or summaries in regulatory assessments, such as International
Agency for Research on Cancer [IARC] documents). If the study appears critical for hazard identification
or MRL derivation, translation into English is requested. Non-peer-reviewed studies that were considered
relevant to the assessment of the health effects of vinyl chloride have undergone peer review by at least
three ATSDR-selected experts who have been screened for conflict of interest. The inclusion criteria
used to identify relevant studies examining the health effects of vinyl chloride are presented in Table B-1.
Table B-1. Inclusion Criteria for the Literature Search and Screen
Health Effects
Species
Human
Laboratory mammals
Route of exposure
Inhalation
Oral
Dermal (or ocular)
Parenteral (these studies will be considered supporting data)
Health outcome
Death
Systemic effects
Body weight effects
Respiratory effects
Cardiovascular effects
Gastrointestinal effects
Hematological effects
Musculoskeletal effects
Hepatic effects
Renal effects
Dermal effects
Ocular effects
Endocrine effects
Immunological effects
Neurological effects
Reproductive effects

VINYL CHLORIDE B-2
APPENDIX B
Table B-1. Inclusion Criteria for the Literature Search and Screen
Developmental effects
Other noncancer effects
Cancer
Toxicokinetics
Absorption
Distribution
Metabolism
Excretion
PBPK models
Biomarkers
Biomarkers of exposure
Biomarkers of effect
Interactions with other chemicals
Potential for human exposure
Releases to the environment
Air
Water
Soil
Environmental fate
Transport and partitioning
Transformation and degradation
Environmental monitoring
Air
Water
Sediment and soil
Other media
Biomonitoring
General populations
Occupation populations
B.1.1 Literature Search
The current literature search was intended to update the draft toxicological profile for vinyl chloride
released for public comment in February 2023; thus, the literature search was restricted to studies
published between January 2020 and May 2023. The following main databases were searched in May
2023:
• PubMed
• National Technical Reports Library (NTRL)
• Scientific and Technical Information Network’s TOXCENTER
The search strategy used the chemical names, Chemical Abstracts Service (CAS) numbers,
synonyms, Medical Subject Headings (MeSH) headings, and keywords for vinyl chloride. The query
strings used for the literature search are presented in Table B-2.

VINYL CHLORIDE B-3
APPENDIX B
The search was augmented by searching the Toxic Substances Control Act Test Submissions (TSCATS),
NTP website, and National Institute of Health Research Portfolio Online Reporting Tools Expenditures
and Results (NIH RePORTER) databases using the queries presented in Table B-3. Additional databases
were searched in the creation of various tables and figures, such as the TRI Explorer, the Substance
Priority List (SPL) resource page, and other items as needed. Regulations applicable to vinyl chloride
were identified by searching international and U.S. agency websites and documents.
Review articles were identified and used for the purpose of providing background information and
identifying additional references. ATSDR also identified reports from the grey literature, which included
unpublished research reports, technical reports from government agencies, conference proceedings and
abstracts, and theses and dissertations.
Table B-2. Database Query Strings
Database
search date
Query string
PubMed
05/2023
(("Vinyl Chloride"[mh] OR 75-01-4[rn] OR (("1-Chloroethene"[tw] OR "1-
Chloroethylene"[tw] OR "Chlorethene"[tw] OR "Chlorethylene"[tw] OR "Chloroethene"[tw]
OR "Chloroethylene"[tw] OR "Ethene, chloro-"[tw] OR "Ethylene monochloride"[tw] OR
"Ethylene, chloro-"[tw] OR "F 1140"[tw] OR "Monochloroethene"[tw] OR
"Monochloroethylene"[tw] OR "Monovinyl chloride"[tw] OR "Trovidur"[tw] OR "Vinyl C
monomer"[tw] OR "Vinyl chloride"[tw] OR "Vinyl chlorine"[tw] OR "Vinylchloride"[tw]) AND
(to[sh] OR po[sh] OR ae[sh] OR pk[sh] OR ai[sh] OR ci[sh] OR bl[sh] OR cf[sh] OR ur[sh]
OR "pharmacology"[sh:noexp] OR "environmental exposure"[mh] OR "endocrine
system"[mh] OR "hormones, hormone substitutes, and hormone antagonists"[mh] OR
"endocrine disruptors"[mh] OR "Computational biology"[mh] OR "Medical Informatics"[mh]
OR Genomics[mh] OR Genome[mh] OR Proteomics[mh] OR Proteome[mh] OR
Metabolomics[mh] OR Metabolome[mh] OR Genes[mh] OR "Gene expression"[mh] OR
Phenotype[mh] OR genetics[mh] OR genotype[mh] OR Transcriptome[mh] OR ("Systems
Biology"[mh] AND ("Environmental Exposure"[mh] OR "Epidemiological Monitoring"[mh]
OR analysis[sh])) OR "Transcription, Genetic "[mh] OR "Reverse transcription"[mh] OR
"Transcriptional activation"[mh] OR "Transcription factors"[mh] OR ("biosynthesis"[sh] AND
(RNA[mh] OR DNA[mh])) OR "RNA, Messenger"[mh] OR "RNA, Transfer"[mh] OR
"peptide biosynthesis"[mh] OR "protein biosynthesis"[mh] OR "Reverse Transcriptase
Polymerase Chain Reaction"[mh] OR "Base Sequence"[mh] OR "Trans-
activators"[mh] OR
"Gene Expression Profiling"[mh] OR (("Neoplasms"[mh] OR "Carcinogens"[mh] OR
"Lymphoproliferative disorders"[mh] OR "Myeloproliferative disorders"[mh] OR "Toxicity
Tests"[mh] OR ((cancer*[tiab] OR carcinogen*[tiab]) AND (risk*[tiab] OR health[tiab]) AND
assessment*[tiab]) OR "Mutagens"[mh] OR "Mutagenicity Tests"[mh] OR "Chromosome
Aberrations"[mh] OR "DNA Damage"[mh] OR "DNA Repair"[mh] OR "DNA
Replication/drug effects"[mh] OR "DNA/drug effects"[mh] OR "DNA/metabolism"[mh] OR
"Genomic Instability"[mh] OR "Salmonella typhimurium/drug effects"[mh] OR "Salmonella
typhimurium/genetics"[mh] OR "Sister Chromatid Exchange"[mh] OR strand-break*[tiab]))
OR (me[sh] AND ("humans"[mh] OR "animals"[mh])) OR toxicokinetics[mh:noexp]))) AND
(2020/10/01:3000[mhda] OR 2020:3000[dp])) OR ((("1-Chloroethene"[tw] OR "1-
Chloroethylene"[tw] OR "Chlorethene"[tw] OR "Chlorethylene"[tw] OR "Chloroethene"[tw]
OR "Chloroethylene"[tw] OR "Ethene, chloro-"[tw] OR "Ethylene monochloride"[tw] OR
"Ethylene, chloro-"[tw] OR "F 1140"[tw] OR "Monochloroethene"[tw] OR
"Monochloroethylene"[tw] OR "Monovinyl chloride"[tw] OR "Trovidur"[tw] OR "Vinyl C
monomer"[tw] OR "Vinyl chloride"[tw] OR "Vinyl chlorine"[tw] OR "Vinylchloride"[tw]) NOT
medline[sb]) AND (2020/10/01:3000[crdt] OR 2020/10/01:3000[edat] OR 2020:3000[dp]))

VINYL CHLORIDE B-4
APPENDIX B
Table B-2. Database Query Strings
Database
search date
Query string
OR ("vinyl chloride"[mh] AND 2022/04/01:2023/05/18[mhda])
NTRL
05/2023
Date limit 2020-2023
Search Titles OR Keywords;
"Chlorethene" OR "Chlorethylene" OR "Chloroethene" OR "Chloroethylene" OR "Ethene,
chloro-" OR "Ethylene monochloride" OR "Ethylene, chloro-" OR "Monochloroethene" OR
"Monochloroethylene" OR "Monovinyl chloride" OR "Trovidur" OR "Vinyl C monomer" OR
"Vinyl chloride" OR "Vinyl chlorine" OR "Vinylchloride" OR "F 1140"
Toxcenter
05/2023
FILE 'TOXCENTER' ENTERED AT 13:34:16 ON 18 MAY 2023
L1 11624 SEA FILE=TOXCENTER 75-01-4
L2 11449 SEA FILE=TOXCENTER L1 NOT TSCATS/FS
L3 10101 SEA FILE=TOXCENTER L2 NOT PATENT/DT
L4 441 SEA FILE=TOXCENTER L3 AND ED>=20201001
ACT TOXQUERY/Q
---------
L5 QUE (CHRONIC OR IMMUNOTOX? OR NEUROTOX? OR TOXICOKIN? OR
BIOMARKER? OR NEUROLOG?)
L6 QUE (PHARMACOKIN? OR SUBCHRONIC OR PBPK OR
EPIDEMIOLOGY/ST,CT,
IT)
L7 QUE (ACUTE OR SUBACUTE OR LD50# OR LD(W)50 OR LC50# OR
LC(W)50)
L8 QUE (TOXICITY OR ADVERSE OR POISONING)/ST,CT,IT
L9 QUE (INHAL? OR PULMON? OR NASAL? OR LUNG? OR RESPIR?)
L10 QUE ((OCCUPATION? OR WORKPLACE? OR WORKER?) AND EXPOS?)
L11 QUE (ORAL OR ORALLY OR INGEST? OR GAVAGE? OR DIET OR DIETS
OR
DIETARY OR DRINKING(W)WATER?)
L12 QUE (MAXIMUM AND CONCENTRATION? AND (ALLOWABLE OR
PERMISSIBLE))
L13 QUE (ABORT? OR ABNORMALIT? OR EMBRYO? OR CLEFT? OR FETUS?)
L14 QUE (FOETUS? OR FETAL? OR FOETAL? OR FERTIL? OR MALFORM?
OR
OVUM?)
L15 QUE (OVA OR OVARY OR PLACENTA? OR PREGNAN? OR PRENATAL?)
L16 QUE (PERINATAL? OR POSTNATAL? OR REPRODUC? OR STERIL? OR
TERATOGEN?)
L17 QUE (SPERM OR SPERMAC? OR SPERMAG? OR SPERMATI? OR
SPERMAS? OR
SPERMATOB? OR SPERMATOC? OR SPERMATOG?)
L18 QUE (SPERMATOI? OR SPERMATOL? OR SPERMATOR? OR
SPERMATOX? OR
SPERMATOZ? OR SPERMATU? OR SPERMI? OR SPERMO?)
L19 QUE (NEONAT? OR NEWBORN? OR DEVELOPMENT OR
DEVELOPMENTAL?)
L20 QUE (ENDOCRIN? AND DISRUPT?)
L21 QUE (ZYGOTE? OR CHILD OR CHILDREN OR ADOLESCEN? OR

VINYL CHLORIDE B-5
APPENDIX B
Table B-2. Database Query Strings
Database
search date
Query string
INFANT?)
L22 QUE (WEAN? OR OFFSPRING OR AGE(W)FACTOR?)
L23 QUE (DERMAL? OR DERMIS OR SKIN OR EPIDERM? OR CUTANEOUS?)
L24 QUE (CARCINOG? OR COCARCINOG? OR CANCER? OR PRECANCER?
OR
NEOPLAS?)
L25 QUE (TUMOR? OR TUMOUR? OR ONCOGEN? OR LYMPHOMA? OR
CARCINOM?)
L26 QUE (GENETOX? OR GENOTOX? OR MUTAGEN? OR
GENETIC(W)TOXIC?)
L27 QUE (NEPHROTOX? OR HEPATOTOX?)
L28 QUE (ENDOCRIN? OR ESTROGEN? OR ANDROGEN? OR HORMON?)
L29 QUE (OCCUPATION? OR WORKER? OR WORKPLACE? OR EPIDEM?)
L30 QUE L5 OR L6 OR L7 OR L8 OR L9 OR L10 OR L11 OR L12 OR L13 OR
L14 OR L15 OR L16 OR L17 OR L18 OR L19 OR L20 OR L21 OR L22 OR
L23 OR L24 OR L25 OR L26 OR L27 OR L28 OR L29
L31 QUE (RAT OR RATS OR MOUSE OR MICE OR GUINEA(W)PIG? OR
MURIDAE
OR DOG OR DOGS OR RABBIT? OR HAMSTER? OR PIG OR PIGS OR
SWINE
OR PORCINE OR MONKEY? OR MACAQUE?)
L32 QUE (MARMOSET? OR FERRET? OR GERBIL? OR RODENT? OR
LAGOMORPHA
OR BABOON? OR CANINE OR CAT OR CATS OR FELINE OR MURINE)
L33 QUE L30 OR L31 OR L32
L34 QUE (NONHUMAN MAMMALS)/ORGN
L35 QUE L33 OR L34
L36 QUE (HUMAN OR HUMANS OR HOMINIDAE OR MAMMALS OR MAMMAL?
OR
PRIMATES OR PRIMATE?)
L37 QUE L35 OR L36
---------
L38 235 SEA FILE=TOXCENTER L4 AND L37
L39 235 SEA FILE=TOXCENTER L4 AND L37
L40 36 SEA FILE=TOXCENTER L38 AND MEDLINE/FS
L41 199 SEA FILE=TOXCENTER L38 NOT MEDLINE/FS
L42 207 DUP REM L40 L41 (28 DUPLICATES REMOVED)
L*** DEL 36 S L38 AND MEDLINE/FS
L*** DEL 36 S L38 AND MEDLINE/FS
L43 36 SEA FILE=TOXCENTER L42
L*** DEL 199 S L38 NOT MEDLINE/FS
L*** DEL 199 S L38 NOT MEDLINE/FS
L44 171 SEA FILE=TOXCENTER L42
L45 171 SEA FILE=TOXCENTER (L43 OR L44) NOT MEDLINE/FS
D SCAN L45

VINYL CHLORIDE B-6
APPENDIX B
Table B-3. Strategies to Augment the Literature Search
Source
Query and number screened when available
TSCATS via
ChemView
05/2023
Compounds searched: 75-01-4
NTP
05/2023
Date limit 2020-2023
"75-01-4" "Vinyl chloride" "Chloroethene" "Chloroethylene"
"Ethylene, chloro-"
"Vinyl C monomer" "Vinyl chlorine" "Vinylchloride" "1-Chloroethene"
"1-Chloroethylene" "Chlorethene" "Chlorethylene" "Ethene, chloro-"
"Ethylene monochloride" "Monochloroethene" "Monochloroethylene" "Monovinyl
chloride"
"F 1140" "Trovidur"
Regulations.gov
05/2023
"Vinyl chloride"
"75-01-4"
"Chloroethene"
"Chloroethylene"
NIH RePORTER
07/2023
Search Criteria
Fiscal Year: Active Projects; Text Search: "1-Chloroethene" OR "1-
Chloroethylene" OR
"Chlorethene" OR "Chlorethylene" OR "Chloroethene" OR "Chloroethylene" OR
"Ethene, chloro-" OR "Ethylene monochloride" OR "Ethylene, chloro-" OR "F 1140" OR
"Monochloroethene" OR "Monochloroethylene" OR "Monovinyl chloride" OR "Trovidur"
OR "Vinyl C monomer" OR "Vinyl chloride" OR "Vinyl chlorine" OR "Vinylchloride"
(advanced); Limit to: Project Title, Project Terms, Project Abstracts
Other
Identified throughout the assessment process
The 2023 results were:
• Number of records identified from PubMed, NTRL, and TOXCENTER (after duplicate
removal): 469
• Number of records identified from other strategies: 48
• Total number of records to undergo literature screening: 517
B
.1.2 Literature Screening
A two-step process was used to screen the literature search to identify relevant studies on vinyl chloride:
• Title and abstract screen
• Full text screen
Title and Abstract Screen. Within the reference library, titles and abstracts were screened manually for
relevance. Studies that were considered relevant (Table B-1 for inclusion criteria) were moved to the
second step of the literature screening process. Studies were excluded when the title and abstract clearly
indicated that the study was not relevant to the toxicological profile.
• Number of titles and abstracts screened: 517
• Number of studies considered relevant and moved to the next step: 119
VINYL CHLORIDE B-7
APPENDIX B
Full Text Screen. The second step in the literature screening process was a full text review of individual
studies considered relevant in the title and abstract screen step. Each study was reviewed to determine
whether it was relevant for inclusion in the toxicological profile.
• Number of studies undergoing full text review: 119
• Number of studies cited in the pre-public draft of the toxicological profile: 602
• Total number of studies cited in the profile: 659
A summary of the results of the literature search and screening is presented in Figure B-1.

VINYL CHLORIDE B-8
APPENDIX B
Figure B-1. May 2023 Literature Search Results and Screen for Vinyl Chloride

VINYL CHLORIDE C-1
APPENDIX C. FRAMEWORK FOR ATSDR’S SYSTEMATIC REVIEW OF
HEALTH EFFECTS DATA FOR VINYL CHLORIDE
To increase the transparency of ATSDR’s process of identifying, evaluating, synthesizing, and
interpreting the scientific evidence on the health effects associated with exposure to vinyl chloride,
ATSDR utilized a slight modification of NTP’s Office of Health Assessment and Translation (OHAT)
systematic review methodology (NTP 2013, 2015; Rooney et al. 2014). ATSDR’s framework is an eight-
step process for systematic review with the goal of identifying the potential health hazards of exposure to
vinyl chloride:
• Step 1. Problem Formulation
• Step 2. Literature Search and Screen for Health Effects Studies
• Step 3. Extract Data from Health Effects Studies
• Step 4. Identify Potential Health Effect Outcomes of Concern
• Step 5. Assess the Risk of Bias for Individual Studies
• Step 6. Rate the Confidence in the Body of Evidence for Each Relevant Outcome
• Step 7. Translate Confidence Rating into Level of Evidence of Health Effects
• Step 8. Integrate Evidence to Develop Hazard Identification Conclusions
C.
1 PROBLEM FORMULATION
The objective of the toxicological profile and this systematic review was to identify the potential health
hazards associated with inhalation, oral, or dermal/ocular exposure to vinyl chloride. The inclusion
criteria used to identify relevant studies examining the health effects of vinyl chloride are presented in
Table C-1.
Data from human and laboratory animal studies were considered relevant for addressing this objective.
Human studies were divided into two broad categories: observational epidemiology studies and
controlled exposure studies. The observational epidemiology studies were further divided: cohort studies
(retrospective and prospective studies), population studies (with individual data or aggregate data), and
case-control studies.
Table C-1. Inclusion Criteria for Identifying Health Effects Studies
Species
Human
Laboratory mammals
Route of exposure
Inhalation
Oral
Dermal (or ocular)
Parenteral (these studies will be considered supporting data)
Health outcome
Death
Systemic effects
Body weight effects
Respiratory effects

VINYL CHLORIDE
APPENDIX C
Table C-1. Inclusion Criteria for Identifying Health Effects Studies
Cardiovascular effects
Gastrointestinal effects
Hematological effects
Musculoskeletal effects
Hepatic effects
Renal effects
Dermal effects
Ocular effects
Endocrine effects
Immunological effects
Neurological effects
Reproductive effects
Developmental effects
Other noncancer effects
Cancer
C.2 LITERATURE SEARCH AND SCREEN FOR HEALTH EFFECTS STUDIES
A literature search and screen were conducted to identify studies examining the health effects of vinyl
chloride. The literature search framework for the toxicological profile is discussed in detail in
Appendix B.
C.2.1 Literature Search
As noted in Appendix B, the current literature search was intended to update the draft toxicological
profile for vinyl chloride released for public comment in January 2023. See Appendix B for the databases
searched and the search strategy.
A total of 517 records relevant to all sections of the toxicological profile were identified (after
duplicate removal).
C.2.2 Literature Screening
As described in Appendix B, a two-step process was used to screen the literature search to identify
relevant studies examining the health effects of vinyl chloride.
Title and Abstract Screen. In the Title and Abstract Screen step, 517 records were reviewed;
10 documents were considered to meet the health effects inclusion criteria in Table C-1 and were moved
to the next step in the process.
Full Text Screen. In the second step in the literature screening process for the systematic review, a full
text review of 208 health effect documents (documents identified in the update literature search and
documents cited in older versions of the profile) was performed. From those 208 documents
(234 studies), 77 documents (89 studies) were included in the qualitative review.

VINYL CHLORIDE
APPENDIX C
C.3 EXTRACT DATA FROM HEALTH EFFECTS STUDIES
Relevant data extracted from the individual studies selected for inclusion in the systematic review were
collected in customized data forms. A summary of the type of data extracted from each study is presented
in Table C-2. For references that included more than one experiment or species, data extraction records
were created for each experiment or species.
Table C-2. Data Extracted From Individual Studies
Citation
Chemical form
Route of exposure (e.g., inhalation, oral, dermal)
Specific route (e.g., gavage in oil, drinking water)
Species
Strain
Exposure duration category (e.g., acute, intermediate, chronic)
Exposure duration
Frequency of exposure (e.g., 6 hours/day, 5 days/week)
Exposure length
Number of animals or subjects per sex per group
Dose/exposure levels
Parameters monitored
Description of the study design and method
Summary of calculations used to estimate doses (if applicable)
Summary of the study results
Reviewer’s comments on the study
Outcome summary (one entry for each examined outcome)
No-observed-adverse-effect level (NOAEL) value
Lowest-observed-adverse-effect level (LOAEL) value
Effect observed at the LOAEL value
A summary of the extracted data for each study is presented in the Supplemental Document for Vinyl
Chloride and overviews of the results of the inhalation and oral exposure studies (no dermal exposure
studies were identified) are presented in Sections 2.2–2.18 of the profile and in the Levels Significant
Exposures tables in Section 2.1 of the profile (Tables 2-1 and 2-2, respectively).
C.4 IDENTIFY POTENTIAL HEALTH EFFECT OUTCOMES OF CONCERN
Overviews of the potential health effect outcomes for vinyl chloride identified in human and animal
studies are presented in Tables C-3 and C-4, respectively. The available human studies evaluating
noncancer effects examined a comprehensive set of endpoints for the inhalation route (no oral or dermal
human studies were located). Occupational studies of inhalation exposure provide a thorough evaluation
of respiratory, cardiovascular, hematological, musculoskeletal, hepatic, dermal, immunological,
neurological, and developmental outcomes with health effects being observed for each outcome (except
developmental). Animal inhalation studies examined a comprehensive set of endpoints, oral animal
studies examined a limited number of health outcomes, and no dermal animal studies were available.
Hepatic, immunological, neurological, developmental, and other noncancer (insulin resistance) effects
VINYL CHLORIDE
APPENDIX C
were considered sensitive noncancer outcomes (i.e., effects were observed at low concentrations or
doses). Studies examining these potential outcomes were carried through to Steps 4–8 of the systematic
review. Human studies that did not estimate exposure or include a comparison group (i.e., occupational
health studies and case reports/series) were not included in the systematic review. Available cohort, case-
control and cross-sectional studies were adequate for evaluating the sensitive health outcomes. There
were 89 studies (published in 77 documents) examining these potential outcomes were carried through to
Steps 4–8 of the systematic review.

VINYL CHLORIDE C-5
APPENDIX C
Table C-3. Overview of the Health Outcomes for Vinyl Chloride Evaluated in Human Studies
Body weight
Respiratory
Cardiovascular
Gastrointestinal
Hematological
Musculoskeletal
Hepatic
Renal
Dermal
Ocular
Endocrine
Immunological
Neurological
Reproductive
Developmental
Other Noncancer
Can
cer
Inhalation studies
Cohort
1
9
11
1
8
5
15
5
1
5
9
4
3
49
1
6
10
1
6
5
14
5
1
5
9
4
0
39
Case control
1
1
5
4
5
1
11
1
1
5
4
0
1
7
Population
1
3
9
1
6
3
4
1
3
0
3
9
1
6
3
0
1
3
Case series
4
6
3
3
6
6
8
4
3
8
12
4
6
3
2
6
6
8
4
2
8
12
Oral studies
Cohort
Case control
Population
Case series
Dermal studies
Cohort
Case control
Population
Case series
Number of studies examining endpoint
0
1
2
3
4
5–9
≥10
Number of studies reporting outcome
0
1
2
3
4
5–9
≥10
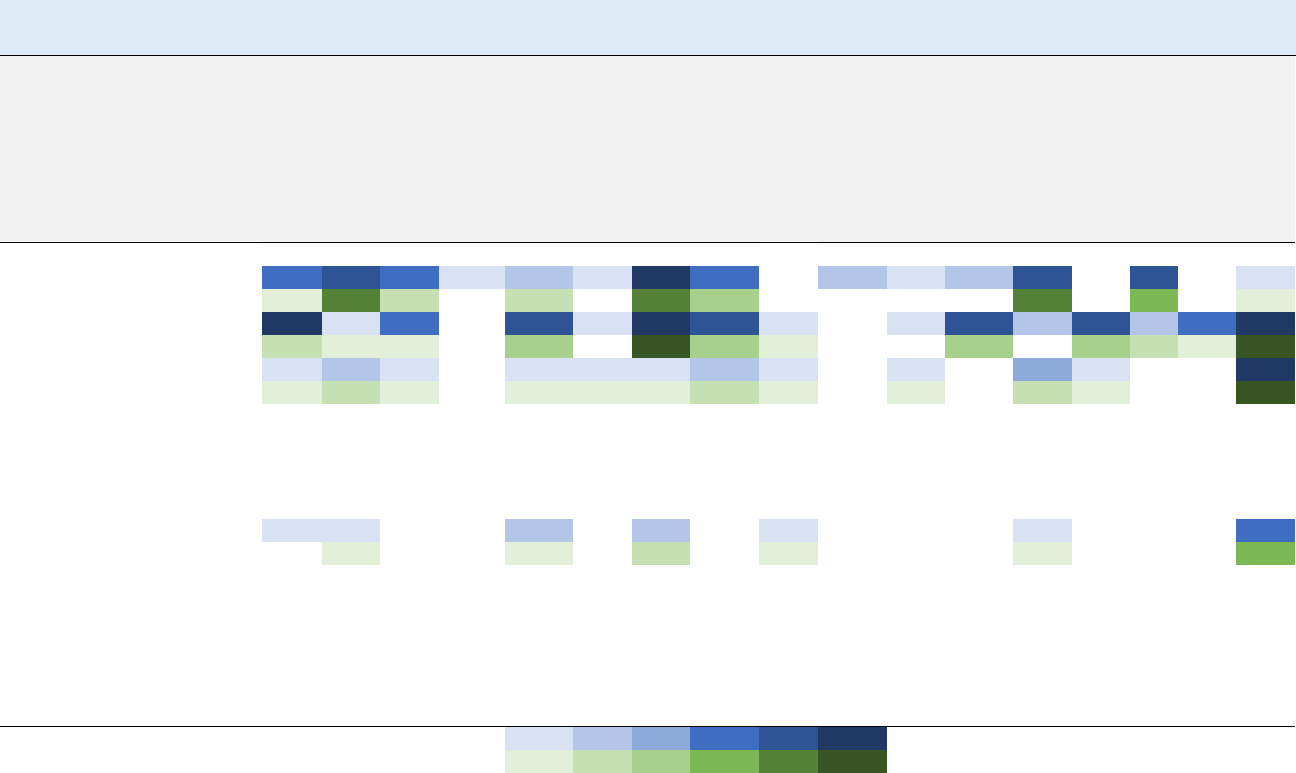
VINYL CHLORIDE C-6
APPENDIX C
Table C-4. Overview of the Health Outcomes for Vinyl Chloride Evaluated in Experimental Animal Studies
Body weight
Respiratory
Cardiovascular
Gastrointestinal
Hematological
Musculoskeletal
Hepatic
Renal
Dermal
Ocular
Endocrine
Immunological
a
Neurological
a
Reproductive
a
Developmental
Other Noncancer
Can
cer
Inhalation studies
Acute-duration
6
5
4
1
2
1
13
4
2
1
2
9
5
1
1
5
2
0
2
0
7
3
0
0
7
4
1
Intermediate-duration
18
1
4
6
1
19
9
1
1
6
2
5
2
4
11
3
1
1
3
0
14
3
1
0
3
0
3
2
1
11
Chronic-duration
1
2
1
1
1
1
2
1
1
3
1
12
1
2
1
1
1
1
2
1
1
2
1
12
Oral studies
Acute-duration
Intermediate-duration
Chronic-duration
1
1
2
2
1
1
4
0
1
1
2
1
1
4
Dermal studies
Acute-duration
Intermediate-duration
Chronic-duration
Number of studies examining endpoint
0
1
2
3
4
5–9
≥10
Number of studies reporting outcome
0
1
2
3
4
5–9
≥10
VINYL CHLORIDE
APPENDIX C
C.5 ASSESS THE RISK OF BIAS FOR INDIVIDUAL STUDIES
C
.5.1 Risk of Bias Assessment
The risk of bias of individual studies was assessed using OHAT’s Risk of Bias Tool (NTP 2015). The
risk of bias questions for observational epidemiology studies, human-controlled exposure studies, and
animal experimental studies are presented in Tables C-5, C-6, and C-7, respectively. Each risk of bias
question was answered on a four-point scale:
• Definitely low risk of bias (++)
• Probably low risk of bias (+)
• Probably high risk of bias (-)
• Definitely high risk of bias (– –)
In general, “definitely low risk of bias” or “definitely high risk of bias” were used if the question could be
answered with information explicitly stated in the study report. If the response to the question could be
inferred, then “probably low risk of bias” or “probably high risk of bias” responses were typically used.
Table C-5. Risk of Bias Questionnaire for Observational Epidemiology Studies
Selection bias
Were the comparison groups appropriate?
Confounding bias
Did the study design or analysis account for important confounding and modifying variables?
Attrition/exclusion bias
Were outcome data complete without attrition or exclusion from analysis?
Detection bias
Is there confidence in the exposure characterization?
Is there confidence in outcome assessment?
Selective reporting bias
Were all measured outcomes reported?
Table C-6. Risk of Bias Questionnaire for Human-Controlled Exposure Studies
Selection bias
Was administered dose or exposure level adequately randomized?
Was the allocation to study groups adequately concealed?
Performance bias
Were the research personnel and human subjects blinded to the study group during the study?
Attrition/exclusion bias
Were outcome data complete without attrition or exclusion from analysis?
Detection bias
Is there confidence in the exposure characterization?
Is there confidence in outcome assessment?
Selective reporting bias
Were all measured outcomes reported?

VINYL CHLORIDE
APPENDIX C
Table C-7. Risk of Bias Questionnaire for Experimental Animal Studies
Selection bias
Was administered dose or exposure level adequately randomized?
Was the allocation to study groups adequately concealed?
Performance bias
Were experimental conditions identical across study groups?
Were the research personnel blinded to the study group during the study?
Attrition/exclusion bias
Were outcome data complete without attrition or exclusion from analysis?
Detection bias
Is there confidence in the exposure characterization?
Is there confidence in outcome assessment?
Selective reporting bias
Were all measured outcomes reported?
After the risk of bias questionnaires were completed for the health effects studies, the studies were
assigned to one of three risk of bias tiers based on the responses to the key questions listed below and the
responses to the remaining questions.
• Is there confidence in the exposure characterization? (only relevant for observational studies)
• Is there confidence in the outcome assessment?
• Does the study design or analysis account for important confounding and modifying variables?
(only relevant for observational studies)
First Tier. Studies placed in the first tier received ratings of “definitely low” or “probably low” risk of
bias on the key questions AND received a rating of “definitely low” or “probably low” risk of bias on the
responses to at least 50% of the other applicable questions.
Second Tier. A study was placed in the second tier if it did not meet the criteria for the first or third tiers.
Third Tier. Studies placed in the third tier received ratings of “definitely high” or “probably high” risk of
bias for the key questions AND received a rating of “definitely high” or “probably high” risk of bias on
the response to at least 50% of the other applicable questions.
The results of the risk of bias assessment for the different types of vinyl chloride health effects studies
(observational epidemiology and animal experimental studies) are presented in Tables C-8, C-9, and
C-10, respectively.

VINYL CHLORIDE C-9
APPENDIX C
Table C-8. Summary of Risk of Bias Assessment for Vinyl Chloride—Observational Epidemiology Studies
Reference
Risk of bias criteria and ratings
Selection
bias
Confounding bias
Attrition /
exclusion
bias
Detection bias
Selective
reporting
bias
Risk of bias tier
Were the
comparison groups
appropriate?
Did the study
design or analysis
account for
important
confounding
and modifying
variables?*
Were outcome
data complete
without attrition or
exclusion from
analysis?
Is there confidence
in the exposure
characterization?*
Is there confidence
in the outcome
assessment?*
Were all measured
outcomes
reported?
Outcome: Hepatic Effects
Inhalation—cohort
Fedeli et al. 2019a
+
+
+
+
++
++
Second
Mundt et al. 2017
++
++
+
+
+
++
First
Hsieh et al. 2007
+
++
+
+
++
++
First
Maroni and Fanetti 2006
+
++
+
+
+
++
First
Zhu et al. 2005a
++
+
+
++
+
+
First
Hsiao et al. 2004
+
++
+
+
++
++
First
Maroni et al. 2003
+
++
+
+
+
++
First
Ward et al. 2001
+
+
+
+
+
+
First
Inhalation—cross-sectional
Lee et al. 2020
–
+
++
++
++
++
First
Yuan et al. 2020
–
++
++
+
++
++
First
Wang et al. 2019b
+
++
+
+
++
++
First
Attarchi et al. 2007
++
+
–
++
+
++
First
Cheng et al. 1999b
–
++
+
+
++
++
First
Du et al. 1995
+
++
+
+
+
++
First
Tamburro et al. 1984
+
–
+
+
+
+
Second
Vihko et al. 1984
– –
– –
–
+
+
++
Second
NIOSH 1977
+
+
–
+
+
++
Second

VINYL CHLORIDE C-10
APPENDIX C
Table C-8. Summary of Risk of Bias Assessment for Vinyl Chloride—Observational Epidemiology Studies
Reference
Risk of bias criteria and ratings
Selection
bias
Confounding bias
Attrition /
exclusion
bias
Detection bias
Selective
reporting
bias
Risk of bias tier
Were the
comparison groups
appropriate?
Did the study
design or analysis
account for
important
confounding
and modifying
variables?*
Were outcome
data complete
without attrition or
exclusion from
analysis?
Is there confidence
in the exposure
characterization?*
Is there confidence
in the outcome
assessment?*
Were all measured
outcomes
reported?
Inhalation—case-control
Cave et al. 2010
++
– –
+
+
+
++
Second
Mastrangelo et al. 2004
++
++
+
+
++
++
First
Du and Wang 1998
+
– –
+
–
+
+
Second
Liss et al. 1985
+
– –
–
–
+
+
Second
Outcome: Immunological Effects
Inhalation—cross-sectional
Saad et al. 2017
++
–
+
–
+
+
Second
Fucic et al. 1998
++
–
+
+
++
++
Second
Fucic et al. 1995
++
–
+
+
+
– –
Second
Bencko et al. 1988
–
–
+
–
+
+
Second
Wagnerova et al. 1988
+
–
+
–
+
–
Second
Bogdanikowa and Zawilska
1984
+
–
+
–
+
–
Second
Inhalation—case-control
Cave et al. 2010
++
– –
+
+
++
++
Second
Black et al. 1983, 1986
++
–
+
–
+
+
Second
Grainger et al. 1980
+
–
+
–
+
+
Second
Outcome: Neurological Effects
Inhalation—cohort
Bove et al. 2014
++
+
+
++
++
++
First
Zhu et al. 2005a
++
+
+
++
–
+
First
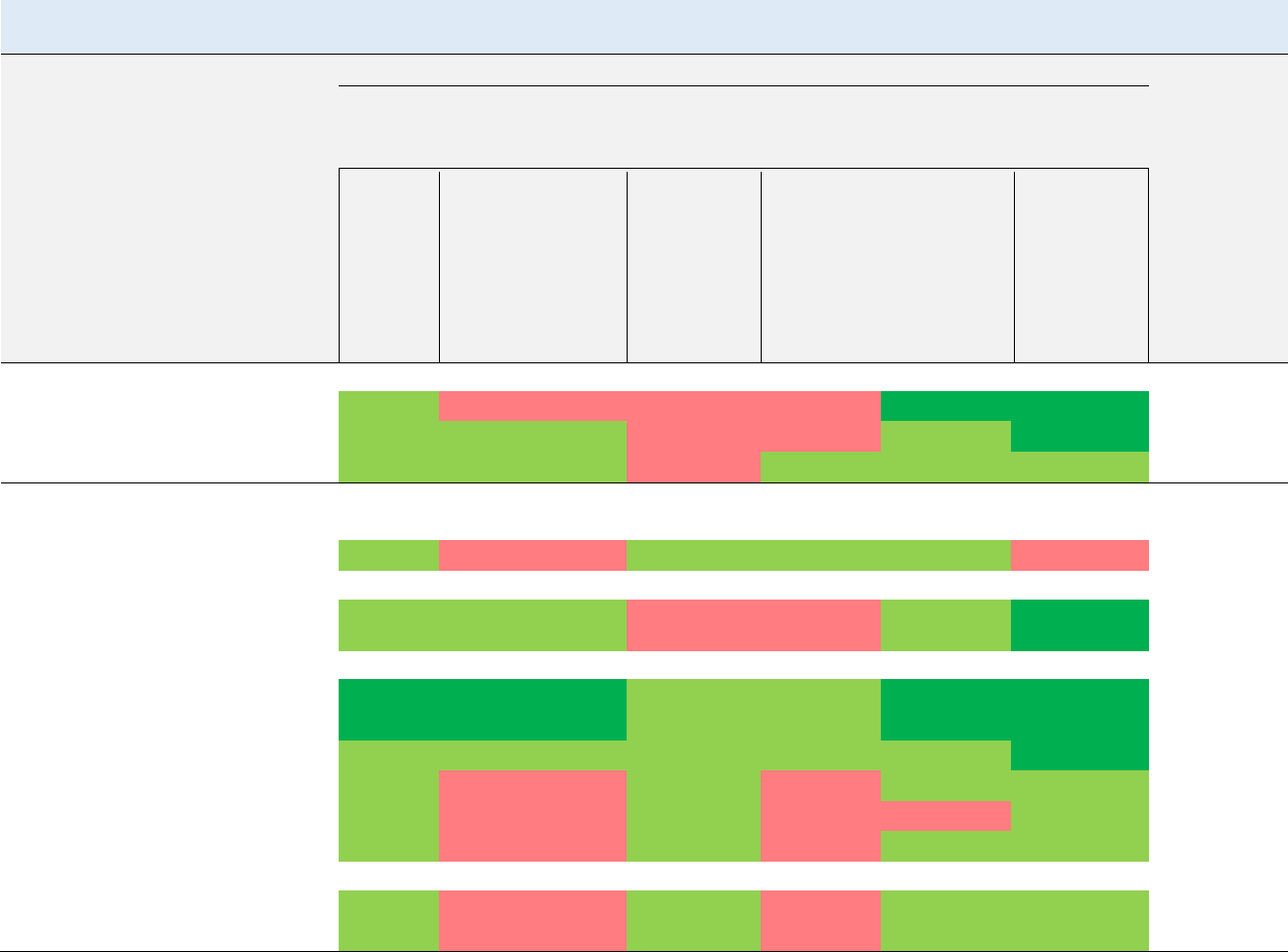
VINYL CHLORIDE C-11
APPENDIX C
Table C-8. Summary of Risk of Bias Assessment for Vinyl Chloride—Observational Epidemiology Studies
Reference
Risk of bias criteria and ratings
Selection
bias
Confounding bias
Attrition /
exclusion
bias
Detection bias
Selective
reporting
bias
Risk of bias tier
Were the
comparison groups
appropriate?
Did the study
design or analysis
account for
important
confounding
and modifying
variables?*
Were outcome
data complete
without attrition or
exclusion from
analysis?
Is there confidence
in the exposure
characterization?*
Is there confidence
in the outcome
assessment?*
Were all measured
outcomes
reported?
Inhalation—cross-sectional
Perticoni et al. 1986
+
–
–
–
++
++
Second
NIOSH 1977
+
+
–
–
+
++
Second
Spirtas et al. 1975
+
+
–
+
+
+
First
Outcome: Developmental Effects
Inhalation—cohort
Bao et al. 1988
+
–
+
+
+
–
Second
Inhalation—cross-sectional
Infante et al. 1976a, 1976b;
NIOSH 1977
+
+
–
–
+
++
Second
Inhalation—case-control
Swartz et al. 2015
++
++
+
+
++
++
First
Talbott et al. 2015
++
++
+
+
++
++
First
Ruckart et al. 2013
+
+
+
+
+
++
First
Rosenman et al. 1989
+
–
+
–
+
+
Second
Theriault et al. 1983
+
–
+
–
–
+
Third
Edmonds et al. 1978
+
–
+
–
+
+
Second
Inhalation—ecological
Infante 1976
+
–
+
–
+
+
Second
Edmonds et al. 1975
+
–
+
–
+
+
Second
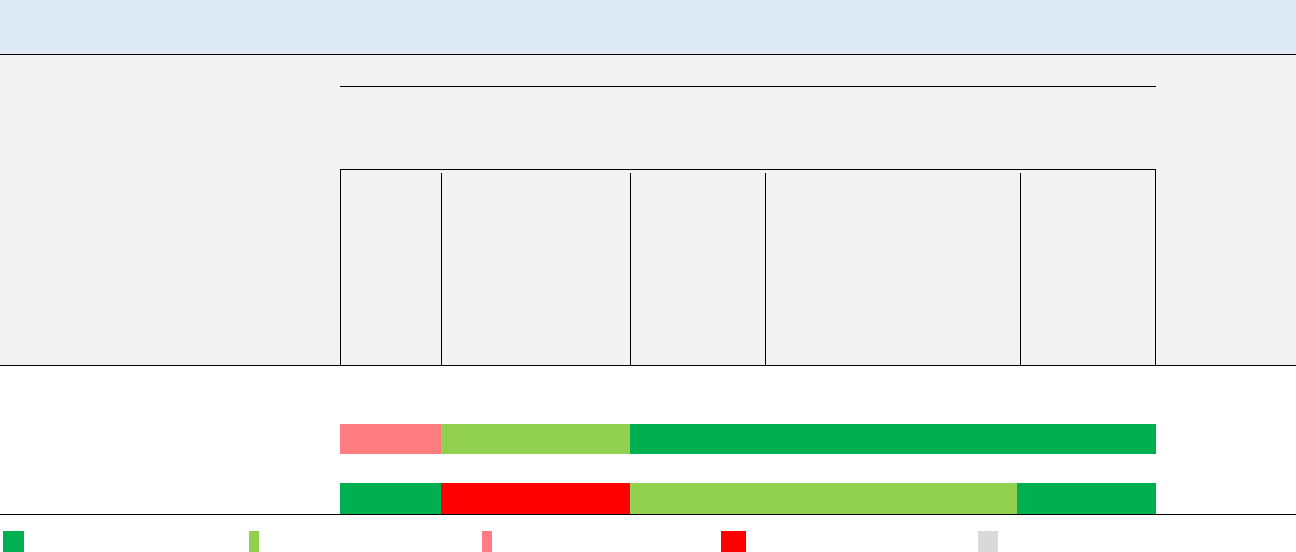
VINYL CHLORIDE C-12
APPENDIX C
Table C-8. Summary of Risk of Bias Assessment for Vinyl Chloride—Observational Epidemiology Studies
Reference
Risk of bias criteria and ratings
Selection
bias
Confounding bias
Attrition /
exclusion
bias
Detection bias
Selective
reporting
bias
Risk of bias tier
Were the
comparison groups
appropriate?
Did the study
design or analysis
account for
important
confounding
and modifying
variables?*
Were outcome
data complete
without attrition or
exclusion from
analysis?
Is there confidence
in the exposure
characterization?*
Is there confidence
in the outcome
assessment?*
Were all measured
outcomes
reported?
Outcome: Other Noncancer (Insulin Resistance)
Inhalation—cross-sectional
Lee et al. 2020
–
+
++
++
++
++
First
Inhalation—case-control
Cave et al. 2010
++
– –
+
+
+
++
Second
++ = definitely low risk of bias; + = probably low risk of bias; – = probably high risk of bias; – – = definitely high risk of bias; na = not applicable

VINYL CHLORIDE C-13
APPENDIX C
Table C-9. Summary of Risk of Bias Assessment for Vinyl Chloride— Human-Controlled Exposure Studies
Reference
Risk of bias criteria and ratings
Selection bias
Performance Bias
Attrition /
exclusion
bias
Detection bias
Selective
reporting
bias
Risk of bias tier
Was administered
dose or exposure
level adequately
randomized?
Was the allocation
to study groups
adequately
concealed?
Were
the
research
personnel and
human subjects
blinded to the
study group
during the
study?*
Outcome data
complete without
attrition or
exclusion from
analysis?
Confidence in
exposure
characterization?
*
Confidence in
outcome
assessment?*
All measured
outcomes
reported?
Outcome: Neurological Effects
Inhalation
Lester et al. 1963
++
+
+
+
+
–
First
Patty et al. 1930
– –
–
+
+
+
–
Second
++ = definitely low risk of bias; + = probably low risk of bias; – = probably high risk of bias; – – = definitely high risk of bias; na = not applicable

VINYL CHLORIDE C-14
APPENDIX C
Table C-10. Summary of Risk of Bias Assessment for Vinyl Chloride—Experimental Animal Studies
Reference
Risk of bias criteria and ratings
Selection bias
Performance bias
Attrition/
exclusio
n bias
Detection bias
Selecti
ve
reporti
ng
bias
Other bias
Was administered dose or
exposure level adequately
randomized?
Was the allocation to study
groups adequately
concealed?
Were experimental
conditions identical across
study groups?
Were the research
personnel blinded to the
study group during the
study?
Were outcome data
complete without attrition or
exclusion from analysis?
Is there confidence in the
exposure characterization?
Is there confidence in the
outcome assessment?*
Were all measured
outcomes reported?
Did the study design or
analysis account for
important confounding and
modifying variables?
Risk of bias tier
Outcome: Hepatic Effects
Inhalation acute-duration exposure
Jaeger et al. 1974 (rat; 1, 5 days)
–
–
+
+
–
–
+
++
NA
Second
John et al. 1977, 1981 (rat; 10 days)
–
–
+
+
++
–
+
++
NA
First
Mastromatteo et al. 1960 (rat; 30
minutes)
– – + + ++ + + ++ NA First
Reynolds et al. 1975a (rat; 1, 5 days)
–
–
+
+
–
–
–
++
NA
Third
Reynolds et al. 1975b (rat; 1 day)
–
–
+
+
–
–
+
++
NA
Second
John et al. 1977, 1981 (mouse; 10 days)
–
–
+
+
++
–
+
++
NA
First
Mastromatteo et al. 1960 (mouse;
30 minutes)
– – + + ++ + + ++ NA First
Mastromatteo et al. 1960 (guinea pig;
30 minutes)
– – + + ++ + + ++ NA First
John et al. 1977, 1981 (rabbit; 13 days)
–
–
+
+
++
–
+
++
NA
First
Ungvary et al. 1978 (rat; 7–9 days)
–
–
+
+
++
–
+
++
NA
First
Hehir et al. 1981 (rat; 1-hour)
–
–
+
+
+
–
+
++
NA
First
Inhalation intermediate-duration exposure
Bi et al. 1985 (rat; 3, 6 months)
+
+
+
+
+
++
+
++
NA
First
Jia et al. 2022 (mice; 13 weeks)
+
+
+
+
++
–
+
+
NA
First

VINYL CHLORIDE C-15
APPENDIX C
Table C-10. Summary of Risk of Bias Assessment for Vinyl Chloride—Experimental Animal Studies
Reference
Risk of bias criteria and ratings
Selection bias
Performance bias
Attrition/
exclusio
n bias
Detection bias
Selecti
ve
reporti
ng
bias
Other bias
Was administered dose or
exposure level adequately
randomized?
Was the allocation to study
groups adequately
concealed?
Were experimental
conditions identical across
study groups?
Were the research
personnel blinded to the
study group during the
study?
Were outcome data
complete without attrition or
exclusion from analysis?
Is there confidence in the
exposure characterization?
Is there confidence in the
outcome assessment?*
Were all measured
outcomes reported?
Did the study design or
analysis account for
important confounding and
modifying variables?
Risk of bias tier
Lester et al. 1963 (rat; 19 days)
–
–
++
+
+
++
+
++
NA
First
Lester et al. 1963 (rat; 92 days)
+
+
++
+
+
++
+
++
NA
First
Liu et al. 2023 (mice; 12 weeks)
–
–
+
+
++
–
+
+
NA
First
Sokal et al. 1980 (rat; 10 months)
–
–
++
+
–
++
+
++
NA
First
Thornton et al. 2002 (rat; 2-generation)
++
+
++
+
++
–
+
++
NA
First
Torkelson et al. 1961 (rat; 6 months)
–
–
++
+
+
+
+
++
NA
First
Wisniewska-Knypl et al. 1980 (rat;
10 months)
– – ++ + – ++ + ++ NA First
Chen et al. 2019 (mouse; 12 weeks)
–
–
++
+
–
–
+
++
NA
Second
Lang et al. 2018 (mouse; 12 weeks)
–
–
++
+
–
–
+
++
NA
Second
Lang et al. 2020 (mouse; 12 weeks)
–
–
++
+
–
–
+
++
NA
Second
Schaffner 1978 (mouse; 6 months)
–
–
+
+
–
–
–
++
NA
Third
Sharma and Gehring 1979 (mouse; 2–
8 weeks)
– – + + – – + ++ NA
Second
Wahlang et al. 2020 (mouse; 12 weeks)
–
–
++
+
–
–
+
++
NA
Second
Wang et al. 2019a (mouse; 16 weeks)
–
–
++
+
–
– –
+
++
NA
Second
Torkelson et al. 1961 (rabbit; 6 months)
–
–
++
+
+
+
+
++
NA
First
Du et al. 1979 (rat; 2–4 weeks)
+
+
++
+
–
– –
+
++
NA
First

VINYL CHLORIDE C-16
APPENDIX C
Table C-10. Summary of Risk of Bias Assessment for Vinyl Chloride—Experimental Animal Studies
Reference
Risk of bias criteria and ratings
Selection bias
Performance bias
Attrition/
exclusio
n bias
Detection bias
Selecti
ve
reporti
ng
bias
Other bias
Was administered dose or
exposure level adequately
randomized?
Was the allocation to study
groups adequately
concealed?
Were experimental
conditions identical across
study groups?
Were the research
personnel blinded to the
study group during the
study?
Were outcome data
complete without attrition or
exclusion from analysis?
Is there confidence in the
exposure characterization?
Is there confidence in the
outcome assessment?*
Were all measured
outcomes reported?
Did the study design or
analysis account for
important confounding and
modifying variables?
Risk of bias tier
Inhalation chronic-duration exposure
Bi et al. 1985 (rat; 12 months)
+
+
+
+
+
++
+
++
NA
First
Oral chronic-duration exposure
Til et al. 1983 (rat; 149 weeks)
++
+
++
+
++
++
+
++
NA
First
Feron et al. 1981 (rat; 2 years)
++
+
++
+
++
++
+
++
NA
First
Outcome: Immunological Effects
Inhalation acute-duration exposure
Mastromatteo et al. 1960 (guinea pig;
30 minutes)
–
–
+
+
++
+
+
++ NA First
Inhalation intermediate-duration exposure
Bi et al. 1985 (rat; 3, 6 months)
+
+
+
+
+
++
+
++
NA
First
Sharma and Gehring 1979 (mouse; 2–
8 weeks)
– – + + – –
+
++ NA
Second
Sharma et al. 1980 (rabbit; 8 weeks)
–
–
+
+
+
–
+
+
NA
First
Sokal et al. 1980 (rat; 10 months)
–
–
++
+
–
++
+
++
NA
Second
Outcome: Neurological Effects
Inhalation acute-duration exposure
Jaeger et al. 1974 (rat; 1, 5 days)
–
–
+
+
–
–
+
++
NA
Second
Lester et al. 1963 (rat; 2 hours)
–
–
++
+
+
++
–
++
NA
Third

VINYL CHLORIDE C-17
APPENDIX C
Table C-10. Summary of Risk of Bias Assessment for Vinyl Chloride—Experimental Animal Studies
Reference
Risk of bias criteria and ratings
Selection bias
Performance bias
Attrition/
exclusio
n bias
Detection bias
Selecti
ve
reporti
ng
bias
Other bias
Was administered dose or
exposure level adequately
randomized?
Was the allocation to study
groups adequately
concealed?
Were experimental
conditions identical across
study groups?
Were the research
personnel blinded to the
study group during the
study?
Were outcome data
complete without attrition or
exclusion from analysis?
Is there confidence in the
exposure characterization?
Is there confidence in the
outcome assessment?*
Were all measured
outcomes reported?
Did the study design or
analysis account for
important confounding and
modifying variables?
Risk of bias tier
Mastromatteo et al. 1960 (rat;
30 minutes)
– – + + ++
+
+
++ NA
First
Hehir et al. 1981 (rat; 2 weeks)
–
–
+
+
+
–
+
++
NA
First
Hehir et al. 1981 (rat; 1 hour)
–
–
+
+
+
–
+
++
NA
First
Hehir et al. 1981 (mouse; 1 hour)
–
–
+
+
+
–
+
++
NA
First
Mastromatteo et al. 1960 (mouse;
30 minutes)
– – + + ++ +
+
++ NA
First
Mastromatteo et al. 1960 (guinea pig;
30 minutes)
– – + + ++ +
+
++ NA
First
Patty et al. 1930 (guinea pig; up to
8 hours)
– – + + + –
+
++ NA
First
Inhalation intermediate-duration exposure
Hehir et al. 1981 (rat; 20 weeks)
–
–
+
+
+
–
+
++
NA
First
Inhalation chronic-duration exposure
Viola 1970 (rat; 12 months)
–
–
–
+
+
–
+
++
NA
Second
Viola et al. 1971 (rat; 12 months)
–
–
+
+
+
+
+
++
NA
First
Feron and Kroes 1979 (rat; 12 months)
–
–
+
+
–
–
+
++
NA
Second

VINYL CHLORIDE C-18
APPENDIX C
Table C-10. Summary of Risk of Bias Assessment for Vinyl Chloride—Experimental Animal Studies
Reference
Risk of bias criteria and ratings
Selection bias
Performance bias
Attrition/
exclusio
n bias
Detection bias
Selecti
ve
reporti
ng
bias
Other bias
Was administered dose or
exposure level adequately
randomized?
Was the allocation to study
groups adequately
concealed?
Were experimental
conditions identical across
study groups?
Were the research
personnel blinded to the
study group during the
study?
Were outcome data
complete without attrition or
exclusion from analysis?
Is there confidence in the
exposure characterization?
Is there confidence in the
outcome assessment?*
Were all measured
outcomes reported?
Did the study design or
analysis account for
important confounding and
modifying variables?
Risk of bias tier
Outcome: Developmental Effects
Inhalation acute-duration exposure
Thornton et al. 2002 (rat; GDs 6–19)
++
+
++
+
++
–
+
+
NA
First
John et al. 1977, 1981 (rat; 10 days)
–
–
+
+
++
–
+
++
NA
First
John et al. 1977, 1981 (mouse; 10 days)
–
–
+
+
++
–
+
++
NA
First
John et al. 1977, 1981 (rabbit; 13 days)
–
–
+
+
++
–
+
++
NA
First
Ungvary et al. 1978 (rat; 7-9 days)
–
–
+
+
++
–
+
++
NA
First
Inhalation intermediate-duration exposure
Sal'nikova and Kotsovskaya 1980 (rat;
21 days)
– – + + – –
+
++ NA
Second
Mirkova et al. 1978
–
–
–
+
–
–
–
–
NA
Third

VINYL CHLORIDE C-19
APPENDIX C
Table C-10. Summary of Risk of Bias Assessment for Vinyl Chloride—Experimental Animal Studies
Reference
Risk of bias criteria and ratings
Selection bias
Performance bias
Attrition/
exclusio
n bias
Detection bias
Selecti
ve
reporti
ng
bias
Other bias
Was administered dose or
exposure level adequately
randomized?
Was the allocation to study
groups adequately
concealed?
Were experimental
conditions identical across
study groups?
Were the research
personnel blinded to the
study group during the
study?
Were outcome data
complete without attrition or
exclusion from analysis?
Is there confidence in the
exposure characterization?
Is there confidence in the
outcome assessment?*
Were all measured
outcomes reported?
Did the study design or
analysis account for
important confounding and
modifying variables?
Risk of bias tier
Outcome: Other Noncancer (Insulin Resistance)
Inhalation intermediate-duration exposure
Chen et al. 2019 (mouse; 12 weeks)
–
–
++
+
–
–
+
++
NA
Second
Lang et al. 2018 (mouse; 12 weeks)
–
–
++
+
–
+
+
++
NA
First
Wahlang et al. 2020 (mouse; 12 weeks)
–
–
++
+
–
–
+
++
NA
Second
++ = definitely low risk of bias; + = probably low risk of bias; – = probably high risk of bias; – – = definitely high risk of bias; na = not applicable
*Key question used to assign risk of bias tier
VINYL CHLORIDE
APPENDIX C
C.6 RATE THE CONFIDENCE IN THE BODY OF EVIDENCE FOR EACH RELEVANT
OUTCOME
Confidences in the bodies of human and animal evidence were evaluated independently for each potential
outcome. ATSDR did not evaluate the confidence in the body of evidence for carcinogenicity; rather, the
Agency defaulted to the cancer weight-of-evidence assessment of other agencies including HHS, EPA,
and IARC. The confidence in the body of evidence for an association or no association between exposure
to vinyl chloride and a particular outcome was based on the strengths and weaknesses of individual
studies. Four descriptors were used to describe the confidence in the body of evidence for effects or when
no effect was found:
• High confidence: the true effect is highly likely to be reflected in the apparent relationship
• Moderate confidence: the true effect may be reflected in the apparent relationship
• Low confidence: the true effect may be different from the apparent relationship
• Very low confidence: the true effect is highly likely to be different from the apparent
relationship
Confidence in the body of evidence for a particular outcome was rated for each type of study: case-
control, case series, cohort, population, human-controlled exposure, and experimental animal. In the
absence of data to the contrary, data for a particular outcome were collapsed across animal species, routes
of exposure, and exposure durations. If species (or strain), route, or exposure duration differences were
noted, then the data were treated as separate outcomes.
C.6.1 Initial Confidence Rating
In ATSDR’s modification to the OHAT approach, the body of evidence for an association (or no
association) between exposure to vinyl chloride and a particular outcome was given an initial confidence
rating based on the key features of the individual studies examining that outcome. The presence of these
key features of study design was determined for individual studies using four “yes or no” questions,
which were customized for epidemiology, human controlled exposure, or experimental animal study
designs. Separate questionnaires were completed for each outcome assessed in a study. The key features
for observational epidemiology (cohort, population, and case-control) studies, human controlled exposure,
and experimental animal studies are presented in Tables C-11, C-12, and C-13, respectively. The initial
confidence in the study was determined based on the number of key features present in the study design:
• High Initial Confidence: Studies in which the responses to the four questions were “yes”.
• Moderate Initial Confidence: Studies in which the responses to only three of the questions
were “yes”.
• Low Initial Confidence: Studies in which the responses to only two of the questions were “yes”.
• Very Low Initial Confidence: Studies in which the response to one or none of the questions
was “yes”.

VINYL CHLORIDE
APPENDIX C
Table C-11. Key Features of Study Design for Observational Epidemiology
Studies
Exposure was experimentally controlled
Exposure occurred prior to the outcome
Outcome was assessed on individual level rather than at the population level
A comparison group was used
Table C-12. Key Features of Study Design for Human-Controlled Exposure
Studies
A comparison group was used or the subjects served as their own control
A sufficient number of subjects were tested
Appropriate methods were used to measure outcomes (i.e., clinically-confirmed outcome versus self-
reported)
Appropriate statistical analyses were performed and reported or the data were reported in such a way to
allow independent statistical analysis
Table C-13. Key Features of Study Design for Experimental Animal Studies
A concurrent control group was used
A sufficient number of animals per group were tested
Appropriate parameters were used to assess a potential adverse effect
Appropriate statistical analyses were performed and reported or the data were reported in such a way to
allow independent statistical analysis
The presence or absence of the key features and the initial confidence levels for studies examining
hepatic, immunological, neurological, developmental and other noncancer (insulin resistance) observed in
the observational epidemiology, human controlled-exposure and animal experimental studies are
presented in Tables C-14, C-15, and C-16, respectively.
Table C-14. Presence of Key Features of Study Design for Vinyl Chloride—
Observational Epidemiology Studies
Reference
Key features
Controlled
exposure
Exposure prior
to outcome
Outcomes
assessed on an
individual level
Comparison
group
Initial
study
confidence
Outcome: Hepatic effects
Inhalation—cohort
Fedeli et al. 2019a
No
Yes
Yes
Yes
Moderate
Mundt et al. 2017
No
Yes
Yes
Yes
Moderate
Hsieh et al. 2007
No
Yes
Yes
Yes
Moderate

VINYL CHLORIDE
APPENDIX C
Table C-14. Presence of Key Features of Study Design for Vinyl Chloride—
Observational Epidemiology Studies
Reference
Key features
Controlled
exposure
Exposure prior
to outcome
Outcomes
assessed on an
individual level
Comparison
group
Initial
study
confidence
Maroni and Fanetti 2006
No
Yes
Yes
Yes
Moderate
Zhu et al. 2005a
No
Yes
Yes
Yes
Moderate
Hsiao et al. 2004
No
Yes
Yes
Yes
Moderate
Maroni et al. 2003
No
Yes
Yes
Yes
Moderate
Ward et al. 2001
No
Yes
Yes
Yes
Moderate
Inhalation—cross-sectional
Lee et al. 2020
No
No
Yes
Yes
Low
Yuan et al. 2020
No
No
Yes
Yes
Low
Wang et al. 2019b
No
No
Yes
Yes
Low
Attarchi et al. 2007
No
No
Yes
Yes
Low
Cheng et al. 1999b
No
No
Yes
Yes
Low
Du et al. 1995
No
No
Yes
Yes
Low
Tamburro et al. 1984
No
No
Yes
Yes
Low
Vihko et al. 1984
No
No
Yes
No
Very low
NIOSH 1977
No
No
Yes
Yes
Low
Inhalation—case-control
Cave et al. 2010
No
Yes
Yes
Yes
Moderate
Mastrangelo et al. 2004
No
Yes
Yes
Yes
Moderate
Du and Wang 1998
No
Yes
Yes
Yes
Moderate
Liss et al. 1985
No
Yes
Yes
Yes
Moderate
Outcome: Immunological effects
Inhalation—cross-sectional
Saad et al. 2017
No
No
Yes
Yes
Moderate
Fucic et al. 1998
No
No
Yes
Yes
Moderate
Fucic et al. 1995
No
No
Yes
Yes
Moderate
Bencko et al. 1988
No
No
Yes
Yes
Moderate
Wagnerova et al. 1988
No
No
Yes
Yes
Moderate
Bogdanikowa and Zawilska
1984
No
No
Yes
Yes
Moderate
Inhalation—case-control
Cave et al. 2010
No
No
Yes
Yes
Low
Black et al. 1983, 1986
No
Yes
Yes
Yes
Moderate
Grainger et al. 1980
No
Yes
Yes
Yes
Moderate

VINYL CHLORIDE
APPENDIX C
Table C-14. Presence of Key Features of Study Design for Vinyl Chloride—
Observational Epidemiology Studies
Reference
Key features
Controlled
exposure
Exposure prior
to outcome
Outcomes
assessed on an
individual level
Comparison
group
Initial
study
confidence
Outcome: Neurological effects
Inhalation—cohort
Bove et al. 2014
No
Yes
Yes
Yes
Moderate
Zhu et al. 2005a
No
Yes
Yes
Yes
Moderate
Inhalation—cross-sectional
Perticoni et al. 1986
No
No
Yes
Yes
Low
NIOSH 1977
No
No
Yes
Yes
Low
Spirtas et al. 1975
No
No
Yes
Yes
Low
Outcome: Developmental effects
Inhalation—cohort
Bao et al. 1988
No
Yes
Yes
Yes
Moderate
Inhalation—cross-sectional
Infante et al. 1976a, 1976b;
NIOSH 1977
No
No
Yes
Yes
Low
Inhalation—case-control
Swartz et al. 2015
No
Yes
Yes
Yes
Moderate
Talbott et al. 2015
No
Yes
Yes
Yes
Moderate
Ruckart et al. 2013
No
Yes
Yes
Yes
Moderate
Rosenman et al. 1989
No
Yes
Yes
Yes
Moderate
Theriault et al. 1983
No
Yes
Yes
Yes
Moderate
Edmonds et al. 1978
No
Yes
Yes
Yes
Moderate
Inhalation—ecological
Infante 1976
No
Yes
Yes
Yes
Moderate
Edmonds et al. 1975
No
Yes
Yes
Yes
Moderate
Other noncancer (insulin resistance)
Inhalation—cross-sectional
Lee et al. 2020
No
No
Yes
Yes
Low
Inhalation—case-control
Cave et al. 2010
No
Yes
Yes
Yes
Moderate

VINYL CHLORIDE
APPENDIX C
Table C-15. Presence of Key Features of Study Design for Vinyl Chloride—
Human-Controlled Exposure Studies
Key features
Reference
Comparison group
Sufficient number of
subjects
Outcomes assessed
with appropriate
methods
Statistical analysis
Initial study
confidence
Outcome: Neurological effects
Inhalation
Lester et al. 1963
Yes
Yes
Yes
No
Moderate
Patty et al. 1930
No
No
Yes
No
Very low
Table C-16. Presence of Key Features of Study Design for Vinyl Chloride—
Experimental Animal Studies
Key feature
Reference
Concurrent control
group
Sufficient number
of animals per
group
Appropriate
parameters to
assess potential
effect
Adequate data for
statistical analysis
Initial study
confidence
Outcome: Hepatic effects
Inhalation acute-duration exposure
Jaeger et al. 1974 (rat; 1, 5 days)
Yes
No
Yes
No
Low
John et al. 1977, 1981 (rat; 10 days)
Yes
Yes
Yes
Yes
High
Mastromatteo et al. 1960 (rat;
30 minutes)
Yes
Yes
Yes
No
Moderate
Reynolds et al. 1975a (rat; 1, 5 days)
No
No
Yes
No
Low
Reynolds et al. 1975b (rat; 1 day)
Yes
No
Yes
No
Low
John et al. 1977, 1981 (mouse;
10 days)
Yes
Yes
Yes
Yes
High
Mastromatteo et al. 1960 (mouse;
30 minutes)
Yes
Yes
Yes
No
Moderate
Mastromatteo et al. 1960 (guinea pig;
30 minutes)
Yes
Yes
Yes
No
Moderate
John et al. 1977, 1981 (rabbit;
13 days)
Yes
Yes
Yes
Yes
High
Ungvary et al. 1978 (rat; 7–9 days)
Yes
Yes
Yes
Yes
High
Hehir et al. 1981 (rat; 1 hour)
Yes
Yes
Yes
No
Moderate

VINYL CHLORIDE
APPENDIX C
Table C-16. Presence of Key Features of Study Design for Vinyl Chloride—
Experimental Animal Studies
Key feature
Reference
Concurrent control
group
Sufficient number
of animals per
group
Appropriate
parameters to
assess potential
effect
Adequate data for
statistical analysis
Initial study
confidence
Inhalation intermediate-duration exposure
Bi et al. 1985 (rat; 3, 6 months)
Yes
Yes
Yes
Yes
High
Jia et al. 2022 (mice; 13 weeks)
Yes
Yes
Yes
Yes
High
Lester et al. 1963 (rat; 19 days)
Yes
Yes
Yes
Yes
High
Lester et al. 1963 (rat; 92 days)
Yes
Yes
Yes
Yes
High
Liu et al. 2023 (mice; 12 weeks)
Yes
Yes
Yes
Yes
High
Sokal et al. 1980 (rat; 10 months)
Yes
Yes
Yes
Yes
High
Thornton et al. 2002 (rat;
2-generation)
Yes
Yes
Yes
Yes
High
Torkelson et al. 1961 (rat; 6 months)
Yes
Yes
Yes
Yes
High
Wisniewska-Knypl et al. 1980 (rat;
10 months)
Yes
Yes
Yes
Yes
High
Chen et al. 2019 (mouse; 12 weeks)
Yes
Yes
Yes
Yes
High
Lang et al. 2018 (mouse; 12 weeks)
Yes
Yes
Yes
Yes
High
Lang et al. 2020 (mouse; 12 weeks)
Yes
Yes
Yes
Yes
High
Schaffner 1978 (mouse; 6 months)
No
Yes
Yes
No
Low
Sharma and Gehring 1979 (mouse;
2–8 weeks)
Yes
No
Yes
Yes
Moderate
Wahlang et al. 2020 (mouse;
12 weeks)
Yes
No
Yes
Yes
Moderate
Wang et al. 2019a (mouse;
16 weeks)
Yes
Yes
Yes
Yes
High
Torkelson et al. 1961 (rabbit;
6 months)
Yes
No
Yes
Yes
Moderate
Du et al. 1979 (rat; 2–4 weeks)
Yes
No
Yes
Yes
Moderate
Inhalation chronic-duration exposure
Bi et al. 1985 (rat; 12 months)
Yes
Yes
Yes
Yes
High
Oral chronic-duration exposure
Til et al. 1983 (rat; 149 weeks)
Yes
Yes
Yes
Yes
High
Feron et al. 1981 (rat; 2 years)
Yes
Yes
Yes
Yes
High
Outcome: Immunological effects
Inhalation acute-duration exposure
Mastromatteo et al. 1960 (guinea pig;
30 minutes)
Yes
Yes
Yes
No
Moderate
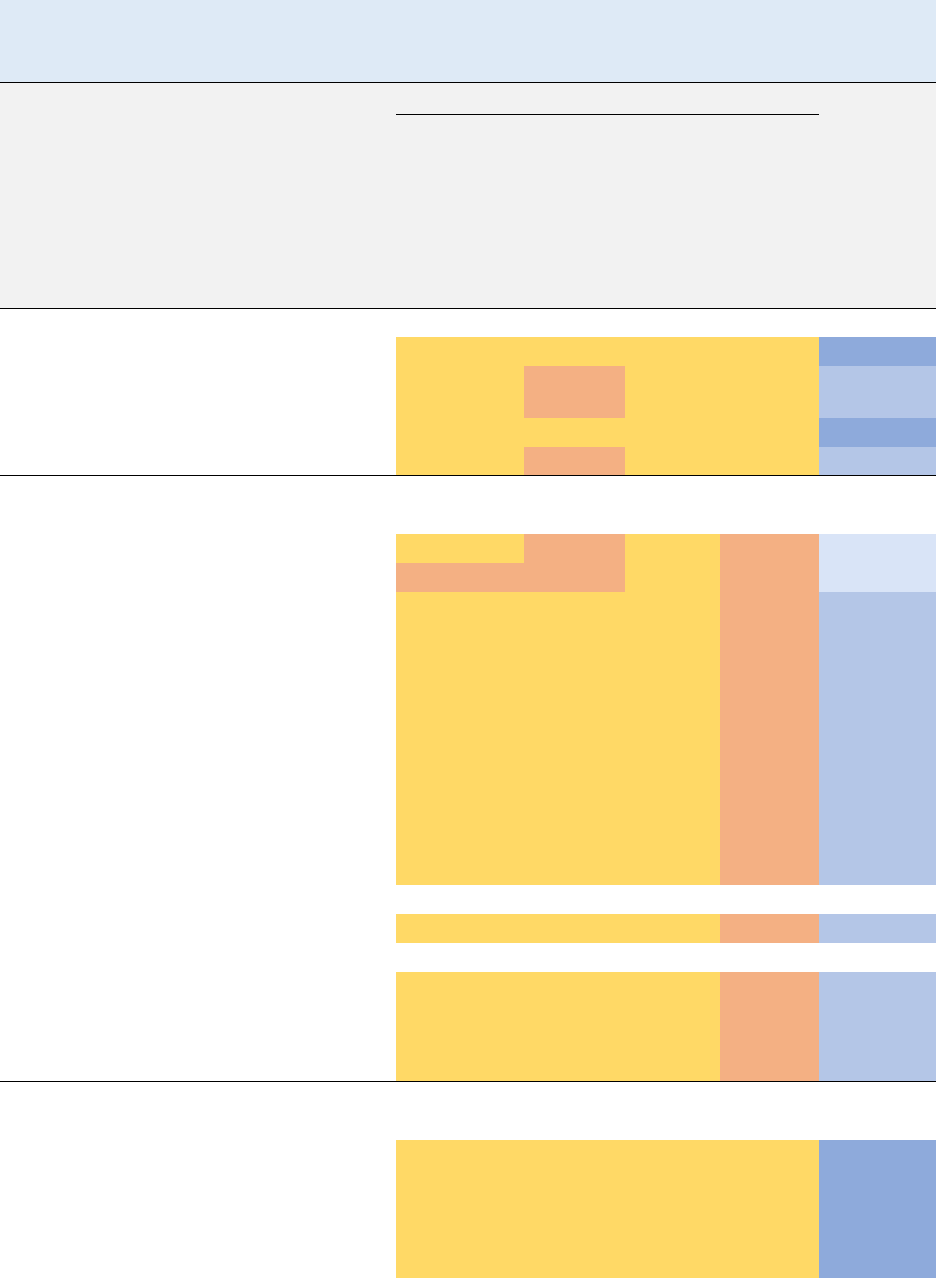
VINYL CHLORIDE
APPENDIX C
Table C-16. Presence of Key Features of Study Design for Vinyl Chloride—
Experimental Animal Studies
Key feature
Reference
Concurrent control
group
Sufficient number
of animals per
group
Appropriate
parameters to
assess potential
effect
Adequate data for
statistical analysis
Initial study
confidence
Inhalation intermediate-duration exposure
Bi et al. 1985 (rat; 3, 6 months)
Yes
Yes
Yes
Yes
High
Sharma and Gehring 1979 (mouse;
2–8 weeks)
Yes
No
Yes
Yes
Moderate
Sharma et al. 1980 (rabbit; 8 weeks)
Yes
Yes
Yes
Yes
High
Sokal et al. 1980 (rat; 10 months)
Yes
Yes
Yes
Yes
High
Outcome: Neurological effects
Inhalation acute-duration exposure
Jaeger et al. 1974 (rat; 1, 5 days)
Yes
No
Yes
No
Low
Lester et al. 1963 (rat; 2 hours)
No
No
Yes
No
Low
Mastromatteo et al. 1960 (rat;
30 minutes)
Yes
Yes
Yes
No
Moderate
Hehir et al. 1981 (rat; 2 weeks)
Yes
Yes
Yes
No
Moderate
Hehir et al. 1981 (rat; 1 hour)
Yes
Yes
Yes
No
Moderate
Hehir et al. 1981 (mouse; 1 hour)
Yes
Yes
Yes
No
Moderate
Mastromatteo et al. 1960 (mouse;
30 minutes)
Yes
Yes
Yes
No
Moderate
Mastromatteo et al. 1960 (guinea pig;
30 minutes)
Yes
Yes
Yes
No
Moderate
Patty et al. 1930 (guinea pig; up to
8 hours)
Yes
Yes
Yes
No
Moderate
Inhalation intermediate-duration exposure
Hehir et al. 1981 (rat; 20 weeks)
Yes
Yes
Yes
No
Moderate
Inhalation chronic-duration exposure
Viola 1970 (rat; 12 months)
Yes
Yes
Yes
No
Moderate
Viola et al. 1971 (rat; 12 months)
Yes
Yes
Yes
No
Moderate
Feron and Kroes 1979 (rat;
12 months)
Yes
Yes
Yes
No
Moderate
Outcome: Developmental effects
Inhalation acute-duration exposure
Thornton et al. 2002 (rat; GDs 6–19)
Yes
Yes
Yes
Yes
High
John et al. 1977, 1981 (rat; 10 days)
Yes
Yes
Yes
Yes
High
John et al. 1977, 1981 (mouse;
10 days)
Yes
Yes
Yes
Yes
High
John et al. 1977, 1981 (rabbit;
Yes
Yes
Yes
Yes
High

VINYL CHLORIDE
APPENDIX C
Table C-16. Presence of Key Features of Study Design for Vinyl Chloride—
Experimental Animal Studies
Key feature
Reference
Concurrent control
group
Sufficient number
of animals per
group
Appropriate
parameters to
assess potential
effect
Adequate data for
statistical analysis
Initial study
confidence
13 days)
Ungvary et al. 1978 (rat; 7–9 days)
Yes
Yes
Yes
Yes
High
Inhalation intermediate-duration exposure
Sal'nikova and Kotsovskaya 1980
(rat; 21 days)
Yes
Yes
Yes
Yes
High
Mirkova et al. 1978 (rat; 21 days)
Yes
Yes
Yes
Yes
High
Other noncancer (insulin resistance)
Inhalation intermediate-duration exposure
Chen et al. 2019 (mouse; 12 weeks)
Yes
Yes
Yes
Yes
High
Lang et al. 2018 (mouse; 12 weeks)
Yes
Yes
Yes
Yes
High
Wahlang et al. 2020 (mouse;
12 weeks)
Yes
No
Yes
Yes
Moderate
A summary of the initial confidence ratings for each outcome is presented in Table C-17. If individual
studies for a particular outcome and study type had different study quality ratings, then the highest
confidence rating for the group of studies was used to determine the initial confidence rating for the body
of evidence; any exceptions were noted in Table C-17.
Table C-17. Initial Confidence Rating for Vinyl Chloride Health Effects Studies
Initial study
confidence
Initial confidence
rating
Outcome: Hepatic effects
Inhalation acute-duration exposure
Animal studies
Jaeger et al. 1974 (rat; 1, 5 days)
Low
High
John et al. 1977, 1981 (rat; 10 days)
High
Mastromatteo et al. 1960 (rat; 30 minutes)
Moderate
Reynolds et al. 1975a (rat; 1, 5 days)
Low
Reynolds et al. 1975b (rat; 1 day)
Low
John et al. 1977, 1981 (mouse; 10 days)
High
Mastromatteo et al. 1960 (mouse; 30 minutes)
Moderate
Mastromatteo et al. 1960 (guinea pig; 30 minutes)
Moderate
John et al. 1977, 1981 (rabbit; 13 days)
High
Ungvary et al. 1978 (rat; 7–9 days)
High

VINYL CHLORIDE
APPENDIX C
Table C-17. Initial Confidence Rating for Vinyl Chloride Health Effects Studies
Initial study
confidence
Initial confidence
rating
Hehir et al. 1981 (rat; 1 hour)
Moderate
Inhalation intermediate-duration exposure
Animal studies
Bi et al. 1985 (rat; 3, 6 months)
High
High
Jia et al. 2022 (mice; 13 weeks)
High
Lester et al. 1963 (rat; 19 days)
High
Lester et al. 1963 (rat; 92 days)
High
Liu et al. 2023 (mice; 12 weeks)
High
Sokal et al. 1980 (rat; 10 months)
High
Thornton et al. 2002 (rat; 2-generation)
High
Torkelson et al. 1961 (rat; 6 months)
High
Wisniewska-Knypl et al. 1980 (rat; 10 months)
High
Chen et al. 2019 (mouse; 12 weeks)
High
Lang et al. 2018 (mouse; 12 weeks)
High
Lang et al. 2020 (mouse; 12 weeks)
High
Schaffner 1978 (mouse; 6 months)
Low
Sharma and Gehring 1979 (mouse; 2–8 weeks)
Moderate
Wahlang et al. 2020 (mouse; 12 weeks)
Moderate
Wang et al. 2019a (mouse; 16 weeks)
High
Torkelson et al. 1961 (rabbit; 6 months)
Moderate
Du et al. 1979 (rat; 2-4 weeks)
Moderate
Inhalation chronic-duration exposure
Human studies
NIOSH 1977
Low
Moderate
Zhu et al. 2005a
Moderate
Liss et al. 1985
Moderate
Tamburro et al. 1984
Low
Vihko et al. 1984
Very low
Du et al. 1995
Low
Cheng et al. 1999b
Low
Ward et al. 2001
Moderate
Du and Wang 1998
Moderate
Mastrangelo et al. 2004
Moderate
Maroni et al. 2003
Moderate
Cave et al. 2010
Moderate
Hsieh et al. 2007
Moderate
Attarchi et al. 2007
Low
Maroni and Fanetti 2006
Moderate
Hsiao et al. 2004
Moderate
Mundt et al. 2017
Moderate
Fedeli et al. 2019a
Moderate

VINYL CHLORIDE
APPENDIX C
Table C-17. Initial Confidence Rating for Vinyl Chloride Health Effects Studies
Initial study
confidence
Initial confidence
rating
Wang et al. 2019b
Low
Lee et al. 2020
Low
Yuan et al. 2020
Animal studies
Bi et al. 1985 (rat; 12 months)
High
High
Oral chronic-duration exposure
Animal studies
Til et al. 1983 (rat; 149 weeks)
High
High
Feron et al. 1981 (rat; 2 years)
High
Outcome: Immunological effects
Inhalation acute-duration exposure
Animal studies
Mastromatteo et al. 1960 (guinea pig; 30 minutes)
Moderate
Moderate
Inhalation intermediate-duration exposure
Animal studies
Bi et al. 1985 (rat; 3, 6 months)
High
High
Sharma and Gehring 1979 (mouse; 2–8 weeks)
Moderate
Sharma et al. 1980 (rabbit; 8 weeks)
High
Sokal et al. 1980 (rat; 10 months)
High
Inhalation chronic-duration exposure
Human studies
Cave et al. 2010
Low
Moderate
Fucic et al. 1995
Moderate
Fucic et al. 1998
Moderate
Wagnerova et al. 1988
Moderate
Bogdanikowa and Zawilska 1984
Moderate
Grainger et al. 1980
Moderate
Black et al. 1983, 1986
Moderate
Saad et al. 2017
Moderate
Bencko et al. 1988
Moderate
Outcome: Neurological effects
Inhalation acute-duration exposure
Human studies
Patty et al. 1930
Very low
Moderate
Lester et al. 1963
Moderate
Animal studies
Jaeger et al. 1974 (rat; 1, 5 days)
Low
Moderate
Lester et al. 1963 (rat; 2 hours)
Low
Mastromatteo et al. 1960 (rat; 30 minutes)
Moderate
Hehir et al. 1981 (rat; 2 weeks)
Moderate

VINYL CHLORIDE
APPENDIX C
Table C-17. Initial Confidence Rating for Vinyl Chloride Health Effects Studies
Initial study
confidence
Initial confidence
rating
Hehir et al. 1981 (rat; 1 hour)
Moderate
Hehir et al. 1981 (mouse; 1 hour)
Moderate
Mastromatteo et al. 1960 (mouse; 30 minutes)
Moderate
Mastromatteo et al. 1960 (guinea pig; 30 minutes)
Moderate
Patty et al. 1930 (guinea pig; up to 8 hours)
Moderate
Inhalation intermediate-duration exposure
Animal studies
Hehir et al. 1981 (rat; 20 weeks)
Moderate
Moderate
Inhalation chronic-duration exposure
Human studies
NIOSH 1977
Low
Moderate
Zhu et al. 2005a
Moderate
Spirtas et al. 1975
Low
Perticoni et al. 1986
Low
Bove et al. 2014
Moderate
Animal studies
Viola 1970 (rat; 12 months)
Moderate
Moderate
Viola et al. 1971 (rat; 12 months)
Moderate
Feron and Kroes 1979 (rat; 12 months)
Moderate
Outcome: Developmental effects
Inhalation acute-duration exposure
Animal studies
Thornton et al. 2002 (rat; GDs 6–19)
High
High
John et al. 1977, 1981 (rat; 10 days)
High
John et al. 1977, 1981 (mouse; 10 days)
High
John et al. 1977, 1981 (rabbit; 13 days)
High
Ungvary et al. 1978 (rat; 7–9 days)
High
Inhalation intermediate-duration exposure
Human studies
Swartz et al. 2015
Moderate
Moderate
Talbott et al. 2015
Moderate
Ruckart et al. 2013
Moderate
Animal studies
Sal'nikova and Kotsovskaya 1980 (rat; 21 days)
High
High
Mirkova et al. 1978 (rat; 21 days)
High
Inhalation chronic-duration exposure
Human studies
NIOSH 1977
Low
Moderate
Edmonds et al. 1975, 1978
Moderate
Infante 1976
Moderate
Rosenman et al. 1989
Moderate

VINYL CHLORIDE
APPENDIX C
Table C-17. Initial Confidence Rating for Vinyl Chloride Health Effects Studies
Initial study
confidence
Initial confidence
rating
Theriault et al. 1983
Moderate
Infante et al. 1976a, 1976b
Low
Bao et al. 1988
Moderate
Outcome: Other noncancer (insulin resistance)
Inhalation intermediate-duration exposure
Animal studies
Chen et al. 2019 (mouse; 12 weeks)
High
High
Lang et al. 2018 (mouse; 12 weeks)
High
Wahlang et al. 2020 (mouse; 12 weeks)
Moderate
Inhalation chronic-duration exposure
Human studies
Lee et al. 2020
Low
Moderate
Cave et al. 2010
Moderate
C
.6.2 Adjustment of the Confidence Rating
The initial confidence rating was then downgraded or upgraded depending on whether there were
substantial issues that would decrease or increase confidence in the body of evidence. The nine properties
of the body of evidence that were considered are listed below. The summaries of the assessment of the
confidence in the body of evidence for hepatic, immunological, neurological, developmental, and other
noncancer (insulin resistance) effects are presented in Table C-18. If the confidence ratings for a
particular outcome were based on more than one type of human study, then the highest confidence rating
was used for subsequent analyses. An overview of the confidence in the body of evidence for all health
effects associated with vinyl chloride exposure is presented in Table C-19.
Five properties of the body of evidence were considered to determine whether the confidence rating
should be downgraded:
• Risk of bias. Evaluation of whether there is substantial risk of bias across most of the studies
examining the outcome. This evaluation used the risk of bias tier groupings for individual studies
examining a particular outcome (Tables C-8, C-9, and C-10). Below are the criteria used to
determine whether the initial confidence in the body of evidence for each outcome should be
downgraded for risk of bias:
o No downgrade if most studies are in the risk of bias first tier
o Downgrade one confidence level if most studies are in the risk of bias second tier
o Downgrade two confidence levels if most studies are in the risk of bias third tier
• U
nexplained inconsistency. Evaluation of whether there is inconsistency or large variability in
the magnitude or direction of estimates of effect across studies that cannot be explained. Below
are the criteria used to determine whether the initial confidence in the body of evidence for each
outcome should be downgraded for unexplained inconsistency:
o No downgrade if there is little inconsistency across studies or if only one study evaluated
the outcome
VINYL CHLORIDE
APPENDIX C
o Downgrade one confidence level if there is variability across studies in the magnitude or
direction of the effect
o Downgrade two confidence levels if there is substantial variability across studies in the
magnitude or direct of the effect
• In
directness. Evaluation of four factors that can affect the applicability, generalizability, and
relevance of the studies:
o Relevance of the animal model to human health—unless otherwise indicated, studies in
rats, mice, and other mammalian species are considered relevant to humans
o Directness of the endpoints to the primary health outcome—examples of secondary
outcomes or nonspecific outcomes include organ weight in the absence of histopathology
or clinical chemistry findings in the absence of target tissue effects
o Nature of the exposure in human studies and route of administration in animal studies—
inhalation, oral, and dermal exposure routes are considered relevant unless there are
compelling data to the contrary
o Duration of treatment in animal studies and length of time between exposure and
outcome assessment in animal and prospective human studies—this should be considered
on an outcome-specific basis
Below are the criteria used to determine whether the initial confidence in the body of evidence for
each outcome should be downgraded for indirectness:
o No downgrade if none of the factors are considered indirect
o Downgrade one confidence level if one of the factors is considered indirect
o Downgrade two confidence levels if two or more of the factors are considered indirect
• Imprecision. Evaluation of the narrowness of the effect size estimates and whether the studies
have adequate statistical power. Data are considered imprecise when the ratio of the upper to
lower 95% confidence intervals (CIs) for most studies is ≥10 for tests of ratio measures (e.g.,
odds ratios) and ≥100 for absolute measures (e.g., percent control response). Adequate statistical
power is determined if the study can detect a potentially biologically meaningful difference
between groups (20% change from control response for categorical data or risk ratio of 1.5 for
continuous data). Below are the criteria used to determine whether the initial confidence in the
body of evidence for each outcome should be downgraded for imprecision:
o No downgrade if there are no serious imprecisions
o Downgrade one confidence level for serious imprecisions
o Downgrade two confidence levels for very serious imprecisions
• Public
ation bias. Evaluation of the concern that studies with statistically significant results are
more likely to be published than studies without statistically significant results.
o Downgrade one level of confidence for cases where there is serious concern with
publication bias

VINYL CHLORIDE C-33
APPENDIX C
Table C-18. Adjustments to the Initial Confidence in the Body of Evidence
Initial confidence
Adjustments to the initial
confidence rating
Final confidence
Outcome: Hepatic
Human studies
Moderate
+1 consistency
High
Animal studies
High
-1 inconsistency
Moderate
Outcome: Immunological
Human studies
Moderate
-1 risk of bias, +1 consistency
Moderate
Animal studies
High
-1 inconsistency, -1 indirectness
Low
Outcome: Neurological
Human Studies
Moderate
None
Moderate
Animal Studies
Moderate
None
Moderate
Outcome: Developmental
Human studies
Moderate
-1 risk of bias
Low
Animal studies
High
None
High
Outcome: Other noncancer (insulin resistance)
Human studies
Moderate
-1 indirectness
Low
Animal studies
High
-1 risk of bias
Moderate

VINYL CHLORIDE C-34
APPENDIX C
Table C-19. Confidence in the Body of Evidence for Vinyl Chloride
Outcome
Confidence in body of evidence
Human studies
Animal studies
Hepatic
High
Moderate
Immunological
Moderate
Low
Neurological
Moderate
Moderate
Developmental
Low
High
Other Noncancer (Insulin resistance)
Low
Moderate
Four properties of the body of evidence were considered to determine whether the confidence rating
should be upgraded:
• Large magnitude of effect. Evaluation of whether the magnitude of effect is sufficiently large
so that it is unlikely to have occurred as a result of bias from potential confounding factors.
o Upgrade one confidence level if there is evidence of a large magnitude of effect in a few
studies, provided that the studies have an overall low risk of bias and there is no serious
unexplained inconsistency among the studies of similar dose or exposure levels;
confidence can also be upgraded if there is one study examining the outcome, provided
that the study has an overall low risk of bias
• Do
se response. Evaluation of the dose-response relationships measured within a study and
across studies. Below are the criteria used to determine whether the initial confidence in the body
of evidence for each outcome should be upgraded:
o Upgrade one confidence level for evidence of a monotonic dose-response gradient
o Upgrade one confidence level for evidence of a non-monotonic dose-response gradient
where there is prior knowledge that supports a non-monotonic dose-response and a non-
monotonic dose-response gradient is observed across studies
• Plausible confounding or other residual biases. This factor primarily applies to human studies
and is an evaluation of unmeasured determinants of an outcome such as residual bias towards the
null (e.g., “healthy worker” effect) or residual bias suggesting a spurious effect (e.g., recall bias).
Below is the criterion used to determine whether the initial confidence in the body of evidence for
each outcome should be upgraded:
o Upgrade one confidence level for evidence that residual confounding or bias would
underestimate an apparent association or treatment effect (i.e., bias toward the null) or
suggest a spurious effect when results suggest no effect
• Consistency in the body of evidence. Evaluation of consistency across animal models and
species, consistency across independent studies of different human populations and exposure
scenarios, and consistency across human study types. Below is the criterion used to determine
whether the initial confidence in the body of evidence for each outcome should be upgraded:
o Upgrade one confidence level if there is a high degree of consistency in the database

VINYL CHLORIDE C-35
APPENDIX C
C.7 TRANSLATE CONFIDENCE RATING INTO LEVEL OF EVIDENCE OF HEALTH
EFFECTS
In the seventh step of the systematic review of the health effects data for vinyl chloride, the confidence in
the body of evidence for specific outcomes was translated to a level of evidence rating. The level of
evidence rating reflected the confidence in the body of evidence and the direction of the effect (i.e.,
toxicity or no toxicity); route-specific differences were noted. The level of evidence for health effects
was rated on a five-point scale:
• High level of evidence: High confidence in the body of evidence for an association between
exposure to the substance and the health outcome
• Moderate level of evidence: Moderate confidence in the body of evidence for an association
between exposure to the substance and the health outcome
• Low level of evidence: Low confidence in the body of evidence for an association between
exposure to the substance and the health outcome
• Evidence of no health effect: High confidence in the body of evidence that exposure to the
substance is not associated with the health outcome
• Inadequate evidence: Low or moderate confidence in the body of evidence that exposure to the
substance is not associated with the health outcome OR very low confidence in the body of
evidence for an association between exposure to the substance and the health outcome
A summary of the level of evidence of health effects for vinyl chloride is presented in Table C-20.
Table C-20. Level of Evidence of Health Effects for Vinyl Chloride
Outcome
Confidence in
body of evidence
Direction of
health effect
Level of evidence
for health effect
Human studies
Hepatic
High
Health effect
High
Immunological
Moderate
Health effect
Moderate
Neurological
Moderate
Health effect
Moderate
Developmental
Low
No health effect
Inadequate
Other noncancer (insulin resistance)
Low
Health effect
Low
Animal studies
Hepatic
Moderate
Health effect
Moderate
Immunological
Low
No health effect
Inadequate
Neurological
Moderate
Health effect
Moderate
Developmental
High
Health effect
High
Other noncancer (insulin resistance)
Moderate
No health effect
Inadequate
VINYL CHLORIDE C-36
APPENDIX C
C.8 INTEGRATE EVIDENCE TO DEVELOP HAZARD IDENTIFICATION CONCLUSIONS
The final step involved the integration of the evidence streams for the human studies and animal studies
to allow for a determination of hazard identification conclusions. For health effects, there were four
hazard identification conclusion categories:
• Known to be a hazard to humans
• Presumed to be a hazard to humans
• Suspected to be a hazard to humans
• Not classifiable as to the hazard to humans
The initial hazard identification was based on the highest level of evidence in the human studies and the
level of evidence in the animal studies; if there were no data for one evidence stream (human or animal),
then the hazard identification was based on the one data stream (equivalent to treating the missing
evidence stream as having low level of evidence). The hazard identification scheme is presented in
Figure C-1 and described below:
• Known: A health effect in this category would have:
o High level of evidence for health effects in human studies AND a high, moderate, or low
level of evidence in animal studies.
• Presumed: A health effect in this category would have:
o Moderate level of evidence in human studies AND high or moderate level of evidence in
animal studies OR
o Low level of evidence in human studies AND high level of evidence in animal studies
• Suspected: A health effect in this category would have:
o Moderate level of evidence in human studies AND low level of evidence in animal
studies OR
o Low level of evidence in human studies AND moderate level of evidence in animal
studies
• Not classifiable: A health effect in this category would have:
o Low level of evidence in human studies AND low level of evidence in animal studies

VINYL CHLORIDE C-37
APPENDIX C
Figure C-1. Hazard Identification Scheme
Other relevant data such as mechanistic or mode-of-action data were considered to raise or lower the level
of the hazard identification conclusion by providing information that supported or opposed biological
plausibility.
Two hazard identification conclusion categories were used when the data indicated that there may be no
health effect in humans:
• Not identified to be a hazard in humans
• Inadequate to determine hazard to humans
If the human level of evidence conclusion of no health effect was supported by the animal evidence of no
health effect, then the hazard identification conclusion category of “not identified” was used. If the
human or animal level of evidence was considered inadequate, then a hazard identification conclusion
category of “inadequate” was used. As with the hazard identification for health effects, the impact of
other relevant data was also considered for no health effect data.
The hazard identification conclusions for vinyl chloride are listed below and summarized in Table C-21.
VINYL CHLORIDE C-38
APPENDIX C
Presumed Health Effects
• Hepatic
o High level of evidence of hepatic effects in humans based on fibrosis, cirrhosis, and
steatosis observed in vinyl chloride workers (Cave et al. 2010; Du and Wang 1998;
Fedeli et al. 2019a; Hsiao et al. 2004; Hsieh et al. 2007; Maroni et al. 2003; Mastrangelo
et al. 2004; Mundt et al. 2017; Ward et al. 2001; Yuan et al. 2020).
o Moderate evidence level in animals including increased liver weight and
histopathological liver lesions in rats and mice following inhalation (Bi et al. 1985; Jia et
al. 2022; Lester et al. 1963; Sokal et al. 1980; Thornton et al. 2002; Torkelson et al. 1961;
Wisniewska-Knypl et al. 1980) and oral exposure (Feron et al. 1981; Til et al. 1983,
1991).
• Neurological
o Moderate level of evidence in humans based on neurological symptoms reported in
human studies (Lester et al. 1963; NIOSH 1977; Patty et al. 1930; Spirtas et al. 1975;
Zhu et al. 2005a) and a single report of peripheral neuropathy (Perticoni et al. 1986).
o Moderate level of evidence in animals based on clinical signs in multiple acute-duration
inhalation studies (Hehir et al. 1981; Jaeger et al. 1974; Lester et al. 1963; Mastromatteo
et al. 1960; Patty et al. 1930)
Suspected Health Effects
• Immunological
o Moderate level of evidence in humans based on occupational worker studies
demonstrating an increase in circulating immune complexes, immunoglobulins,
complement factors, and levels of inflammatory cytokines (Bencko et al. 1988,
Bogdanikowa and Zawilska 1984; Cave et al. 2010; Grainger et al. 1980; Saad et al.
2017; Wagnerova et al. 1988; Ward 1976).
o Inadequate evidence in animals due to limited information available on increased spleen
weight in rats (Bi et al. 1985; Sokal et al. 1980) and a splenic lymphocyte proliferation
assay in mice and rabbits (Sharma and Gehring 1979, Sharma et al. 1980)
• Developmental
o Inadequate evidence in humans due to the absence of demonstrated developmental effects
in a small number of ecological and case-control studies of birth defects (Edmonds et al.
1978; Infante 1976; Infante et al. 1976a, 1976b; NIOSH 1977; Rosenman et al. 1989;
Ruckart et al. 2013; Swartz et al. 2015; Talbott et al. 2015; Theriault et al. 1983).
o High level of evidence in animals based on developmental effects occurring at low
concentrations in inhalation studies (John et al. 1977, 1981).
Not Classifiable
• Other noncancer (insulin resistance)
o Low level of evidence level in humans based on two epidemiology studies with serum
markers of increased insulin resistance (Cave et al. 2010; Lee et al. 2020).
o Several intermediate-duration inhalation studies using glucose, insulin, and pyruvate
tolerance tests (Chen et al. 2019; Lang et al. 2018) and measures of fasting blood glucose
and glycogen storage (Wahlang et al. 2020). These studies used a single low
concentration of vinyl chloride (0.85 ppm) and did not evaluate effects at higher
concentrations.
VINYL CHLORIDE C-39
APPENDIX C
Table C-21. Hazard Identification Conclusions for Vinyl Chloride
Outcome
Hazard identification
Hepatic
Presumed health effect
Immunological
Suspected health effect
Neurological
Presumed health effect
Developmental
Suspected health effect
Other noncancer (insulin resistance)
Not classifiable
VINYL CHLORIDE D-1
APPENDIX D. USER'S GUIDE
Chapter 1. Relevance to Public Health
This chapter provides an overview of U.S. exposures, a summary of health effects based on evaluations of
existing toxicologic, epidemiologic, and toxicokinetic information, and an overview of the minimal risk
levels. This is designed to present interpretive, weight-of-evidence discussions for human health
endpoints by addressing the following questions:
1. What effects are known to occur in humans?
2. What effects observed in animals are likely to be of concern to humans?
3. What exposure conditions are likely to be of concern to humans, especially around hazardous
waste sites?
Minimal Risk Levels (MRLs)
Where sufficient toxicologic information is available, ATSDR derives MRLs for inhalation and oral
routes of entry at each duration of exposure (acute, intermediate, and chronic). These MRLs are not
meant to support regulatory action, but to acquaint health professionals with exposure levels at which
adverse health effects are not expected to occur in humans.
MRLs should help physicians and public health officials determine the safety of a community living near
a hazardous substance emission, given the concentration of a contaminant in air or the estimated daily
dose in water. MRLs are based largely on toxicological studies in animals and on reports of human
occupational exposure.
MRL users should be familiar with the toxicologic information on which the number is based.
Section 1.2, Summary of Health Effects, contains basic information known about the substance. Other
sections, such as Section 3.2 Children and Other Populations that are Unusually Susceptible and
Section 3.4 Interactions with Other Substances, provide important supplemental information.
MRL users should also understand the MRL derivation methodology. MRLs are derived using a
modified version of the risk assessment methodology that the Environmental Protection Agency (EPA)
provides (Barnes and Dourson 1988) to determine reference doses (RfDs) for lifetime exposure.
To derive an MRL, ATSDR generally selects the most sensitive endpoint which, in its best judgement,
represents the most sensitive human health effect for a given exposure route and duration. ATSDR
cannot make this judgement or derive an MRL unless information (quantitative or qualitative) is available
for all potential systemic, neurological, and developmental effects. If this information and reliable
quantitative data on the chosen endpoint are available, ATSDR derives an MRL using the most sensitive
species (when information from multiple species is available) with the highest no-observed-adverse-effect
level (NOAEL) that does not exceed any adverse effect levels. When a NOAEL is not available, a
lowest-observed-adverse-effect level (LOAEL) can be used to derive an MRL, and an uncertainty factor
of 10 must be employed. Additional uncertainty factors of 10 must be used both for human variability to
protect sensitive subpopulations (people who are most susceptible to the health effects caused by the
substance) and for interspecies variability (extrapolation from animals to humans). In deriving an MRL,
these individual uncertainty factors are multiplied together. The product is then divided into the
inhalation concentration or oral dosage selected from the study. Uncertainty factors used in developing a

VINYL CHLORIDE D-2
APPENDIX D
substance-specific MRL are provided in the footnotes of the levels of significant exposure (LSE) tables
that are provided in Chapter 2. Detailed discussions of the MRLs are presented in Appendix A.
Chapter 2. Health Effects
Tables and Figures for Levels of Significant Exposure (LSE)
Tables and figures are used to summarize health effects and illustrate graphically levels of exposure
associated with those effects. These levels cover health effects observed at increasing dose
concentrations and durations, differences in response by species and MRLs to humans for noncancer
endpoints. The LSE tables and figures can be used for a quick review of the health effects and to locate
data for a specific exposure scenario. The LSE tables and figures should always be used in conjunction
with the text. All entries in these tables and figures represent studies that provide reliable, quantitative
estimates of NOAELs, LOAELs, or Cancer Effect Levels (CELs).
The legends presented below demonstrate the application of these tables and figures. Representative
examples of LSE tables and figures follow. The numbers in the left column of the legends correspond to
the numbers in the example table and figure.
TABLE LEGEND
See Sample LSE Table (page D-5)
(1) Route of exposure. One of the first considerations when reviewing the toxicity of a substance
using these tables and figures should be the relevant and appropriate route of exposure.
Typically, when sufficient data exist, three LSE tables and two LSE figures are presented in the
document. The three LSE tables present data on the three principal routes of exposure
(i.e., inhalation, oral, and dermal). LSE figures are limited to the inhalation and oral routes. Not
all substances will have data on each route of exposure and will not, therefore, have all five of the
tables and figures. Profiles with more than one chemical may have more LSE tables and figures.
(2) Exposure period. Three exposure periods—acute (<15 days), intermediate (15–364 days), and
chronic (≥365 days)—are presented within each relevant route of exposure. In this example, two
oral studies of chronic-duration exposure are reported. For quick reference to health effects
occurring from a known length of exposure, locate the applicable exposure period within the LSE
table and figure.
(3) Figure key. Each key number in the LSE table links study information to one or more data points
using the same key number in the corresponding LSE figure. In this example, the study
represented by key number 51 identified NOAELs and less serious LOAELs (also see the three
"51R" data points in sample LSE Figure 2-X).
(4) Species (strain) No./group. The test species (and strain), whether animal or human, are identified
in this column. The column also contains information on the number of subjects and sex per
group. Chapter 1, Relevance to Public Health, covers the relevance of animal data to human
toxicity and Section 3.1, Toxicokinetics, contains any available information on comparative
toxicokinetics. Although NOAELs and LOAELs are species specific, the levels are extrapolated
to equivalent human doses to derive an MRL.
(5) Exposure parameters/doses. The duration of the study and exposure regimens are provided in
these columns. This permits comparison of NOAELs and LOAELs from different studies. In
this case (key number 51), rats were orally exposed to “Chemical X” via feed for 2 years. For a

VINYL CHLORIDE D-3
APPENDIX D
more complete review of the dosing regimen, refer to the appropriate sections of the text or the
original reference paper (i.e., Aida et al. 1992).
(6) Parameters monitored. This column lists the parameters used to assess health effects. Parameters
monitored could include serum (blood) chemistry (BC), biochemical changes (BI), body weight
(BW), clinical signs (CS), developmental toxicity (DX), food intake (FI), gross necropsy (GN),
hematology (HE), histopathology (HP), immune function (IX), lethality (LE), neurological
function (NX), organ function (OF), ophthalmology (OP), organ weight (OW), reproductive
function (RX), urinalysis (UR), and water intake (WI).
(7) Endpoint. This column lists the endpoint examined. The major categories of health endpoints
included in LSE tables and figures are death, body weight, respiratory, cardiovascular,
gastrointestinal, hematological, musculoskeletal, hepatic, renal, dermal, ocular, endocrine,
immunological, neurological, reproductive, developmental, other noncancer, and cancer. "Other
noncancer" refers to any effect (e.g., alterations in blood glucose levels) not covered in these
systems. In the example of key number 51, three endpoints (body weight, hematological, and
hepatic) were investigated.
(8) NOAEL. A NOAEL is the highest exposure level at which no adverse effects were seen in the
organ system studied. The body weight effect reported in key number 51 is a NOAEL at
25.5 mg/kg/day. NOAELs are not reported for cancer and death; with the exception of these two
endpoints, this field is left blank if no NOAEL was identified in the study.
(9) LOAEL. A LOAEL is the lowest dose used in the study that caused an adverse health effect.
LOAELs have been classified into "Less Serious" and "Serious" effects. These distinctions help
readers identify the levels of exposure at which adverse health effects first appear and the
gradation of effects with increasing dose. A brief description of the specific endpoint used to
quantify the adverse effect accompanies the LOAEL. Key number 51 reports a less serious
LOAEL of 6.1 mg/kg/day for the hepatic system, which was used to derive a chronic exposure,
oral MRL of 0.008 mg/kg/day (see footnote "c"). MRLs are not derived from serious LOAELs.
A cancer effect level (CEL) is the lowest exposure level associated with the onset of
carcinogenesis in experimental or epidemiologic studies. CELs are always considered serious
effects. The LSE tables and figures do not contain NOAELs for cancer, but the text may report
doses not causing measurable cancer increases. If no LOAEL/CEL values were identified in the
study, this field is left blank.
(10) Reference. The complete reference citation is provided in Chapter 8 of the profile.
(11) Footnotes. Explanations of abbreviations or reference notes for data in the LSE tables are found
in the footnotes. For example, footnote "c" indicates that the LOAEL of 6.1 mg/kg/day in key
number 51 was used to derive an oral MRL of 0.008 mg/kg/day.
FIGURE LEGEND
See Sample LSE Figure (page D-6)
LSE figures graphically illustrate the data presented in the corresponding LSE tables. Figures help the
reader quickly compare health effects according to exposure concentrations for particular exposure
periods.
(12) Exposure period. The same exposure periods appear as in the LSE table. In this example, health
effects observed within the chronic exposure period are illustrated.

VINYL CHLORIDE D-4
APPENDIX D
(13) Endpoint. These are the categories of health effects for which reliable quantitative data exist.
The same health effect endpoints appear in the LSE table.
(14) Levels of exposure. Concentrations or doses for each health effect in the LSE tables are
graphically displayed in the LSE figures. Exposure concentration or dose is measured on the log
scale "y" axis. Inhalation exposure is reported in mg/m
3
or ppm and oral exposure is reported in
mg/kg/day.
(15) LOAEL. In this example, the half-shaded circle that is designated 51R identifies a LOAEL
critical endpoint in the rat upon which a chronic oral exposure MRL is based. The key number
51 corresponds to the entry in the LSE table. The dashed descending arrow indicates the
extrapolation from the exposure level of 6.1 mg/kg/day (see entry 51 in the sample LSE table) to
the MRL of 0.008 mg/kg/day (see footnote "c" in the sample LSE table).
(16) CEL. Key number 59R is one of studies for which CELs were derived. The diamond symbol
refers to a CEL for the test species (rat). The number 59 corresponds to the entry in the LSE
table.
(17) Key to LSE figure. The key provides the abbreviations and symbols used in the figure.

VINYL CHLORIDE D-5
APPENDIX D

VINYL CHLORIDE D-6
APPENDIX D

VINYL CHLORIDE E-1
APPENDIX E. QUICK REFERENCE FOR HEALTH CARE PROVIDERS
Toxicological Profiles are a unique compilation of toxicological information on a given hazardous
substance. Each profile reflects a comprehensive and extensive evaluation, summary, and interpretation
of available toxicologic and epidemiologic information on a substance. Health care providers treating
patients potentially exposed to hazardous substances may find the following information helpful for fast
answers to often-asked questions.
Primary Chapters/Sections of Interest
Chapter 1: Relevance to Public Health: The Relevance to Public Health Section provides an overview
of exposure and health effects and evaluates, interprets, and assesses the significance of toxicity
data to human health. A table listing minimal risk levels (MRLs) is also included in this chapter.
Chapter 2: Health Effects: Specific health effects identified in both human and animal studies are
reported by type of health effect (e.g., death, hepatic, renal, immune, reproductive), route of
exposure (e.g., inhalation, oral, dermal), and length of exposure (e.g., acute, intermediate, and
chronic).
NOTE: Not all health effects reported in this section are necessarily observed in the clinical
setting.
Pediatrics:
Section 3.2 Children and Other Populations that are Unusually Susceptible
Section 3.3 Biomarkers of Exposure and Effect
ATSDR Information Center
Phone: 1-800-CDC-INFO (800-232-4636) or 1-888-232-6348 (TTY)
Internet: http://www.atsdr.cdc.gov
ATSDR develops educational and informational materials for health care providers categorized by
hazardous substance, clinical condition, and/or by susceptible population. The following additional
materials are available online:
Clinician Briefs and Overview discuss health effects and approaches to patient management in a
brief/factsheet style. They are narrated PowerPoint presentations with Continuing Education
credit available (see https://www.atsdr.cdc.gov/emes/health_professionals/clinician-briefs-
overviews.html).
Managing Hazardous Materials Incidents is a set of recommendations for on-scene (prehospital) and
hospital medical management of patients exposed during a hazardous materials incident (see
https://www.atsdr.cdc.gov/MHMI/index.asp).
Fact Sheets (ToxFAQs™) provide answers to frequently asked questions about toxic substances (see
https://www.atsdr.cdc.gov/toxfaqs/Index.asp).

VINYL CHLORIDE E-2
APPENDIX E
Other Agencies and Organizations
The National Center for Environmental Health (NCEH) focuses on preventing or controlling disease,
injury, and disability related to the interactions between people and their environment outside the
workplace. Contact: NCEH, Mailstop F-29, 4770 Buford Highway, NE, Atlanta, GA
30341-3724 • Phone: 770-488-7000 • FAX: 770-488-7015 • Web Page:
https://www.cdc.gov/nceh/.
The National Institute for Occupational Safety and Health (NIOSH) conducts research on occupational
diseases and injuries, responds to requests for assistance by investigating problems of health and
safety in the workplace, recommends standards to the Occupational Safety and Health
Administration (OSHA) and the Mine Safety and Health Administration (MSHA), and trains
professionals in occupational safety and health. Contact: NIOSH, 395 E Street, S.W., Suite 9200,
Patriots Plaza Building, Washington, DC 20201 • Phone: 202-245-0625 or 1-800-CDC-INFO
(800-232-4636) • Web Page: https://www.cdc.gov/niosh/.
The National Institute of Environmental Health Sciences (NIEHS) is the principal federal agency for
biomedical research on the effects of chemical, physical, and biologic environmental agents on
human health and well-being. Contact: NIEHS, PO Box 12233, 104 T.W. Alexander Drive,
Research Triangle Park, NC 27709 • Phone: 919-541-3212 • Web Page:
https://www.niehs.nih.gov/.
Clinical Resources (Publicly Available Information)
The Association of Occupational and Environmental Clinics (AOEC) has developed a network of clinics
in the United States to provide expertise in occupational and environmental issues. Contact:
AOEC, 1010 Vermont Avenue, NW, #513, Washington, DC 20005 • Phone: 202-347-4976
The American College of Occupational and Environmental Medicine (ACOEM) is an association of
physicians and other health care providers specializing in the field of occupational and
environmental medicine. Contact: ACOEM, 25 Northwest Point Boulevard, Suite 700, Elk
Grove Village, IL 60007-1030 • Phone: 847-818-1800 • FAX: 847-818-9266 • Web Page:
http://www.acoem.org/.
The American College of Medical Toxicology (ACMT) is a nonprofit association of physicians with
recognized expertise in medical toxicology. Contact: ACMT, 10645 North Tatum Boulevard,
Suite 200-111, Phoenix AZ 85028 • Phone: 844-226-8333 • FAX: 844-226-8333 • Web Page:
http://www.acmt.net.
The Pediatric Environmental Health Specialty Units (PEHSUs) is an interconnected system of specialists
who respond to questions from public health professionals, clinicians, policy makers, and the
public about the impact of environmental factors on the health of children and reproductive-aged
adults. Contact information for regional centers can be found at http://pehsu.net/findhelp.html.
The American Association of Poison Control Centers (AAPCC) provide support on the prevention and
treatment of poison exposures. Contact: AAPCC, 515 King Street, Suite 510, Alexandria VA
22314 • Phone: 701-894-1858 • Poison Help Line: 1-800-222-1222 • Web Page:
http://www.aapcc.org/.
VINYL CHLORIDE F-1
APPENDIX F. GLOSSARY
Absorption—The process by which a substance crosses biological membranes and enters systemic
circulation. Absorption can also refer to the taking up of liquids by solids, or of gases by solids or liquids.
Acute Exposure—Exposure to a chemical for a duration of ≤14 days, as specified in the Toxicological
Profiles.
Adsorption—The adhesion in an extremely thin layer of molecules (as of gases, solutes, or liquids) to the
surfaces of solid bodies or liquids with which they are in contact.
Adsorption Coefficient (K
oc
)—The ratio of the amount of a chemical adsorbed per unit weight of
organic carbon in the soil or sediment to the concentration of the chemical in solution at equilibrium.
Adsorption Ratio (Kd)—The amount of a chemical adsorbed by sediment or soil (i.e., the solid phase)
divided by the amount of chemical in the solution phase, which is in equilibrium with the solid phase, at a
fixed solid/solution ratio. It is generally expressed in micrograms of chemical sorbed per gram of soil or
sediment.
Benchmark Dose (BMD) or Benchmark Concentration (BMC)—is the dose/concentration
corresponding to a specific response level estimate using a statistical dose-response model applied to
either experimental toxicology or epidemiology data. For example, a BMD
10
would be the dose
corresponding to a 10% benchmark response (BMR). The BMD is determined by modeling the dose-
response curve in the region of the dose-response relationship where biologically observable data are
feasible. The BMDL or BMCL is the 95% lower confidence limit on the BMD or BMC.
Bioconcentration Factor (BCF)—The quotient of the concentration of a chemical in aquatic organisms
at a specific time or during a discrete time period of exposure divided by the concentration in the
surrounding water at the same time or during the same period.
Biomarkers—Indicators signaling events in biologic systems or samples, typically classified as markers
of exposure, effect, and susceptibility.
Cancer Effect Level (CEL)—The lowest dose of a chemical in a study, or group of studies, that
produces significant increases in the incidence of cancer (or malignant tumors) between the exposed
population and its appropriate control.
Carcinogen—A chemical capable of inducing cancer.
Case-Control Study—A type of epidemiological study that examines the relationship between a
particular outcome (disease or condition) and a variety of potential causative agents (such as toxic
chemicals). In a case-control study, a group of people with a specified and well-defined outcome is
identified and compared to a similar group of people without the outcome.
Case Report—A report that describes a single individual with a particular disease or exposure. These
reports may suggest some potential topics for scientific research, but are not actual research studies.
Case Series—Reports that describe the experience of a small number of individuals with the same
disease or exposure. These reports may suggest potential topics for scientific research, but are not actual
research studies.
VINYL CHLORIDE F-2
APPENDIX F
Ceiling Value—A concentration that must not be exceeded.
Chronic Exposure—Exposure to a chemical for ≥365 days, as specified in the Toxicological Profiles.
Clastogen—A substance that causes breaks in chromosomes resulting in addition, deletion, or
rearrangement of parts of the chromosome.
Cohort Study—A type of epidemiological study of a specific group or groups of people who have had a
common insult (e.g., exposure to an agent suspected of causing disease or a common disease) and are
followed forward from exposure to outcome, and who are disease-free at start of follow-up. Often, at
least one exposed group is compared to one unexposed group, while in other cohorts, exposure is a
continuous variable and analyses are directed towards analyzing an exposure-response coefficient.
Cross-sectional Study—A type of epidemiological study of a group or groups of people that examines
the relationship between exposure and outcome to a chemical or to chemicals at a specific point in time.
Data Needs—Substance-specific informational needs that, if met, would reduce the uncertainties of
human health risk assessment.
Developmental Toxicity—The occurrence of adverse effects on the developing organism that may result
from exposure to a chemical prior to conception (either parent), during prenatal development, or
postnatally to the time of sexual maturation. Adverse developmental effects may be detected at any point
in the life span of the organism.
Dose-Response Relationship—The quantitative relationship between the amount of exposure to a
toxicant and the incidence of the response or amount of the response.
Embryotoxicity and Fetotoxicity—Any toxic effect on the conceptus as a result of prenatal exposure to
a chemical; the distinguishing feature between the two terms is the stage of development during which the
effect occurs. Effects include malformations and variations, altered growth, and in utero death.
Epidemiology—The investigation of factors that determine the frequency and distribution of disease or
other health-related conditions within a defined human population during a specified period.
Excretion—The process by which metabolic waste products are removed from the body.
Genotoxicity—A specific adverse effect on the genome of living cells that, upon the duplication of
affected cells, can be expressed as a mutagenic, clastogenic, or carcinogenic event because of specific
alteration of the molecular structure of the genome.
Half-life—A measure of rate for the time required to eliminate one-half of a quantity of a chemical from
the body or environmental media.
Health Advisory—An estimate of acceptable drinking water levels for a chemical substance derived by
EPA and based on health effects information. A health advisory is not a legally enforceable federal
standard, but serves as technical guidance to assist federal, state, and local officials.
Immediately Dangerous to Life or Health (IDLH)—A condition that poses a threat of life or health, or
conditions that pose an immediate threat of severe exposure to contaminants that are likely to have
adverse cumulative or delayed effects on health.
VINYL CHLORIDE F-3
APPENDIX F
Immunotoxicity—Adverse effect on the functioning of the immune system that may result from
exposure to chemical substances.
Incidence—The ratio of new cases of individuals in a population who develop a specified condition to
the total number of individuals in that population who could have developed that condition in a specified
time period.
Intermediate Exposure—Exposure to a chemical for a duration of 15–364 days, as specified in the
Toxicological Profiles.
In Vitro—Isolated from the living organism and artificially maintained, as in a test tube.
In Vivo—Occurring within the living organism.
Lethal Concentration
(LO)
(LC
LO
)—The lowest concentration of a chemical in air that has been reported
to have caused death in humans or animals.
Lethal Concentration
(50)
(LC
50
)—A calculated concentration of a chemical in air to which exposure for
a specific length of time is expected to cause death in 50% of a defined experimental animal population.
Lethal Dose
(LO)
(LD
Lo
)—The lowest dose of a chemical introduced by a route other than inhalation that
has been reported to have caused death in humans or animals.
Lethal Dose
(50)
(LD
50
)—The dose of a chemical that has been calculated to cause death in 50% of a
defined experimental animal population.
Lethal Time
(50)
(LT
50
)—A calculated period of time within which a specific concentration of a chemical
is expected to cause death in 50% of a defined experimental animal population.
Lowest-Observed-Adverse-Effect Level (LOAEL)—The lowest exposure level of chemical in a study,
or group of studies, that produces statistically or biologically significant increases in frequency or severity
of adverse effects between the exposed population and its appropriate control.
Lymphoreticular Effects—Represent morphological effects involving lymphatic tissues such as the
lymph nodes, spleen, and thymus.
Malformations—Permanent structural changes that may adversely affect survival, development, or
function.
Metabolism—Process in which chemical substances are biotransformed in the body that could result in
less toxic and/or readily excreted compounds or produce a biologically active intermediate.
Minimal LOAEL—Indicates a minimal adverse effect or a reduced capacity of an organ or system to
absorb additional toxic stress that does not necessarily lead to the inability of the organ or system to
function normally.
Minimal Risk Level (MRL)—An estimate of daily human exposure to a hazardous substance that is
likely to be without an appreciable risk of adverse noncancer health effects over a specified route and
duration of exposure.
VINYL CHLORIDE F-4
APPENDIX F
Modifying Factor (MF)—A value (greater than zero) that is applied to the derivation of a Minimal Risk
Level (MRL) to reflect additional concerns about the database that are not covered by the uncertainty
factors. The default value for a MF is 1.
Morbidity—The state of being diseased; the morbidity rate is the incidence or prevalence of a disease in
a specific population.
Mortality—Death; the mortality rate is a measure of the number of deaths in a population during a
specified interval of time.
Mutagen—A substance that causes mutations, which are changes in the DNA sequence of a cell’s DNA.
Mutations can lead to birth defects, miscarriages, or cancer.
Necropsy—The gross examination of the organs and tissues of a dead body to determine the cause of
death or pathological conditions.
Neurotoxicity—The occurrence of adverse effects on the nervous system following exposure to a
hazardous substance.
No-Observed-Adverse-Effect Level (NOAEL)—The dose of a chemical at which there were no
statistically or biologically significant increases in frequency or severity of adverse effects seen between
the exposed population and its appropriate control. Although effects may be produced at this dose, they
are not considered to be adverse.
Octanol-Water Partition Coefficient (K
ow
)—The equilibrium ratio of the concentrations of a chemical
in n-octanol and water, in dilute solution.
Odds Ratio (OR)—A means of measuring the association between an exposure (such as toxic substances
and a disease or condition) that represents the best estimate of relative risk (risk as a ratio of the incidence
among subjects exposed to a particular risk factor divided by the incidence among subjects who were not
exposed to the risk factor). An odds ratio that is greater than 1 is considered to indicate greater risk of
disease in the exposed group compared to the unexposed group.
Permissible Exposure Limit (PEL)—An Occupational Safety and Health Administration (OSHA)
regulatory limit on the amount or concentration of a substance not to be exceeded in workplace air
averaged over any 8-hour work shift of a 40-hour workweek.
Pesticide—General classification of chemicals specifically developed and produced for use in the control
of agricultural and public health pests (insects or other organisms harmful to cultivated plants or animals).
Pharmacokinetics—The dynamic behavior of a material in the body, used to predict the fate
(disposition) of an exogenous substance in an organism. Utilizing computational techniques, it provides
the means of studying the absorption, distribution, metabolism, and excretion of chemicals by the body.
Pharmacokinetic Model—A set of equations that can be used to describe the time course of a parent
chemical or metabolite in an animal system. There are two types of pharmacokinetic models: data-based
and physiologically-based. A data-based model divides the animal system into a series of compartments,
which, in general, do not represent real, identifiable anatomic regions of the body, whereas the
physiologically-based model compartments represent real anatomic regions of the body.
VINYL CHLORIDE F-5
APPENDIX F
Physiologically Based Pharmacodynamic (PBPD) Model—A type of physiologically based dose-
response model that quantitatively describes the relationship between target tissue dose and toxic
endpoints. These models advance the importance of physiologically based models in that they clearly
describe the biological effect (response) produced by the system following exposure to an exogenous
substance.
Physiologically Based Pharmacokinetic (PBPK) Model—A type of physiologically based dose-
response model that is comprised of a series of compartments representing organs or tissue groups with
realistic weights and blood flows. These models require a variety of physiological information, including
tissue volumes, blood flow rates to tissues, cardiac output, alveolar ventilation rates, and possibly
membrane permeabilities. The models also utilize biochemical information, such as blood:air partition
coefficients, and metabolic parameters. PBPK models are also called biologically based tissue dosimetry
models.
Prevalence—The number of cases of a disease or condition in a population at one point in time.
Prospective Study—A type of cohort study in which a group is followed over time and the pertinent
observations are made on events occurring after the start of the study.
Recommended Exposure Limit (REL)—A National Institute for Occupational Safety and Health
(NIOSH) time-weighted average (TWA) concentration for up to a 10-hour workday during a 40-hour
workweek.
Reference Concentration (RfC)—An estimate (with uncertainty spanning perhaps an order of
magnitude) of a continuous inhalation exposure to the human population (including sensitive subgroups)
that is likely to be without an appreciable risk of deleterious noncancer health effects during a lifetime.
The inhalation RfC is expressed in units of mg/m
3
or ppm.
Reference Dose (RfD)—An estimate (with uncertainty spanning perhaps an order of magnitude) of the
daily oral exposure of the human population to a potential hazard that is likely to be without risk of
deleterious noncancer health effects during a lifetime. The oral RfD is expressed in units of mg/kg/day.
Reportable Quantity (RQ)—The quantity of a hazardous substance that is considered reportable under
the Comprehensive Environmental Response, Compensation, and Liability Act (CERCLA). RQs are
(1) ≥1 pound or (2) for selected substances, an amount established by regulation either under CERCLA or
under Section 311 of the Clean Water Act. Quantities are measured over a 24-hour period.
Reproductive Toxicity—The occurrence of adverse effects on the reproductive system that may result
from exposure to a hazardous substance. The toxicity may be directed to the reproductive organs and/or
the related endocrine system. The manifestation of such toxicity may be noted as alterations in sexual
behavior, fertility, pregnancy outcomes, or modifications in other functions that are dependent on the
integrity of this system.
Retrospective Study—A type of cohort study based on a group of persons known to have been exposed
at some time in the past. Data are collected from routinely recorded events, up to the time the study is
undertaken. Retrospective studies are limited to causal factors that can be ascertained from existing
records and/or examining survivors of the cohort.
Risk—The possibility or chance that some adverse effect will result from a given exposure to a hazardous
substance.
VINYL CHLORIDE F-6
APPENDIX F
Risk Factor—An aspect of personal behavior or lifestyle, an environmental exposure, existing health
condition, or an inborn or inherited characteristic that is associated with an increased occurrence of
disease or other health-related event or condition.
Risk Ratio/Relative Risk—The ratio of the risk among persons with specific risk factors compared to the
risk among persons without risk factors. A risk ratio that is greater than 1 indicates greater risk of disease
in the exposed group compared to the unexposed group.
Serious LOAEL—A dose that evokes failure in a biological system and can lead to morbidity or
mortality.
Short-Term Exposure Limit (STEL)—A STEL is a 15-minute TWA exposure that should not be
exceeded at any time during a workday.
Standardized Mortality Ratio (SMR)—A ratio of the observed number of deaths and the expected
number of deaths in a specific standard population.
Target Organ Toxicity—This term covers a broad range of adverse effects on target organs or
physiological systems (e.g., renal, cardiovascular) extending from those arising through a single limited
exposure to those assumed over a lifetime of exposure to a chemical.
Teratogen—A chemical that causes structural defects that affect the development of an organism.
Threshold Limit Value (TLV)—An American Conference of Governmental Industrial Hygienists
(ACGIH) concentration of a substance to which it is believed that nearly all workers may be repeatedly
exposed, day after day, for a working lifetime without adverse effect. The TLV may be expressed as a
Time-Weighted Average (TLV-TWA), as a Short-Term Exposure Limit (TLV-STEL), or as a ceiling
limit (TLV-C).
Time-Weighted Average (TWA)—An average exposure within a given time period.
Toxicokinetic—The absorption, distribution, metabolism, and elimination of toxic compounds in the
living organism.
Toxics Release Inventory (TRI)—The TRI is an EPA program that tracks toxic chemical releases and
pollution prevention activities reported by industrial and federal facilities.
Uncertainty Factor (UF)—A factor used in operationally deriving the Minimal Risk Level (MRL),
Reference Dose (RfD), or Reference Concentration (RfC) from experimental data. UFs are intended to
account for (1) the variation in sensitivity among the members of the human population, (2) the
uncertainty in extrapolating animal data to the case of human, (3) the uncertainty in extrapolating from
data obtained in a study that is of less than lifetime exposure, and (4) the uncertainty in using lowest-
observed-adverse-effect level (LOAEL) data rather than no-observed-adverse-effect level (NOAEL) data.
A default for each individual UF is 10; if complete certainty in data exists, a value of 1 can be used;
however, a reduced UF of 3 may be used on a case-by-case basis (3 being the approximate logarithmic
average of 10 and 1).
Xenobiotic—Any substance that is foreign to the biological system.
VINYL CHLORIDE G-1
APPENDIX G. ACRONYMS, ABBREVIATIONS, AND SYMBOLS
AAPCC American Association of Poison Control Centers
ACGIH American Conference of Governmental Industrial Hygienists
ACOEM American College of Occupational and Environmental Medicine
ACMT American College of Medical Toxicology
ADI acceptable daily intake
ADME absorption, distribution, metabolism, and excretion
AEGL Acute Exposure Guideline Level
AIC Akaike’s information criterion
AIHA American Industrial Hygiene Association
ALT alanine aminotransferase
AOEC Association of Occupational and Environmental Clinics
AP alkaline phosphatase
AST aspartate aminotransferase
atm atmosphere
ATSDR Agency for Toxic Substances and Disease Registry
AWQC Ambient Water Quality Criteria
BCF bioconcentration factor
BMD/C benchmark dose or benchmark concentration
BMD
X
dose that produces a X% change in response rate of an adverse effect
BMDL
X
95% lower confidence limit on the BMD
X
BMDS Benchmark Dose Software
BMR benchmark response
BUN blood urea nitrogen
C centigrade
CAA Clean Air Act
CAS Chemical Abstract Services
CDC Centers for Disease Control and Prevention
CEL cancer effect level
CERCLA Comprehensive Environmental Response, Compensation, and Liability Act
CFR Code of Federal Regulations
Ci curie
CI confidence interval
cm centimeter
CPSC Consumer Products Safety Commission
CWA Clean Water Act
DNA deoxyribonucleic acid
DOD Department of Defense
DOE Department of Energy
DWEL drinking water exposure level
EAFUS Everything Added to Food in the United States
ECG/EKG electrocardiogram
EEG electroencephalogram
EPA Environmental Protection Agency
ERPG emergency response planning guidelines
F Fahrenheit
F1 first-filial generation
FDA Food and Drug Administration
FEMA Federal Emergency Management Agency
FIFRA Federal Insecticide, Fungicide, and Rodenticide Act
VINYL CHLORIDE G-2
APPENDIX G
FR Federal Register
FSH follicle stimulating hormone
g gram
GC gas chromatography
gd gestational day
GGT γ-glutamyl transferase
GRAS generally recognized as safe
HEC human equivalent concentration
HED human equivalent dose
HHS Department of Health and Human Services
HPLC high-performance liquid chromatography
HSDB Hazardous Substances Data Bank
IARC International Agency for Research on Cancer
IDLH immediately dangerous to life and health
IRIS Integrated Risk Information System
JECFA Joint FAO/WHO Expert Committee on Food Additives
Kd adsorption ratio
kg kilogram
kkg kilokilogram; 1 kilokilogram is equivalent to 1,000 kilograms and 1 metric ton
K
oc
organic carbon partition coefficient
K
ow
octanol-water partition coefficient
L liter
LC liquid chromatography
LC
50
lethal concentration, 50% kill
LC
Lo
lethal concentration, low
LD
50
lethal dose, 50% kill
LD
Lo
lethal dose, low
LDH lactate dehydrogenase
LH luteinizing hormone
LOAEL lowest-observed-adverse-effect level
LSE Level of Significant Exposure
LT
50
lethal time, 50% kill
m meter
mCi millicurie
MCL maximum contaminant level
MCLG maximum contaminant level goal
MF modifying factor
mg milligram
mL milliliter
mm millimeter
mmHg millimeters of mercury
mmol millimole
MRL Minimal Risk Level
MS mass spectrometry
MSHA Mine Safety and Health Administration
Mt metric ton
NAAQS National Ambient Air Quality Standard
NAS National Academy of Science
NCEH National Center for Environmental Health
ND not detected
ng nanogram
VINYL CHLORIDE G-3
APPENDIX G
NHANES National Health and Nutrition Examination Survey
NIEHS National Institute of Environmental Health Sciences
NIOSH National Institute for Occupational Safety and Health
NLM National Library of Medicine
nm nanometer
nmol nanomole
NOAEL no-observed-adverse-effect level
NPL National Priorities List
NR not reported
NRC National Research Council
NS not specified
NTP National Toxicology Program
OR odds ratio
OSHA Occupational Safety and Health Administration
PAC Protective Action Criteria
PAH polycyclic aromatic hydrocarbon
PBPD physiologically based pharmacodynamic
PBPK physiologically based pharmacokinetic
PEHSU Pediatric Environmental Health Specialty Unit
PEL permissible exposure limit
PEL-C permissible exposure limit-ceiling value
pg picogram
PND postnatal day
POD point of departure
ppb parts per billion
ppbv parts per billion by volume
ppm parts per million
ppt parts per trillion
REL recommended exposure limit
REL-C recommended exposure level-ceiling value
RfC reference concentration
RfD reference dose
RNA ribonucleic acid
SARA Superfund Amendments and Reauthorization Act
SCE sister chromatid exchange
SD standard deviation
SE standard error
SGOT serum glutamic oxaloacetic transaminase (same as aspartate aminotransferase or AST)
SGPT serum glutamic pyruvic transaminase (same as alanine aminotransferase or ALT)
SIC standard industrial classification
SLOAEL serious lowest-observed-adverse-effect level
SMR standardized mortality ratio
sRBC sheep red blood cell
STEL short term exposure limit
TLV threshold limit value
TLV-C threshold limit value-ceiling value
TRI Toxics Release Inventory
TSCA Toxic Substances Control Act
TWA time-weighted average
UF uncertainty factor
U.S. United States
VINYL CHLORIDE G-4
APPENDIX G
USDA United States Department of Agriculture
USGS United States Geological Survey
USNRC U.S. Nuclear Regulatory Commission
VOC volatile organic compound
WBC white blood cell
WHO World Health Organization
> greater than
≥ greater than or equal to
= equal to
< less than
≤ less than or equal to
% percent
α alpha
β beta
γ gamma
δ delta
μm micrometer
μg microgram
q
1
*
cancer slope factor
– negative
+ positive
(+) weakly positive result
(–) weakly negative result
